Abstract
Combining the FDA Adverse Event Reporting System (FAERS) and the Cancer Genome Atlas (TCGA) databases, we aim to explore the factors that influence anti-programmed cell death protein-1 inhibitors/programmed death-ligand-1 (PD-1/PD-L1) related severe cardiac adverse events (cAEs). We obtained anti-PD-1/PD-L1 adverse event reports from January 2014 to December 2022 from the FAERS database. Disproportionality analysis was performed to find anti-PD-1/PD-L1-related cAEs using the proportional reporting ratio (PRR). We were exploring influencing factors based on multivariate logistic regression analysis. Finally, we utilized a strategy that combines FAERS and TCGA databases to explore the potential immune and genetic influencing factors associated with anti-PD-1/PD-L1-related severe cAEs. Reports of severe cAEs accounted for 7.10% of the overall anti-PD-1/PD-L1 adverse event reports in the FAERS database. Immune-mediated myocarditis (PRR = 77.01[59.77–99.23]) shows the strongest toxic signal. The elderly group (65–74: OR = 1.34[1.23–1.47], ≥ 75: OR = 1.64[1.49–1.81]), male (OR = 1.14[1.05–1.24]), anti-PD-L1 agents (OR = 1.17[1.03–1.33]), patients with other adverse events (OR = 2.38[2.17–2.60]), and the concomitant use of proton pump inhibitor (OR = 1.29[1.17–1.43]), nonsteroidal anti-inflammatory drugs (OR = 1.17[1.04–1.31]), or antibiotics (OR = 1.24[1.08–1.43]) may increase the risk of severe cAEs. In addition, PD-L1 mRNA (Rs = 0.71, FDR = 2.30 × 10− 3) and low-density lipoprotein receptor-related protein 3 (LRP3) (Rs = 0.82, FDR = 2.17 × 10− 2) may be immune and genetic influencing factors for severe cAEs. Severe cAEs may be related to antigen receptor-mediated signalling pathways. In this study, we found that age, gender, anti-PD-1/PD-L1 agents, concomitant other adverse events, concomitant medication, PD-L1 mRNA, and LRP3 may be influencing factors for anti-PD-1/PD-L1-related severe cAEs. However, our findings still require a large-scale prospective cohort validation.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICI) represent a significant advancement in tumour immunotherapy, as they precisely target and inhibit the function of immune checkpoint molecules, thereby facilitating the restoration and augmentation of the body’s immune response against tumour cells1. Based on varying therapeutic targets, ICI can be categorized into several groups, encompassing anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), anti-programmed cell death protein-1 (PD-1), and anti-programmed death-ligand-1 (PD-L1). T-cell receptors (TCR) activate T cells, whereas CTLA-4, a molecule upregulated on the surface of activated T cells, functions to prevent excessive TCR-mediated stimulation. CTLA-4 competitively inhibits CD28, a TCR costimulatory receptor, from binding to ligands such as B7-1 and B7-2, thereby impeding CD28-mediated T-cell activation2. Similarly, PD-1 is upregulated on activated T cells and binds to its ligand, PD-L1, transmitting a negative costimulatory signal that restricts T-cell activation2. The oncogenic and immunosuppressive phenotype of the tumour microenvironment is marked by the overexpression of PD-L1 by cancer cells and the upregulation of PD-1 and CTLA-4 by T cells3. Blocking these molecules elicits an immune-mediated anti-tumor response.
Since the release of the world’s first anti-PD-1 in 2014, multiple anti-PD-1/PD-L1 monoclonal antibodies have been approved by the Food and Drug Administration (FDA). At present, seven anti-PD-1/PD-L1 agents have been approved by the FDA and applied in clinical practice, including nivolumab, pembrolizumab, cemiplimab, dostarlimab, atezolizumab, durvalumab, and avelumab2. The approval of these drugs is based on a large amount of clinical trial data, demonstrating their safety and efficacy in various types of tumours4,5,6. Significant therapeutic effects have been shown, especially in treating advanced solid tumours, recurrent or refractory tumours, and specific molecule expression positive tumours7,8. Its mechanism of action involves blocking immunosuppressive signals, enhancing anti-tumour immune responses, and reshaping the tumour microenvironment, providing new therapeutic hope for patients9.
Although anti-PD-1/PD-L1 has achieved significant therapeutic effects in anti-tumour, its use also comes with some safety issues. Immune-related adverse events (irAEs) can involve any organ or system, usually with low impact, and are treatable and reversible. However, some adverse effects can be severe and lead to permanent illness10. Anti-PD-1/PD-L1-related cardiac adverse events (cAEs), although not typical, have a higher mortality rate. Common anti-PD-1/PD-L1-related cAEs include myocardial disease, pericardial disease, and vasculitis disease. It currently occurs in approximately 1-1.5% of patients, but its mortality rate is relatively high11,12. According to reports, the incidence of myocarditis using anti-PD-1 or anti-PD-L1 alone was 0.5% and 2.4%, respectively12,13. A large pharmacovigilance study indicated that more than 80% of anti-PD-1/PD-L1-related cAEs were severe cases, with a mortality rate of up to 50% for myocarditis, 21.1% for pericardial disease, and 6.1% for vasculitis11. Anti-PD-1/PD-L1-related cAEs may cause the interruption, termination of immunotherapy, or even the death of the patients and are limiting factors in immunotherapy. In order to reduce the incidence and mortality rate, it is necessary to strengthen patient monitoring and management and adopt personalized treatment strategies. Meanwhile, further research is needed to explore the mechanisms and preventive measures of anti-PD-1/PD-L1-related cAEs.
Some studies have conducted pharmacovigilance analysis on the cardiac adverse events of ICI14,15,16. However, these studies focus on evaluating the risk and epidemiological characteristics of cardiac adverse events caused by ICI, lacking further exploration of potential risk factors for severe cAEs at the clinical, immunological, and genetic levels. This study combined the FDA Adverse Event Reporting System (FAERS) and the Cancer Genome Atlas (TCGA) databases to explore influencing factors of anti-PD-1/PD-L1-related severe cAEs. The main objectives of this study are as follows: (1) Based on the updated FAERS database, analyze the risk of severe cardiac adverse events caused by anti-PD-1/PD-L1, describe the main characteristics of patients, including gender, age, outcome, and time of occurrence, in order to comprehensively understand the epidemiological characteristics of anti-PD-1/PD-L1-related severe cAEs and provide a reference for safe clinical medication. (2) Combining FAERS with the TCGA database, we will explore the clinical, immunological, and genetic factors influencing anti-PD-1/PD-L1-related severe cAEs and provide a basis for personalized treatment.
Methods
Data source and collection
We conducted a pharmacovigilance study on severe cardiac adverse events associated with anti-PD1/PD-L1 based on the FAERS database. The FAERS database is a comprehensive data system used to collect, organize, and analyze adverse events and medication error information related to the use of drugs and therapeutic biologics. These data provide the crucial regulatory basis for the FDA to monitor the safety of marketed drugs and promptly identify and evaluate potential safety risks17. Anti-PD-1/PD-L1, including anti-PD-1-agents (nivolumab, pembrolizumab, cemiplimab, dostarlimab), and anti-PD-L1-agents (atezolizumab, avelumab, and durvalumab), were used as keywords to obtain report data of anti-PD-1/PD-L1 from the FAERS Publish Dashboard for the nine-year 2014–2022 (January 1, 2014–December 31, 2022). We limit the level of suspicion in the report to “primary suspect”.
Adverse events in the FAERS database are coded using preferred term (PT) codes from the Medical Dictionary for Regulatory Activities (MedDRA). This dictionary is an international medical terminology set for clinical validation created by the International Coordinating Committee on Human Drug Registration Technology, specifically designed for regulatory agencies and the regulated biopharmaceutical industry. It is used to standardize data entry, retrieval, evaluation, and display throughout the regulatory process from pre-market to post-market18. This dictionary has five levels of hierarchical terminology, including System Organ Class (SOC), High-Level Term (HLT), High-Level Group Term (HLGT), Preferred Term (PT), and Lowest Level Term (LLT). SOC is the highest level classification in the MedDRA terminology set, which divides medical terminology into different system or organ categories based on human anatomy and physiological characteristics. HLT is a further subdivision under SOC, representing a group of medical conditions, diseases, or diagnoses with similar properties or functions. HLGT is a further grouping of HLT, typically representing a set of medical terms with broader standard features. PT is the fundamental unit in the MedDRA terminology set, representing a single term with a clear medical meaning. LLT is mentioned in some cases but is not a standard classification level in the MedDRA terminology set. In the context of MedDRA, PT is often considered the lowest-level term as it provides the most specific and detailed medical description. Different PTs are grouped into different SOCs, but there is a primary SOC, a feature called multiaxiality. According to system organ class (SOC) = “cardiac disorders” and primary SOC = “Yes”, PTs of all cardiac adverse events in MedDRA (version 4.5.0) were obtained and used for the subsequent analysis, thus ensuring that the analyzed PTs were actual cardiac adverse events from a clinical point of view.
Data processing procedure
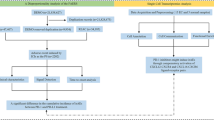
We followed the FDA’s recommended method of removing duplicate reports for data cleaning and analysis. The detailed screening process is shown in Fig. 1. Select the most recent FDA DT for reports with the same case number. Select the report with the higher ISR number for those cases with the same case number and FDA DT. Reports with the same value in fields including age, sex, country, event date, adverse event, drug, and indication were identified as duplicate reports. Delete duplicate records and keep only the latest version of the report. In addition, we retained only adverse event reports for those aged 18 years or older and excluded records of gender and age deficiencies. The remaining reports underwent further screening to exclude reports of cardiac adverse events that other factors could cause. The following criteria were used for the screening process:
-
1.
Reports regarding treatment indications for heart diseases, with keywords “benign cardiac neoplasm”, “cardiac neoplasm malignant”, “cardiac myxoma”, “pericarditis malignant”, “pericardial mesothelioma malignant”, “pericardial mesothelioma malignant recurrent”, “pericardial effusion malignant”, “metastases to heart”, “cardiac neoplasm unspecified”, “pericardial neoplasm”, “malignant pericardial neoplasm”, “benign pericardium neoplasm”, “cardiac fibroma”, “cardiac haemangioma benign”, “cardiac neurofibroma”, “cardiac teratoma”, “cardiac valve fibroelastoma”, “primary cardiac lymphoma”, “leukemic cardiac infiltration”, “pericardial lipoma”, and “cardiac lipoma”.
-
2.
Concomitant drug prescriptions that may cause cardiac adverse reactions, such as anthracycline drugs (doxorubicin, epirubicin, doxorubicin) and tyrosine kinase inhibitors (afatinib, alectinib, brigatinib, ceritinib, crizotinib, erlotinib, gefitinib, lorlatinib, and osimertinib).
-
3.
Reports accompanying anti-CTLA-4 agents (ipilimumab and tremelimumab).
After the above steps of deduplication as well as screening of anti-PD-1/PD-L1 data, the overall adverse event reports of patients treated with anti-PD-1/PD-L1 in the FAERS database used for further analysis were finally obtained (N = 60,766).
Disproportionality analysis
In pharmacovigilance studies, disproportionality analysis primarily evaluates possible associations between a specific adverse event and a particular drug19. We performed a disproportionality analysis to assess the potential association between cardiac adverse events and anti-PD-1/PD-L1 by calculating the PRR20. The control group consists of all other drugs. The following formula was used to calculate the PRR and 95% confidence interval (CI):
Among them, a represents the number of cases of cardiac adverse events after using anti-PD-1/PD-L1 agents, b represents the number of cases of other adverse events after using anti-PD-1/PD-L1 agents, c represents the number of cases of cardiac adverse events after using other drugs, and d represents the number of cases of other adverse events after using other drugs. Suppose the number of reports of cardiac adverse events is equal to or greater than three, and the lower limit of the 95% confidence interval (CI) of the proportional reporting ratio (PRR) is greater than one. The cardiac adverse event signal will be considered valid and strongly associated with anti-PD-1/PD-L1 agents. Overall, PTs of cardiac disorders that meet the conditions above were defined as anti-PD-1/PD-L1-related cAEs21.
Descriptive analysis
After the screening, we performed a descriptive analysis of the clinical characteristics of reports with anti- PD-1/PD-L1-related cAEs. It included sex, age, age group, region, anti-PD-1/PD-L1 categories, indication, concomitant medication, outcome, and other AEs. The time to onset of severe anti-PD-1/PD-L1-related cAEs was obtained by subtracting the event start time from the therapy start time. Cumulative distribution curves were used to present time-to-onset information for severe anti-PD-1/PD-L1-related cAEs in different groups22.
Analysis of clinical influencing factors in FAERS
The following factors can have a significant impact on the microbiota. They may lead to adverse events when taking anti-PD-1/PD-L1 medication: chemotherapy, targeted therapy, and accompanying medications such as antibiotics, proton pump inhibitors (PPIs), immunopotentiators, immunosuppressants, non-steroidal anti-inflammatory drugs (NSAIDs), insulin, metformin, aspirin, statin, glucocorticoids, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB)23. Multifactor logistic regression analysis was used to calculate the odds ratio (OR) for the occurrence of anti-PD-1/PD-L1-related severe cAEs under different exposures. We are adjusting for age, gender, region, anti-PD-1/PD-L1 treatment strategy, and cancer type. Due to intrinsic limitations in the pharmacovigilance database, we can only calculate potential risk through OR24.
Analysis of immune and genetic influencing factors in TCGA
Gene expression and somatic mutation across 27 cancer types were downloaded from the TCGA data portal [https://portal.gdc.cancer.gov/]. Tumour mutation burden was calculated by the number of nonsilent somatic mutations per sample25. In addition, T cell receptor diversity, B cell receptor diversity, neoantigen load, intratumor heterogeneity, and estimated immune cell abundance were downloaded from the GDC PanImmune Data Portal [https://gdc.cancer.gov/about-data/publications/panimmune]26. TCGA is an international research project aiming to better understand cancer occurrence, development, and metastasis mechanisms through large-scale genome sequencing and molecular feature analysis. TCGA constructs a comprehensive cancer genome map by collecting genomic data from various types of cancer, including gene mutations, gene expression, protein expression, DNA methylation, and other aspects27.
The variables studied include PD-L1 mRNA, PD-1 mRNA, PD-L2 mRNA, T cell receptor (TCR) diversity, B cell receptor (BCR) diversity, lymphocytes, stromal fraction, leukocyte fraction, CD8 + T cell, dendritic cells resting, T cells follicular helper, macrophage M1, dendritic cells, interferon-γ response, CD4 + T cells memory activated, T cells regulatory Tregs, plasma cells, B cells naive, transforming growth factor-β response, T helper 17 cells, T helper 1 cells, T helper 2 cells, tumour mutation burden, single-nucleotide variant neoantigens, neutrophils, intratumor heterogeneity, dendritic cells activated, macrophage M0, T cells γδ, mast cells activated, natural killer cells activated, B cells memory, natural killer cells resting, CD4 + T cells memory resting, mast cells resting, macrophage M2, CD4 + T cells naive, eosinophils and monocytes.
We adopted an approach described in a previous study that evaluated the correlation between single variables and response rate28,29. We then added variables to obtain bivariate models for better performance. The median values of each factor were calculated for each cancer type. Based on the cancer types, we correlated the average expression level of single genes with the PRR of anti-PD-1/PD-L1-related severe cAEs29. After obtaining the optimal single factor, we combined two or three genes in sequence for linear regression to test the predictive performance of the multifactor model. By conducting pathway enrichment analysis on significant genes, we aim to identify potential factors and biological mechanisms associated with anti-PD-1/PD-L1-related severe cAEs.
Statistical analysis
Categorical variables were expressed as numbers or percentages and assessed using the Chi-square or Fisher’s exact test according to different sample sizes. Log-rank tests were used to compare the median time to onset between different groups within cases of anti-PD-1/PD-L1-related severe cAEs. The predictive performance was estimated using Spearman rank correlation coefficient (Rs) and unexplained variance (1-Rs2). We use the log-likelihood ratio test to compare the goodness of fit between different models. We used the variance inflation factor to assess multicollinearity. Pathway enrichment was conducted using the R package cluster profiler30. The ‘p.adjust’ function was used to apply the Benjamini-Hochberg adjustment to multiple comparisons. Statistical significance was defined as two-sided P < 0.05 and false discovery rate (FDR) < 0.05. All of the above analyses were performed using R (version 4.3.0). This study is reported per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline31.
Results
Cardiac adverse events among anti-PD-1/PD-L1 patients in the FAERS, 2014–2022
After eliminating the cases where severe cAEs might have happened due to concurrent medications and relevant treatment indications, a total of 60,766 adverse events associated with anti-PD-1/PD-L1 agents were reported from the first quarter of 2014 to the fourth quarter of 2022. The detailed data processing procedure is shown in Fig. 1. Severe cardiac adverse events accounted for only a tiny fraction of the total adverse reactions in the anti-PD-1/PD-L1 reports, with a case number of 4313, which accounted for 7.10% (4313/60,766) of the overall cases. In addition, the number of cases per year was only a small but relatively constant proportion (4.93 -7.70%) of the overall cases for that year (Fig. 2A). The incidence of cAEs also varied among different anti-PD-1/PD-L1 treatment strategies (Fig. 2B). The number of cases with cAEs treated with anti-PD-1 was 7.30%, while anti-PD-L1 was 6.54%. In anti-PD-1, nivolumab has the highest proportion of cases of cAEs, at 7.78%. Overall, the proportion of cAEs under various anti-PD-1/PD-L1 treatment strategies indicates that it is a non-negligible part of the adverse events that may be associated with anti-PD-1/PD-L1.
Statistics on the occurrence of severe cardiac adverse events in anti-PD-1/PD-L1 reports from the FAERS database during 2014–2022. (A) The upper bar plot shows the number of anti-PD-1/PD-L1 reports with cardiac adverse events versus anti-PD-1/PD-L1 reports without cardiac adverse events in the FAERS database during 2014 and 2022, as well as the overall situation. The proportional bar plot below shows the number of anti-PD-1/PD-L1 reports with cardiac adverse events versus anti-PD-1/PD-L1 reports without cardiac adverse events in the FAERS database during 2014 and 2022, as well as the overall situation. (B) The upper bar plot shows the number of anti-PD-1/PD-L1 reports with cardiac adverse events versus anti-PD-1/PD-L1 reports without cardiac adverse events for different anti-PD-1/PD-L1 treatment strategies in the FAERS database from 2014 to 2022 and the overall situation. The proportional bar plot below shows the amount of anti-PD-1/PD-L1 reports with cardiac adverse events versus anti-PD-1/PD-L1 reports without cardiac adverse events for different anti-PD-1/PD-L1 treatment strategies in the FAERS database from 2014 to 2022 and overall situation. AEs: adverse events.
Scanning for anti-PD-1/PD-L1-related severe cardiac adverse events
Generally, anti-PD-1/PD-L1 agents were significantly associated with the occurrence of cAEs (PRR: 1.39, 95%CI: 1.36–1.42). In addition, there were differences between treatment strategies for anti-PD-1/PD-L1. When comparing anti-PD-1 agents to anti-PD-L1 agents, the latter demonstrates more signals of disproportionate reporting with a PRR of 1.52 and a 95% confidence interval of 1.45–1.59. However, among anti-PD-1 agents, dostarlimab has the most significant signal of disproportionate reporting with a PRR of 1.88 and a 95% confidence interval of 1.24–2.86. On the other hand, among anti-PD-L1 agents, avelumab has the most substantial signal of disproportionate reporting with a PRR of 1.75 and a 95% confidence interval of 1.46–2.10 (Fig. 3B). We counted the categories of severe cAEs and the number of cases in the reports of anti-PD-1/PD-L1 (Supplementary Table S1). Myocarditis (N = 620, 11.53%), cardiac failure (N = 541, 10.06%), atrial fibrillation (N = 453, 8.42%), pericardial effusion (N = 395, 7.34%) and myocardial infarction (N = 276, 5.13%) were the five categories of cAEs with the highest numbers of cases. We performed a disproportionality analysis by calculating the PRR of PTs with no less than 3 cases (N = 99) in the above cAEs, using the complete FAERS database as a comparator. The different potential cardiac safety signal spectra of anti-PD-1/PD-L1 strategies are shown in Fig. 3A. We define the forty-three categories of cardiac adverse events (PTs) that display potential safety signals as anti-PD-1/PD-L1-related severe cAEs and conduct subsequent analyses on them. We found that compared to anti-PD-L1, the spectrum of anti-PD-1 is more comprehensive, with 39 potential cardiac safety signals. In the overall context, the top five severe cardiac adverse events PTs highly correlated with anti-PD-1/PD-L1 treatments are immune-mediated myocarditis (PRR: 77.01, 95%CI: 59.77–99.23), autoimmune pericarditis (PRR: 57.90, 95%CI: 16.34-205.17), autoimmune myocarditis (PRR: 50.30, 95%CI: 36.19–69.91), myocarditis (PRR: 21.78, 95%CI: 20.37–23.29) and endocarditis non-informative (PRR: 11.45, 95%CI: 5.20–25.20) (Supplementary Table S2).
Scanning for anti-PD-/PD-L1-related cardiac adverse events based on the FAERS database. (A) The heatmap shows the PRR for 99 severe cardiac adverse events (with cases no less than 3) in the FAERS database under different anti-PD-1/PD-L1 treatment strategies (including overall, anti-PD-1, and anti-PD-L1). The heatmap was generated by Microsoft Office Excel 365 (https://www.microsoft.com/). Severe cardiac adverse events labelled with dark blue colour meet the criteria that the lower limit of the 95% confidence interval for the PRR is greater than one, and the number of cases occurring is no less than 3. The numbers in the figure represent the number of severe cardiac adverse events. (B) The forest plot shows the PRR of anti-PD-1/PD-L1-related severe cAEs. The forest plot was generated by Microsoft Office Excel 365. (C) The time interval between drug initiation and anti-PD-1/PD-L1-related severe cardiac adverse events. The bar chart was generated by Microsoft Office Excel 365. IQR: interquartile rang. (D) The cumulative distribution curves demonstrate the onset time of anti-PD-1/PD-L1-related severe cardiac adverse events after treatment with anti-PD-1/PD-L1 in different subgroups (the order is whether other adverse reactions accompany, whether it is fatal, and whether it is combined with metformin or targeted treatment drugs). The cumulative distribution curves was generated by R software (https://www.r-project.org/; version 4.3.0).
Descriptive analysis of cases with anti-PD-1/PD-L1-related cardiac adverse events
After screening the reports of anti-PD-1/PD-L1 in the FAERS database, we obtained cases with anti-PD-1/PD-L1-related severe cAEs (N = 3012) and statistically described the clinical characteristics of these cases. All demographic and clinical characteristics are presented in Table 1. The overall reporting rate of anti-PD1/PD-L1-related cAEs shows an increasing trend, with slight fluctuations yearly. Physicians and pharmacists reported the vast majority of incidents. The median age of the patients was 69 years (interquartile range [IQR] 61–75). After dividing the patients into three age groups using 65 and 75 years as the cut-off, we found that most patients (N = 2023, 67.2%) were over 65. This means that patients aged 65 years or older are more likely to be affected by anti-PD-1/PD-L1 induced cardiac adverse events. Most reported cases were male (N = 2005, 66.6%), and most were reported from Asia (N = 1117, 37.1%). Among the cases with anti-PD-1/PD-L1, cases with indications for lung cancer (43.2%, 1301/3012) accounted for the majority. More than half of the anti-PD-1/PD-L1 agents are concentrated in nivolumab and pembrolizumab, accounting for 74%. Compared to anti-PD-L1 agents, anti-PD-1 agents had a higher proportion of cardiac adverse events (75.1%, N = 2261). In combination therapy, chemotherapy drugs comprise the highest proportion (17.7%, N = 533), while immunopotentiators are relatively scarce (0.3%, N = 9). Reports of accompanying other adverse events accounted for 73.5% of anti-PD-1/PD-L1-related severe cAEs.
In cases where anti-PD-1/PD-L1-related severe cAEs occurred, more than 30% of cases had a fatal outcome. There are some notable differences between patients who died and those who survived. Firstly, males (P = 0.006) and patients in the higher age group (P < 0.001) were more commonly found in the fatal group. Secondly, there were significant differences in the number of cases among patients from different regions (P = 0.043). Thirdly, patients who experienced other adverse events (P < 0.001) were more likely to be in the fatal group. However, there was no significant difference in the distribution of the number of cases with different cancer original sites between the two groups (P = 0.882).
Furthermore, the two groups had no statistically significant difference in the distribution of cases with different anti-PD-1/PD-L1 treatment strategies (P = 0.063). Lastly, there was no significant difference in the proportion of cases treated with combination therapy between the fatal and non-fatal groups (Table 1).
Time to onset analysis
More than 65% of cases of anti-PD-1/PD-L1-related severe cAEs occurred during the first two months of initiating anti-PD-1/PD-L1 treatments, with a median onset time of 30 days (IQR 13–94) and 5.2% of cardiac adverse events occurring one year after drug initiation (Fig. 3C). The median onset time of patients without adverse events was 31.5 days longer than those with other adverse events (Days: 63 vs. 31.5, P = 0.020) (Fig. 3D). In addition, there are significant differences in the median onset time regarding whether the patient is fatal, whether combined with metformin or targeted therapy drugs (Fig. 3D). However, we did not observe that age, gender, anti-PD-1/PD-L1 treatment, or other concomitant medication significantly impacted the time to onset (Supplementary Fig. S1).
Commonly reported adverse reactions of anti-PD-1/PD-L1-related severe cAEs
Out of the 3012 cases of anti-PD-1/PD-L1-related severe cAEs that were analyzed, 73.5% of the cases had other adverse events along with them. In the fatal group, 82.8% of cases had other adverse events. Among cases with concomitant adverse events, respiratory, thoracic, and mediastinal disorders were the most commonly reported, accounting for 38.78% of cases. General disorders and administration site conditions were present in 29.48% of cases, while co-reported arteriosclerosis, stenosis, vascular insufficiency, and necrosis were present in only 0.18% of cases (Fig. 4A). In addition, malignant neoplasm progression (15.08%), dyspnoea (9.35%), pleural fusion (8.76%), and pneumonia (8.13%) were the most typical combined reported adverse events associated with anti-PD-1/PD-L1 (Fig. 4B).
The influencing factors of anti-PD-1/PD-L1-related severe cardiac adverse events
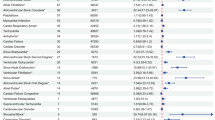
We further explored factors that might influence the occurrence of anti-PD-1/PD-L1-related severe cAEs by multifactor logistic regression analysis based on the total anti-PD-1/PD-L1 reports. After adjusting for confounding factors (Fig. 4C), compared to the odds of anti-PD-1/PD-L1-related severe cAEs for patients under 65, patients aged 65–70 have 1.34 times higher odds (OR = 1.34 [1.23–1.47], P < 0.001) and those over 75 years old have 1.64 times higher odds (OR = 1.64 [1.49–1.81], P < 0.001). Compared with anti-PD-1 agents, patients using anti-PD-L1 agents have 1.17 times higher odds (OR = 1.17 [1.03–1.33], P = 0.019) of developing cardiac adverse events. Increased potential risk of anti-PD-1/PD-L1-related severe cAEs in male patients (OR: 1.14, 95%CI: 1.05–1.24, P = 0.002) and patients with other adverse events (OR: 2.38, 95%CI: 2.17–2.60, P < 0.001). The simultaneous use of PPI (OR: 1.29, 95% CI: 1.17–1.43, P < 0.001), NSAIDs (OR: 1.17, 95% CI: 1.04–1.31, P = 0.009), metformin (OR: 1.43, 95% CI: 1.17–1.75, P < 0.001), other oral antidiabetic drugs (OR: 1.35, 95% CI: 1.09–1.67, P = 0.006), aspirin (OR: 1.53, 95% CI: 1.32–1.78, P < 0.001), statins (OR: 1.61, 95% CI: 1.42–1.82, P < 0.001), glucocorticoids (OR: 1.14, 95% CI: 1.09–1.29, P = 0.032), ACEI/ARB (OR: 1.58, 95% CI: 1.41–1.77, P < 0.001), or antibiotics (OR: 1.24, 95% CI: 1.08–1.43, P = 0.003) can be the causative factors for anti-PD-1/PD-L1-related severe cAEs.
Anti-PD-1/PD-L1-related severe cardiac adverse events. ALL figures was generated by Microsoft Office Excel 365. (A) The bar plot shows the statistics of the top 28 PTs of co-reported adverse events. The colour indicates the SOC of the corresponding PT. The percentage values in the figure represent the proportion of cases with such adverse events out of the total anti-PD-1/PD-L1-related severe cardiac adverse event cases with co-reported adverse events. (B) The bar plot shows the SOC statistics regarding PTs of co-reported adverse events. The percentage values in the figure represent the proportion of cases with such adverse events out of the total anti-PD-1/PD-L1-related severe cardiac adverse event cases with co-reported adverse events. (C) The forest plot shows the multifactor logistic regression analysis results regarding the factors influencing anti-PD-1/PD-L1-related severe cardiac adverse events. The variables represented in red and bold have statistical significance. ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, AEs: adverse events, Chemo: chemotherapy, NSAIDs: non-steroidal anti-inflammatory drugs, OR: odds ratio, PPI: proton pump inhibitor, PT: preferred term, SOC: systemic organ class, TTD: targeted therapeutic drugs.
Analysis of the immune influencing factors associated with anti-PD-1/PD-L1 related severe cardiac adverse events
To determine the immune and genetic influencing factors associated with the occurrence of severe cardiac adverse events related to anti-PD-1/PD-L1, we used linear regression to evaluate the association between multiple omics factors and the PRR of anti-PD-1/PD-L1-related severe cAEs for different cancer types. The PRR of anti-PD-1/PD-L1-related severe cAEs varied by tumour type, and the highest anti-PD-1/PD-L1-related severe cAEs PRR observed for thymoma (THYM) (4.30, 95% CI: 2.88–6.42), while the lowest value was observed for uveal melanoma (UVM) (0.54, 95% CI: 0.12–1.60) (Fig. 5A and Supplementary Table S3).
We collected 39 immune factors related to irAEs, including PD-1 mRNA, PD-L1 mRNA, PD-L2 mRNA, TCR/BCR diversity, neoantigen load, intratumor heterogeneity, and immune cell abundance. We identified eleven potential immune influencing factors, including PD-L1 mRNA (Rs = 0.71, FDR = 2.30 × 10− 3), TCR Diversity (Rs = 0.67, FDR = 4.16 × 10− 3), PD-1 mRNA (Rs = 0.63, FDR = 7.03 × 10− 3), Lymphocytes (Rs = 0.53, FDR = 3.18 × 10− 2), BCR Diversity (Rs = 0.52, FDR = 3.18 × 10− 2), Stromal Fraction (Rs = 0.52, FDR = 3.18 × 10− 2), Leukocyte Fraction (Rs = 0.52, FDR = 3.18 × 10− 2), CD8 + T Cells (Rs = 0.50, FDR = 3.44 × 10− 2), Dendritic Cells Resting (Rs = 0.50, FDR = 3.44 × 10− 2), T Cells Follicular Helper (Rs = 0.49, FDR = 3.44 × 10− 2), Macrophages M1 (Rs = 0.48, FDR = 4.02 × 10− 2) (Fig. 5B and Supplementary Table S4).
To identify more powerful predictive models, we combined these eleven factors. We evaluated the performance of bivariate models by Spearman correlation and goodness of fit by the log-likelihood ratio test28. Combining PD-L1 mRNA with most factors significantly improved the model’s goodness of fit compared to single factors (Fig. 5C and Supplementary Table S5). In particular, the combination of PD-L1 mRNA and CD8 + T cells achieved maximum predictive efficacy (Rs = 0.81, FDR = 9.46 × 10− 8) (Fig. 5D). The correlation coefficient (Rs, 0.81) suggested that this bivariate regression model explained 66% (Rs2, 0.66) of observed anti-PD-1/PD-L1-related severe cAEs PRR. We assessed the multicollinearity of these eleven factors by the variance inflation factor and observed no multicollinearity for PD-L1 mRNA and CD8 + T cells32 (Supplementary Table S6). We also found that the abundance of PD-L1 mRNA and CD8 + T cells exhibited no significant correlation (P = 0.15), suggesting the independent influencing factor of anti-PD-1/PD-L1-related severe cAEs. We further evaluated the performance of the combinations of other factors with PD-L1 mRNA and CD8 + T cells abundance bivariate model. No trivariate models achieved higher correlation coefficients or increased accuracy (Supplementary Table S5).
(A) Anatomic sites of cancer types (left panel) and anti-PD-1/PD-L1-related severe cAEs PRR across 27 cancer types (right panel). The anatomic illustration was generated by R package gganatogram v1.130. (B) Spearman correlation between anti-PD-1/PD-L1-related severe cAEs PRR and 39 factors for positive correlation (right) and negative correlation (left). The correlation bar chart was generated by Microsoft Office Excel 365. * indicates significant correlation (FDR < 0.05); PD-L1 mRNA FDR = 2.30 × 10− 3, TCR Diversity FDR = 4.16 × 10− 3, PD-1 mRNA FDR = 7.03 × 10− 3, Lymphocytes FDR = 3.18 × 10− 2, BCR Diversity FDR = 3.18 × 10− 2, Stromal Fraction FDR = 3.18 × 10− 2, Leukocyte Fraction FDR = 3.18 × 10− 2, CD8 + T Cells FDR = 3.44 × 10− 2, Dendritic Cells Resting FDR = 3.44 × 10− 2, T Cells Follicular Helper FDR = 3.44 × 10− 2, Macrophages M1 FDR = 4.02 × 10− 2; (C) Comparison of performance of bivariate models in predicting anti-PD-1/PD-L1-related severe cAEs for all combinations of eleven significantly correlated variables. The heat map of bivariate combination model was generated by Microsoft Office Excel 365. Spearman R (Rs) was calculated between predicted and observed anti-PD-1/PD-L1-related severe cAEs PRR. The shade of the square indicates the Rs, and the size indicates the significance of the log-likelihood ratio test. (D) The combined effect of PD-L1 mRNA and CD8 + T Cells bivariate model (Spearman correlation, Rs = 0.81, FDR = 9.46 × 10− 8). The scatter plot was generated by Microsoft Office Excel 365. The equation of the bivariate model is -0.3164 + 0.6891 × PD-L1 mRNA + 7.5227 × CD8 + T Cells. ACC: adrenocortical carcinoma, AEs: adverse events, BCR Diversity: B cell receptor diversity, BLCA: bladder urothelial carcinoma, BRCA: breast invasive carcinoma, CESC: cervical squamous cell carcinoma and endocervical adenocarcinoma, CHOL: cholangiocarcinoma, COAD: colon adenocarcinoma, ESCA: esophageal carcinoma, FDR: false discovery rate, GBM: glioblastoma multiforme, HNSC: head and neck squamous cell carcinoma, IFN-γ response: interferon-γ response, KICH: Kidney Chromophobe, KIRC: kidney renal clear cell carcinoma, KIRP: Kidney renal papillary cell carcinoma, LGG: brain lower-grade glioma, LIHC: liver hepatocellular carcinoma, LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma, MESO: mesothelioma, OV: ovarian serous cystadenocarcinoma, PAAD: pancreatic adenocarcinoma, PRAD: prostate adenocarcinoma, PRR: proportional reporting ratio, READ: rectum adenocarcinoma, SARC: sarcoma, SKCM: skin cutaneous melanoma, SNV neoantigens: single-nucleotide variant neoantigens, STAD: stomach adenocarcinoma, TCR Diversity: T cell receptor diversity, TGF-β response: transforming growth factor-β response, THCA: thyroid carcinoma, Th1 Cells: T helper 1 cells, Th2 Cells: T helper 2 cells, Th17 Cells: T helper 17 cells, THYM: Thymoma, TMB: tumour mutation burden, UVM: uveal melanoma.
Analysis of the genetic influencing factors associated with anti-PD-1/PD-L1-related severe cAEs
We observed 220 genes significantly associated with anti-PD-1/PD-L1, including 198 coding genes and 22 non-coding genes (Supplementary Table S7). We conducted signal pathway enrichment analysis on the top 30 genes with significant correlation (Supplementary Table S8). They are highly enriched in the immune response process, with the most significant pathway being the antigen receptor-mediated signalling pathway, with an enrichment score of 0.3 (p.adjust = 2.99 × 10− 5) (Fig. 6A). The low-density lipoprotein receptor-related protein 3 (LRP3), which is involved in fat metabolism, achieved the highest correlation coefficient (Rs = 0.82, FDR = 2.17 × 10− 2, Fig. 6B). Combinations between any two of the top ten significant correlated genes suggested that the combination of LRP3 with most of the other genes achieved better predictive performance (Fig. 6C and Supplementary Table S9). Among these, the combination of LRP3 and T Cell Receptor Alpha Variable 27 (TRAV27) achieved the best predictive performance among all bivariate linear regression models (Rs = 0.88, FDR = 4.05 × 10− 10, Fig. 6D). We evaluated the multicollinearity of these top ten genes by the variance inflation factor and observed no multicollinearity for LRP3 and TRAV27 (Supplementary Table S10). Combinations of the third gene did not improve the predictive value of the LRP3 and TRAV27 bivariate model (Supplementary Table S10). We further screened the combination of significant immune factors and genes to identify more powerful combinations and did not discover models that had better performance (Supplementary Table S11).
Evaluation of the association between anti-PD-1/PD-L1-related severe cAEs and gene-related factors. ALL figures was generated by Microsoft Office Excel 365. (A) Pathway enrichment of the top thirty genes significantly correlated with anti-PD-1/PD-L1-related severe cAEs across multiple cancer types. (B) Linear correlation model between LRP3 expression and anti-PD-1/PD-L1-related severe cAEs. (C) Comparison of performance of bivariate models in predicting anti-PD-1/PD-L1-related severe cAEs for all combinations of the top ten anti-PD-1/PD-L1-related severe cAEs PRR significantly correlated genes. Spearman correlation (Rs) was calculated between the predicted and observed anti-PD-1/PD-L1-related severe cAEs PRR. The shade of the square indicates the Rs, and the size indicates the significance of the log-likelihood ratio test. (D) The combined effect of LRP3 and TRAV27 bivariate model (Spearman correlation, Rs = 0.88, FDR = 4.05 × 10− 10). The equation of the bivariate regression model is 0.727 + 0.538 × LRP3 + 1.399 × TRAV27. PRR: proportional reporting ratio, FDR: false discovery rate, ACC: adrenocortical carcinoma, BLCA: bladder urothelial carcinoma, BRCA: breast invasive carcinoma, CESC: cervical squamous cell carcinoma and endocervical adenocarcinoma, CHOL: cholangiocarcinoma, COAD: colon adenocarcinoma, ESCA: esophageal carcinoma, FDR: false discovery rate, GBM: glioblastoma multiforme, HNSC: head and neck squamous cell carcinoma, KICH: Kidney Chromophobe, KIRC: kidney renal clear cell carcinoma, KIRP: kidney renal papillary cell carcinoma, LGG: brain lower-grade glioma, LIHC: liver hepatocellular carcinoma, LRP3: low-density lipoprotein receptor-related protein 3, LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma, MESO: mesothelioma, OV: ovarian serous cystadenocarcinoma, PAAD: pancreatic adenocarcinoma, PRAD: prostate adenocarcinoma, PRR: proportional reporting ratio, READ: rectum adenocarcinoma, SARC: sarcoma, SKCM: skin cutaneous melanoma, STAD: stomach adenocarcinoma, THCA: thyroid carcinoma, THYM: Thymoma, T TRAV27: Cell Receptor Alpha Variable 27, UVM: uveal melanoma..
Discussion
The adverse events associated with anti-PD-1/PD-L1 have been widely studied and reported, but there needs to be more comprehensive research on the factors influencing anti-PD-1/PD-L1-related severe cAEs. Previous pharmacovigilance studies on cardiac adverse events of ICIs have focused more on risk mining and epidemiological characterization, but they lack exploration of clinical, immunological, and genetic influencing factors14,15,16. This study combines real-world data from FAERS and TCGA databases to explore comprehensively the clinical characteristics and influencing factors of anti-PD-1/PD-L-rerelated severe cAEs. The results of this study supplement previous research findings. This is the first study to systematically explore the influencing factors of anti-PD-1/PD-L1-related severe cAEs by combining pharmacovigilance data and multi-omics data.
Anti-PD-1/PD-L1-related severe cAEs are a type of adverse event with a lower incidence but a higher mortality rate. According to the FAERS database, from 2014 to 2022, 7.10% (4.93-7.70%) of the reports of receiving anti-PD-1/PD-L1 treatment had severe cardiac adverse events, of which more than 30% were fatal. The proportion of severe cardiac adverse events reported by patients receiving anti-PD-1 treatment was slightly higher than that reported by patients receiving anti-PD-L1 treatment in anti-PD-1/PD-L1 reports (7.30% vs. 6.54%). Previous studies have shown that anthracycline drugs and tyrosine kinase inhibitors are also associated with cardiac adverse events, and anti-PD-1 and anti-PD-L1 are often used in combination with them33,34,35. We screened the anti-PD-1/PD-L1 reports by excluding potential factors that may induce cardiac adverse events to ensure we screened for actual cardiac adverse events associated with anti-PD-1/PD-L1 treatment. While we can somewhat mitigate the impact of these non-anti-PD-1/PD-L1 factors, eliminating their effects is impossible. After screening of anti-PD-1/PD-L1 data, we found that immune-mediated myocarditis, autoimmune pericarditis, autoimmune myocarditis, myocarditis, and endocarditis noninfective are the top five severe cardiac adverse reactions PTs highly associated with anti-PD-1/PD-L1 treatment. It is worth noting that three out of the top five highly correlated PTs are myocarditis, revealing the importance of myocarditis in anti-PD-1/PD-L1-related severe cAEs. Multiple studies have shown that myocarditis is the most common and fatal cardiac adverse event to immunotherapy13,36. Therefore, it is necessary to strengthen vigilance against immune-mediated myocarditis in clinical practice.
In a further investigation of factors affecting anti-PD-1/PD-L1-related severe cAEs, we observed that patients over 65 have a higher probability of developing anti-PD-1/PD-L1-related severe cAEs. Patients over the age of 75 are more susceptible to fatal cardiac adverse events. Previous studies have been controversial regarding the impact of age on irAEs. For example, studies have shown that irAEs in elderly patients have not increased significantly compared with young patients37. However, other studies have shown that age impacts irAEs38,39. A review discussed the complex relationship between age and irAEs, and the results varied with the age cut-off chosen40. There is also evidence for organ-specificity with age37,41. It is undeniable that heart disease is common in the elderly population, but aging may promote the development of anti-PD-1/PD-L1-related severe cAEs42. Although it may be difficult to distinguish the impact of age on the development of cardiac irAEs, we should identify and intervene as soon as possible to improve the outcome of elderly patients.
In addition, we found that male patients have an increased risk of anti-PD-/PD-L1-related severe cAEs. Studies have shown that gender and irAEs risk may depend on the ICI43,44. The kind of irAEs also seems to differ in men and women45. Retrospective reviews on the cardiac events in ICI therapy reported that men are at high risk of cardiac irAEs46,47. These findings suggest that there may be a specific correlation between gender and cardiac irAEs. Therefore, gender should be regarded as an essential factor in the future research and clinical treatment of cardiac irAEs.
In previous studies, scholars have suggested that because anti-PD-L1 does not block the signal transduction of the PD-L2 pathway, their incidence of adverse events may be lower than that of anti-PD-148,49. In this study, the risk of cardiotoxicity of anti-PD-L1 was slightly higher than that of anti-PD-1. Due to the lack of research on immune-related cAEs, further exploration and elucidation of the underlying mechanisms behind the differences in cardiac safety signals of anti-PD-1/PD-L1 are needed. There has been controversy over whether the combination of PD-1/PD-L1 inhibitors with chemotherapy or targeted therapy increases the risk of immune-related adverse reactions. This study’s multiple logistic regression analysis showed that combination chemotherapy or targeted therapy did not increase the risk of cardiac irAEs. However, due to the limitations of the FAERS database, the data on combination therapy may not be comprehensive, and this result is uncertain. Previous studies have suggested that conventional chemotherapy drugs and targeted therapy drugs may induce cardiac toxicity by directly damaging myocardial cells50. Alternatively, increasing the expression of PD-L1 and synergistically inducing immune-related cardiac adverse events with ICI therapy51,52. There are also studies suggesting that combination chemotherapy may induce the release of cancer cell antigens, which cross-react with cardiac tissue and increase the risk of severe immune-related cardiac adverse events53,54. Therefore, it is still necessary to be vigilant about the increased risk of cardiac toxicity in immunotherapy combined with chemotherapy or targeted therapy.
In addition, we found that concomitant use of PPI, NSAIDs, metformin, other oral antidiabetic drugs, aspirin, statins, glucocorticoids, ACEI/ARB, or antibiotics may be influencing factors for anti-PD-1/PD-L1-related severe cAEs. These drugs are associated with significant changes in microbiome composition23,55. Microbiota is now recognized as a significant intrinsic regulator of the immune response, and its composition influences response to ICI in both humans and mice, mainly through the induction of IFNγ + CD8 T cells and NKT cells56,57,58. More and more literature on the relationship between tumour response and irAEs59,60. Microbiota may also play a role in anti-PD-1/PD-L1-related cAEs development. It is unknown whether their impact on anti-PD-1/PD-L1-related cAEs is entirely mediated by changes in microbiota or other immune regulatory characteristics.
Our findings indicate that in 73.5% of the cases, anti-PD-1/PD-L1-related severe cAEs were accompanied by other adverse events. From this, anti-PD-1/PD-L1-related severe cAEs may be directly or indirectly related to the occurrence of other accompanying adverse events. It may also be the occurrence of accompanying adverse events caused by cardiac irAEs. The causal relationship between the two is still uncertain. For example, 6.54% of myositis was observed in cases with concomitant adverse events. There has been an increase in reports of myasthenia gravis, myositis, and myocarditis overlap syndrome induced by ICI. The incidence of immune-related myocarditis is about 1%, with 25% of patients developing myositis and 11% developing myasthenia gravis12,13,61,62,63,64. It may be related to the regulatory role of the PD-1 signalling pathway in the autoimmune response of these tissues.
Our study identified severe cardiac adverse events that were highly associated with anti-PD-1/PD-L1 based on real-world data. However, unlike irAEs, we could only perform a preliminary exploration of the immune and genetic influencing factors of anti-PD-1/PD-L1-related severe cAEs by combining TCGA pan-cancer transcriptome data. According to the results, the PPR value of anti-PD-1/PD-L1-related severe cAEs was higher in thymoma patients than in other tumours. According to reports, severe irAEs are less than 10% in ICI therapy for most solid tumours65. However, in thymoma, the risk of severe irAEs such as myasthenia gravis, myocarditis, hepatitis, and myositis increases66. Due to self-antigen-specific T cells in thymomas, they may enter the circulatory system, leading to disorder in the composition of circulating T cell subsets and increasing the risk of irAEs67. Therefore, clinical attention should be paid to the possible cardiac irAEs in thymoma patients after ICI therapy.
In the study of immune and genetic influencing factors, we found that the expression levels of PD-L1 mRNA and PD-1 mRNA in tumours are correlated with cardiac irAEs, and the higher their expression levels, the higher the risk of cardiac irAEs. The expression level of tumour PD-L1 protein is a widely validated therapeutic biomarker against PD-1/PD-L1, indicating to some extent that patients may benefit from immunotherapy68. Research has shown that patients who experience irAEs after receiving anti-PD-1/PD-L1 treatment have better efficacy69. Therefore, PD-L1 expression may also be associated with cardiac irAEs. Moreover, immune factors such as TCR Diversity, Lymphocytes, BCR Diversity, Strategic Fraction, Leukocyte Fraction, CD8 + T Cells, Dendritic Cells Resting, T Cells Follicle Helper and Macrophages M1 may be influencing factors for anti-PD-1/PD-L1-related severe cAEs. This suggests that tumour T cell and B cell infiltration play an essential role in the occurrence of cardiac irAEs.
In addition, we found that low-density lipoprotein-associated receptor protein 3 (LRP3) (Rs = 0.82, FDR = 2.17 × 10− 2) was highly correlated with PRR of anti-PD-1/PD-L1-related severe cAEs. The combined linear model of LRP3 and TRAV27 achieved the best prediction efficiency (Rs = 0.88, FDR = 4.05 × 10− 10). As far as we know, no study has reported that LRP3 is associated with immunotherapy response. LRP3 is a member of the low-density lipoproteins receptor (LDLR) family, which regulates the differentiation of adipocytes and osteoblasts. Recent studies have found lipid metabolism a critical regulator of T-cell responses70,71,72. However, it is not clear whether it is related to cardiac irAEs. Gene enrichment analysis showed that the antigen receptor-mediated signalling pathway correlated highly with the PRR of anti-PD-1/PD-L1-related severe cAEs. Most of the genes annotated by this pathway regulate immune response signalling. The available evidence proposes a potential biological mechanism that could be linked to anti-PD-1/PD-L1-related severe cAEs. In summary, whether anti-PD-1/PD-L1 is associated with these cardiac AEs and what mechanism still needs further exploration and verification through more clinical studies.
This study has some limitations. Firstly, as the FAERS database is based on a spontaneously reported adverse event reporting system, it has some inherent selection biases, such as the race and geographical ___location of reported cases, approval and market penetration times for different drugs, public awareness of specific adverse events, and not all severe adverse event reports will be collected. Therefore, we cannot obtain a causal relationship between anti-PD-1/PD-L1 and cardiac adverse events nor calculate the incidence of cardiac adverse events. Secondly, while the study attempts to mitigate the impact of non-anti-PD-1/PD-L1 factors, it cannot entirely eliminate their effects. Furthermore, we have not provided substantial evidence demonstrating the potential biological mechanisms associated with anti-PD-1/PD-L1-related severe cAEs. Finally, as this study is a case analysis and exploratory study, the findings regarding anti-PD-1/PD-L1-related severe cAEs still need to be validated in large-scale prospective studies.
Conclusions
In this study, we found that the elderly, male, anti-PD-L1 treatment, patients with other adverse events, and the concomitant use of PPI, NSAIDs, metformin, other oral antidiabetic drugs, aspirin, statins, glucocorticoids, ACEI/ARB, or antibiotics may be clinical influencing factors for anti-PD-1/PD-L1-related severe cardiac adverse events. In addition, PD-L1 mRNA and LRP3 may be immune and genetic influencing factors for anti-PD-1/PD-L1-related severe cardiac adverse events. The results of this study provide a better basis for understanding anti-PD-1/PD-L1-related severe cardiac adverse events. Due to the limitations, verifying our findings through prospective research is necessary to manage risks better.
Data availability
Individual safety records were downloaded from FAERS Public Dashboard [https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis]. The TCGA data were downloaded from TCGA data portal [https://portal.gdc.cancer.gov/] and GDC PanImmune Data Portal [https://gdc.cancer.gov/about-data/publications/panimmune]. All the remaining data are available within the article, in supplementary information files, or from the author upon reasonable request.
Abbreviations
- ACEI/ARB:
-
Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker
- BCR diversity:
-
B cell receptor diversity
- cAEs:
-
Cardiac adverse events
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein-4
- FAERS:
-
FDA Adverse Event Reporting System
- FDA:
-
Food and Drug Administration
- FDR:
-
False discovery rate
- ICI:
-
Immune checkpoint inhibitor
- irAEs:
-
Immune-related adverse events
- LRP3:
-
Lipoprotein receptor related protein 3
- MedDRA:
-
Medical dictionary of regulated drug activity
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OR:
-
Odds ratio
- PD-1:
-
Programmed cell death protein-1
- PD-L1:
-
Programmed death-ligand-1
- PPI:
-
Proton pump inhibitor
- PRR:
-
Proportional reporting ratio
- PT:
-
Preferred term
- SOC:
-
System Organ Class
- TCGA:
-
The Cancer Genome Atlas
- TCR diversity:
-
t cell receptor diversity
- TRAV27:
-
T Cell Receptor Alpha Variable 27
References
Naimi, A. et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell. Commun. Signal.20, 44. https://doi.org/10.1186/s12964-022-00854-y (2022).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086. https://doi.org/10.1158/2159-8290.CD-18-0367 (2018).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol.8, 86. https://doi.org/10.3389/fonc.2018.00086 (2018).
Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 401, 1853–1865. https://doi.org/10.1016/S0140-6736(23)00727-4 (2023).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 398, 27–40. https://doi.org/10.1016/S0140-6736(21)00797-2 (2021).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high Advanced Colorectal Cancer. N Engl. J. Med.383, 2207–2218. https://doi.org/10.1056/NEJMoa2017699 (2020).
Yoon, H. H. et al. Association of PD-L1 expression and other variables with Benefit from Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic review and Meta-analysis of 17 phase 3 Randomized clinical trials. JAMA Oncol.8, 1456–1465. https://doi.org/10.1001/jamaoncol.2022.3707 (2022).
Wu, Q., Qian, W., Sun, X. & Jiang, S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J. Hematol. Oncol.15, 143. https://doi.org/10.1186/s13045-022-01362-9 (2022).
Wang, Y. et al. New developments in the mechanism and application of immune checkpoint inhibitors in cancer therapy (review). Int. J. Oncol.63https://doi.org/10.3892/ijo.2023.5534 (2023).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers. 6https://doi.org/10.1038/s41572-020-0160-6 (2020).
Salem, J. E. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol.19, 1579–1589. https://doi.org/10.1016/S1470-2045(18)30608-9 (2018).
Mahmood, S. S. et al. Myocarditis in patients treated with Immune Checkpoint inhibitors. J. Am. Coll. Cardiol.71, 1755–1764. https://doi.org/10.1016/j.jacc.2018.02.037 (2018).
Moslehi, J. J., Salem, J. E., Sosman, J. A., Lebrun-Vignes, B. & Johnson, D. B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 391, 933. https://doi.org/10.1016/S0140-6736(18)30533-6 (2018).
Wang, F., Wei, Q. & Wu, X. Cardiac arrhythmias associated with immune checkpoint inhibitors: a comprehensive disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol.13, 986357. https://doi.org/10.3389/fphar.2022.986357 (2022).
Wang, F. & Wu, X. Cardiovascular toxicities associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. J. Clin. Pharm. Ther.47, 1576–1584. https://doi.org/10.1111/jcpt.13707 (2022).
Chen, C. et al. Cardiotoxicity Induced by Immune Checkpoint inhibitors: a Pharmacovigilance Study from 2014 to 2019 based on FAERS. Front. Pharmacol.12, 616505. https://doi.org/10.3389/fphar.2021.616505 (2021).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci.10, 796–803. https://doi.org/10.7150/ijms.6048 (2013).
Brown, E. G., Wood, L. & Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf.20, 109–117. https://doi.org/10.2165/00002018-199920020-00002 (1999).
Caster, O., Aoki, Y., Gattepaille, L. M. & Grundmark, B. Disproportionality Analysis for Pharmacovigilance Signal Detection in small databases or subsets: recommendations for limiting false-positive associations. Drug Saf.43, 479–487. https://doi.org/10.1007/s40264-020-00911-w (2020).
Bate, A. & Evans, S. J. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf.18, 427–436. https://doi.org/10.1002/pds.1742 (2009).
Zhou, C. et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine59, 101967. https://doi.org/10.1016/j.eclinm.2023.101967 (2023).
Cirmi, S., El Abd, A., Letinier, L., Navarra, M. & Salvo, F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: an analysis of the FDA adverse event reporting System Database (FAERS). Cancers (Basel). 12https://doi.org/10.3390/cancers12040826 (2020).
Maier, L. & Typas, A. Systematically investigating the impact of medication on the gut microbiome. Curr. Opin. Microbiol.39, 128–135. https://doi.org/10.1016/j.mib.2017.11.001 (2017).
Jing, Y. et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother Cancer. 10. https://doi.org/10.1136/jitc-2021-003779 (2022).
Hellmann, M. D. et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell.35, 329. https://doi.org/10.1016/j.ccell.2019.01.011 (2019).
Thorsson, V. et al. The Immune Landscape of Cancer. Immunity48, 812–830 e814. https://doi.org/10.1016/j.immuni.2018.03.023 (2018).
Tomczak, K., Czerwinska, P. & Wiznerowicz, M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp. Oncol. (Pozn). 19, A68–77. https://doi.org/10.5114/wo.2014.47136 (2015).
Lee, J. S. & Ruppin, E. Multiomics Prediction of Response Rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 Ligand 1. JAMA Oncol.5, 1614–1618. https://doi.org/10.1001/jamaoncol.2019.2311 (2019).
Jing, Y. et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun.11, 4946. https://doi.org/10.1038/s41467-020-18742-9 (2020).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16, 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
von Elm, E. et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 370, 1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X (2007).
Oshima, Y., Tanimoto, T., Yuji, K. & Tojo, A. EGFR-TKI-Associated interstitial pneumonitis in Nivolumab-treated patients with Non-small Cell Lung Cancer. JAMA Oncol.4, 1112–1115. https://doi.org/10.1001/jamaoncol.2017.4526 (2018).
Shyam Sunder, S., Sharma, U. C. & Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal. Transduct. Target. Ther.8, 262. https://doi.org/10.1038/s41392-023-01469-6 (2023).
Barbieri, M. A. et al. Adverse drug reactions with HER2-Positive breast Cancer Treatment: an analysis from the Italian Pharmacovigilance database. Drugs Real. World Outcomes. 9, 91–107. https://doi.org/10.1007/s40801-021-00278-z (2022).
Barbieri, M. A. et al. Safety profile of tyrosine kinase inhibitors used in non-small-cell lung cancer: an analysis from the Italian pharmacovigilance database. Front. Oncol.12, 1005626. https://doi.org/10.3389/fonc.2022.1005626 (2022).
Lehmann, L. H. et al. Clinical strategy for the diagnosis and treatment of Immune Checkpoint inhibitor-Associated myocarditis: a narrative review. JAMA Cardiol.6, 1329–1337. https://doi.org/10.1001/jamacardio.2021.2241 (2021).
Samani, A. et al. Impact of age on the toxicity of immune checkpoint inhibition. J. Immunother Cancer. 8. https://doi.org/10.1136/jitc-2020-000871 (2020).
Baldini, C. et al. Impact of aging on immune-related adverse events generated by anti-programmed death (ligand)PD-(L)1 therapies. Eur. J. Cancer. 129, 71–79. https://doi.org/10.1016/j.ejca.2020.01.013 (2020).
Huang, X. et al. Age-Associated changes in adverse events arising from Anti-PD-(L)1 therapy. Front. Oncol.11, 619385. https://doi.org/10.3389/fonc.2021.619385 (2021).
Wong, S. K., Nebhan, C. A. & Johnson, D. B. Impact of patient age on clinical efficacy and toxicity of checkpoint inhibitor therapy. Front. Immunol.12, 786046. https://doi.org/10.3389/fimmu.2021.786046 (2021).
Betof, A. S. et al. Impact of age on outcomes with immunotherapy for patients with Melanoma. Oncologist. 22, 963–971. https://doi.org/10.1634/theoncologist.2016-0450 (2017).
Hid Cadena, R. et al. Checks and balances in Autoimmune Vasculitis. Front. Immunol.9, 315. https://doi.org/10.3389/fimmu.2018.00315 (2018).
Valpione, S. et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl Med.16, 94. https://doi.org/10.1186/s12967-018-1467-x (2018).
Delaunay, M. et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir J.50https://doi.org/10.1183/13993003.00050-2017 (2017).
Triggianese, P. et al. Immune checkpoint inhibitors-induced autoimmunity: The impact of gender. Autoimmun. Rev.19, 102590. https://doi.org/10.1016/j.autrev.2020.102590 (2020).
Lal, J. C., Brown, S. A., Collier, P. & Cheng, F. A retrospective analysis of cardiovascular adverse events associated with immune checkpoint inhibitors. Cardiooncology. 7, 19. https://doi.org/10.1186/s40959-021-00106-x (2021).
Kazama, S. et al. Prognostic impact of immune-related adverse events on patients with and without cardiovascular disease: a retrospective review. Cardiooncology. 7, 26. https://doi.org/10.1186/s40959-021-00112-z (2021).
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L. & Hansen, A. R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol.28, 2377–2385. https://doi.org/10.1093/annonc/mdx286 (2017).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol.16, 563–580. https://doi.org/10.1038/s41571-019-0218-0 (2019).
Rosner, M. H. & Perazella, M. A. Acute kidney Injury in patients with Cancer. N Engl. J. Med.376, 1770–1781. https://doi.org/10.1056/NEJMra1613984 (2017).
Thompson, J. A. et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN Clinical Practice guidelines in Oncology. J. Natl. Compr. Canc Netw.20, 387–405. https://doi.org/10.6004/jnccn.2022.0020 (2022).
Peng, J. et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the Nuclear factor-kappab to Foster an immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res.75, 5034–5045. https://doi.org/10.1158/0008-5472.CAN-14-3098 (2015).
Lyon, A. R., Yousaf, N., Battisti, N. M. L., Moslehi, J. & Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol.19, e447–e458. https://doi.org/10.1016/S1470-2045(18)30457-1 (2018).
Rubio-Infante, N. et al. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur. J. Heart Fail.23, 1739–1747. https://doi.org/10.1002/ejhf.2289 (2021).
Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut. 65, 740–748. https://doi.org/10.1136/gutjnl-2015-310376 (2016).
Amoroso, C. et al. The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation. Cells. 9. https://doi.org/10.3390/cells9051234 (2020).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 360https://doi.org/10.1126/science.aan5931 (2018).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 565, 600–605. https://doi.org/10.1038/s41586-019-0878-z (2019).
Toi, Y. et al. Association of Immune-related adverse events with Clinical Benefit in patients with Advanced Non-small-cell Lung Cancer treated with Nivolumab. Oncologist. 23, 1358–1365. https://doi.org/10.1634/theoncologist.2017-0384 (2018).
Haratani, K. et al. Association of Immune-related adverse events with Nivolumab Efficacy in Non-small-cell Lung Cancer. JAMA Oncol.4, 374–378. https://doi.org/10.1001/jamaoncol.2017.2925 (2018).
Aldrich, J. et al. Inflammatory myositis in Cancer patients receiving Immune Checkpoint inhibitors. Arthritis Rheumatol.73, 866–874. https://doi.org/10.1002/art.41604 (2021).
Fazel, M., Jedlowski, P. M. & Severe Myositis Myocarditis, and Myasthenia Gravis with Elevated Anti-Striated Muscle Antibody following Single Dose of Ipilimumab-Nivolumab Therapy in a Patient with Metastatic Melanoma. Case Reports Immunol 2539493. https://doi.org/10.1155/2019/2539493 (2019).
Bawek, S. J., Ton, R., McGovern-Poore, M., Khoncarly, B. & Narvel, R. Nivolumab-Induced Myasthenia Gravis Concomitant with myocarditis, myositis, and Hepatitis. Cureus. 13, e18040. https://doi.org/10.7759/cureus.18040 (2021).
Jeyakumar, N. et al. The terrible Triad of Checkpoint Inhibition: a Case Report of Myasthenia Gravis, Myocarditis, and Myositis Induced by Cemiplimab in a patient with metastatic cutaneous squamous cell carcinoma. Case Rep. Immunol. 2020 (5126717). https://doi.org/10.1155/2020/5126717 (2020).
Wang, P. F. et al. Immune-related adverse events Associated with Anti-PD-1/PD-L1 treatment for malignancies: A Meta-analysis. Front. Pharmacol.8, 730. https://doi.org/10.3389/fphar.2017.00730 (2017).
Giaccone, G. et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol.19, 347–355. https://doi.org/10.1016/S1470-2045(18)30062-7 (2018).
Tateo, V. et al. Immunobiology of thymic epithelial tumors: Implications for immunotherapy with Immune Checkpoint inhibitors. Int. J. Mol. Sci.21https://doi.org/10.3390/ijms21239056 (2020).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol.18, 345–362. https://doi.org/10.1038/s41571-021-00473-5 (2021).
Hussaini, S. et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - a systematic review and meta-analysis. Cancer Treat. Rev.92, 102134. https://doi.org/10.1016/j.ctrv.2020.102134 (2021).
Lim, S. A., Su, W., Chapman, N. M. & Chi, H. Lipid metabolism in T cell signaling and function. Nat. Chem. Biol.18, 470–481. https://doi.org/10.1038/s41589-022-01017-3 (2022).
Bian, Y. et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 585, 277–282. https://doi.org/10.1038/s41586-020-2682-1 (2020).
Chang, C. H. et al. Metabolic competition in the Tumor Microenvironment is a driver of Cancer Progression. Cell. 162, 1229–1241. https://doi.org/10.1016/j.cell.2015.08.016 (2015).
Funding
This work was supported by the Fujian Provincial Science and Technology Plan Project (Grant number: 2023Y41010075) and the Natural Science Foundation of Fujian Province (Grant number: 2021J011304).
Author information
Authors and Affiliations
Contributions
Concept and design: J.Y. and X.T.C. Collection and assembly of data: X.T.C., J.R.L., and B.T.W. Data analysis and interpretation: X.T.C., S.M.H., J.R.L., and B.T.W.. Manuscript writing: All authors. Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, X., Lin, J., Wang, B. et al. Clinical characteristics and influencing factors of anti-PD-1/PD-L1-related severe cardiac adverse event: based on FAERS and TCGA databases. Sci Rep 14, 22199 (2024). https://doi.org/10.1038/s41598-024-72864-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72864-4