Abstract
Recent studies indicate that CISD3 is crucial in mitochondrial function and tumorigenesis. Using various databases, we systematically analyzed its expression, prognostic value, and immune activity. Our findings show CISD3 is mainly expressed in tumor cells across cancers, with higher mRNA but lower protein levels, degraded post-translationally via the lysosomal pathway. In certain cancers, CISD3 expression is positively correlated with tumor-infiltrating immune cells. Prognostic analysis suggests dual roles as both protective and risk factors, notably an independent prognostic predictor in renal cell carcinoma (RCC). CISD3 copy number variations are linked to homologous recombination defects and tumor-specific neoantigens, negatively correlated with methylation levels. Pathway analysis reveals CISD3 involvement in oncogenic processes, such as proliferation inhibition and epithelial-mesenchymal transition. Protein interactions underline its role in mitochondrial metabolism and redox balance. Experiments confirm low CISD3 expression in cancers, with overexpression reducing proliferation, migration, invasion, and tumor growth in mice. Mechanistic studies indicate CISD3 overexpression disrupts mitochondrial function, increases ROS levels, decreases GSH/GSSG ratios and mitochondrial membrane potential, inhibiting antioxidant activity and promoting cell damage and ferroptosis, thus impeding cancer progression. This study highlights CISD3’s potential as a prognostic biomarker and therapeutic target.
Similar content being viewed by others
Introduction
Cancer remains one of the most significant global health challenges, with treatment strategies often constrained by the complexity of tumor biology1. Despite advances in medical technology, cancer therapies frequently face obstacles such as drug resistance and tumor heterogeneity, leading to inconsistent treatment outcomes2,3. Consequently, there is a critical and ongoing need to identify and develop novel biomarkers and therapeutic targets that can offer more precise and effective treatment options for cancer patients.
CISD3, a member of the NEET protein family, plays a crucial role in human cells, particularly in mitochondrial function, iron homeostasis regulation, and cell death mechanisms4,5,6. It is one of the NEET proteins encoded by three different genes, and shares the CDGSH ___domain with mitoNEET (CISD1) and NAF-1 (CISD2). This ___domain enables the binding of [Fe2S2] clusters7,8. What distinguishes CISD3 is its presence as a soluble protein within the mitochondrial matrix, where it plays a critical role in regulating mitochondrial homeostasis and ferroptosis9,10. The methylation changes in the CpG islands surrounding the CISD3 gene, along with its expression patterns in various cancer types, suggest its potential as a biomarker and therapeutic target. Therefore, further research on CISD3 could significantly enhance our understanding of tumorigenesis and provide a scientific basis for developing new therapeutic strategies11,12,13. Focusing exclusively on a single cancer type may overlook the broader regulatory mechanisms of CISD3 in cancer. Thus, a systematic evaluation of CISD3’s role across different cancers is necessary. To our knowledge, there has been no comprehensive bioinformatics analysis of CISD3 in the context of pan-cancer.
In this study, we conducted a comprehensive analysis of CISD3 in pan-cancer for the first time using multiple public databases, including gene expression, genomic alterations, prognostic and diagnostic value, immune infiltration, functional enrichment analysis, and more. These analyses enhance our comprehension of the potential functions of CISD3 in cancer biology and offer novel directions for further research on this gene. Additionally, experimental exploration revealed the potential role of CISD3 in inhibiting proliferation and metastasis in renal cell carcinoma (RCC).
Materials and methods
mRNA and protein expression analysis
The pan-cancer CISD3 expression matrix was obtained from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases14. Analysis was conducted to compare CISD3 mRNA levels between tumor and normal tissues, and the data were visualized using the R ggplot2 package. SangerBox3.0 was used to analyze the relationship between CISD3 expression and staging and grading in the TCGA pan-cancer dataset15. The UALCAN database was utilized to analyze the protein expression of CISD3 in normal and cancer tissues16. The Human Protein Atlas (HPA) database was accessed to retrieve IHC images of CISD3 expression in normal and cancer tissues, as well as mRNA and protein expression data of CISD3 in human tissues17. Transcriptional level data of CISD3 transcripts were obtained from the Ensembl website18. The COMPARTMENTS Subcellular Localization Database was used to obtain a schematic representation of CISD3 subcellular localization19.
Single-cell and spatial transcriptomics data analysis
The TISCH database was utilized to analyze subcellular expression patterns of CISD3 in the pan-cancer single-cell dataset20. Spatial transcriptomics data for lung squamous cell carcinoma (LUSC), colorectal cancer (CRC), and kidney renal clear cell carcinoma (KIRC) were collected. The R software (R: A Language and Environment for Statistical Computing, version 4.1.1, URL: https://www.r-project.org/) Seurat package was used for data preprocessing. The SelectIntegrationFeatures, PrepSCTIntegration, FindIntegrationAnchors, and IntegrateData functions were used to integrate ST data. Unsupervised clustering methods were employed to perform cluster analysis on similar ST points. The Cottrazm package, including the get_enrichment_matrix and enrichment_analysis functions, was utilized to generate enrichment score matrices. The SpatialFeaturePlot function was used to visualize the enrichment scores for each cell type. The SpatialDimPlot function in the Seurat package was employed to visualize the maximum cell composition of each micro-region, while the SpatialFeaturePlot function was used to visualize the expression of CISD3 in each micro-region. Spearman correlation analysis was conducted to calculate the correlation between cell content and cell content, as well as between cell content and CISD3 expression, across all spots. The linkET package was used to visualize the results.
Prognostic and diagnostic value analysis
The prognostic and diagnostic value of CISD3 was evaluated using the GEPIA21and The Kaplan Meier Plotter online platforms22. Specifically, the evaluation encompassed overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS), first progression (FP), post-progression survival (PPS), and disease-free survival (DFS). The R “pROC” package was employed to generate receiver operating characteristic (ROC) curves, and the results were visualized using ggplot2. The R “survival” package was utilized for proportional hazards assumption testing and Cox regression analysis. Samples meeting the predefined p-value threshold (p < 0.05) in univariate analysis were included in multivariate Cox regression analysis. Additionally, the “rms” package was employed to construct and visualize nomogram-related models.
Genomic instability and epigenetic analysis
Copy number variation (CNV) data for 11,495 samples were downloaded from The Cancer Genome Atlas (TCGA) database and processed to identify significantly altered amplifications or deletions regions using GISTIC2.023. Illumina HumanMmethylation 450k level 3 data were also downloaded from the TCGA database. The R package “survival” was utilized to assess differences in CISD3 CNV and methylation among different groups. SangerBox3.0 was employed to analyze differences in CISD3 mRNA expression among different CNV alteration types, homologous recombination deficiency (HRD), and the correlation between 45 mRNA modification methylation regulators and CISD3 expression in TCGA pan-cancer tissues. Neoantigen data for each tumor were obtained from previous studies24, and correlation analysis was conducted by integrating the samples’ Neoantigen and gene expression data.
Protein-protein interaction analysis and enrichment analysis
Data on 14 cancer-related functional states were obtained from the CancerSEA database25. The Z-score algorithm within the R package gene set variation analysis (GSVA) was employed to calculate z-scores for 14 gene sets representing various functional states. Pearson correlation analysis was conducted to assess the statistical correlation between CISD3 and the z-scores of each gene set. The ComPPI database was employed to construct a network of interacting proteins with CISD3 that share common subcellular localization26. The cytohubba module in Cytoscape was used to select the top 20 interacting proteins. The generated gene sets underwent functional enrichment analysis using the DAVID database27, and visualization was performed using the R “ggplot2” package.
Immune correlation analysis
Data on CISD3 expression and immune scores in pan-cancer were obtained from the ESTIMATE database28. The R package ggplot2 was utilized to visualize the results. The online platform TIMER 2.0 was employed to analyze the correlation between CISD3 expression and immune infiltrating cells29. The SangerBox3.0 website was utilized to analyze the correlation between CISD3 expression and immune checkpoint genes, microsatellite instability (MSI), tumor mutational burden (TMB), and neoantigen expression.
Cell culture
Human kidney renal clear cell carcinoma (KIRC) cell lines 786-O and Caki-1 were obtained from Wuhan Punuo Sai Biotechnology Co., Ltd., China. The human renal cortical proximal tubule epithelial cells HK2, and RCC cell lines ACHN and A498 were generously provided by Dr. Kan Gong’s laboratory at the Urology Research Institute of Peking University. Human normal colon epithelial cells NCM460 and colorectal cancer (CRC) cell line HCT116 were purchased from Wuhan Boster Biological Technology. The human CRC cell line LoVo was acquired from Cyagen Biosciences (Shanghai) Co., Ltd. Human normal bronchial epithelial cells BEAS-2B and non-small cell lung cancer (NSCLC) cells A549 and H1299 were obtained from Procell Life Science & Technology Co., Ltd. in Wuhan.
The RCC cell lines A498 and ACHN were generously provided by Gong Kan’s lab at Peking University’s Institute of Urology. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, PM150210, Procell, China) supplemented with 10% fetal bovine serum (FBS, 164210, Procell, China) and 1% penicillin-streptomycin (PB180120, Procell, China). The cells were maintained in a humidified incubator at 37 °C with 5% CO2. Cells at the logarithmic growth phase were harvested for further experiments.
CCK-8 assay
The proliferation of control groups and cells with CISD3 overexpression or knockdown was assessed using the CCK-8 kit (C6005, NCM Biotech, China). Cells were seeded at a density of 1 × 103 cells per well in a 96-well plate and measured every 1–5 days. Once the CCK-8 reagent was added, the plates were placed in a cell culture incubator at 37 °C for 2 h. The absorbance at 450 nm was measured using a microplate reader.
Colony formation test
Renal cancer cells stably transfected with CISD3 overexpression or knockdown were seeded in 6-well plates, with 1000 cells per well, and incubated for 7–10 days. The culture medium was aspirated, fixed with 4% paraformaldehyde for 20 min, and stained with 0.1% crystal violet for 20 min. Gently rinse twice with PBS, air-dry, take pictures, and count and evaluate using ImageJ software(ImageJ: Image Processing and Analysis in Java, version 2.3.0/1.53q, URL: https://imagej.nih.gov/ij/).
Wound healing assay
The control groups and cells stably transfected with CISD3 overexpression or knockdown were seeded in 6-well plates. When the cell density reached 100%, a sterile 10 µl pipette tip was used to create scratches across the cell monolayer in each group. The cells were then gently washed with PBS to remove any non-adherent cell debris. Photographs were taken of the scratch area at 24–48 h after cell culture, and the scratch area was compared to that at 0 h.
Cell migration and invasion assay
The control group and cells stably transfected with CISD3 overexpression or knockdown were seeded in the upper chambers either with Matrigel (0827045, ABW Bio, China) for the invasion assay or without Matrigel for the transwell migration assay. The lower chamber was filled with 500 µl of complete medium containing 10% FBS. After incubation in a cell culture incubator for 24 h, the upper chamber was removed, and non-invading cells were wiped off. The cells were then fixed with 4% paraformaldehyde for 20 min and stained with 0.1% crystal violet for another 20 min. Three random fields were selected under a microscope for photography, and the number of invading cells was quantified.
Western blot analysis
The tissue samples used for experimental verification were collected from RCC patients diagnosed at the Department of Urology, Qingdao University Affiliated Hospital, China. The clinical and pathological information is summarized in Supplementary Table 1. The study followed the guiding principles of the Declaration of Helsinki and was approved by the Ethics Committee of Qingdao University Hospital (Qingdao, China, QYFYWZLL 26556). All patients expressed their understanding and signed written informed consent. Proteins were extracted from 8 pairs of frozen RCC tumor tissues and adjacent non-tumor tissues, and CISD3 protein levels were detected. RCC cells were treated with either MG132 (MCE, HY-13259 C, USA, 10µM) or chloroquine (CQ, MCE, HY-17589 A, USA, 50µM) for 24 h, and cell lysates were collected. The total protein from RCC, CRC, and NSCLC cells was extracted using RIPA lysis buffer (P0013B, Beyotime, China) containing protease and phosphatase inhibitors. The protein concentration was determined using a BCA protein assay kit (E-BC-K318-M, Elabscience, China). The proteins were separated by SDS-PAGE and transferred onto PVDF membranes (10600023, Cytiva, USA). The membranes were then blocked with 5% non-fat milk in TBST at room temperature for 1 h. Primary antibodies employed in the study included anti-CISD3 (1:1000; 30480-1-AP, Sanying, China) and anti-GAPDH (1:1000, E-AB-48016, Elabscience, China). The primary antibodies were incubated overnight at 4 °C, followed by washing with TBST, and incubation with secondary antibodies (1:10000, E-AB-1003, Elabscience, China) at room temperature for 1 h. Finally, chemiluminescent substrate was used to visualize the protein bands, and ImageJ software was utilized for quantitative analysis based on grayscale values.
Reactive oxygen species (ROS) detection
We employed a detection kit containing the fluorescent probe DCFH-DA (S0033S, Beyotime, China) to measure ROS fluorescence. Following the manufacturer’s instructions, cells in the logarithmic growth phase were collected and suspended in DCFH-DA at a final concentration of 10 micromoles per liter. The suspension was incubated at 37 °C for 20 min in a cell culture incubator. Subsequently, the cells were washed three times with serum-free culture medium and analyzed for ROS using flow cytometry.
Glutathione/oxidized glutathione ratio (GSH/GSSG)
The intracellular GSH/GSSG ratio was determined using a total GSH/GSSG colorimetric assay kit (E-BC-K097-M, Elabscience, China), following the manufacturer’s instructions. The GSH/GSSG levels within the cells were evaluated.
Mitochondrial membrane potential detection
Mitochondrial membrane potential was assessed using the enhanced JC-1 Mitochondrial Membrane Potential Assay Kit (C2003S, Beyotime, China). All steps were carried out according to the protocol provided by the manufacturer.
In vivo assay
Tumor formation experiments were conducted using control groups, renal cancer cells with stable CISD3 overexpression or knockdown, and CRC cells with CISD3 overexpression. Log-phase cells were harvested and digested with trypsin, followed by centrifugation and counting. The cells were then resuspended in PBS. Subsequently, 5 × 10^6 tumor cells were subcutaneously injected into the right axillary region of the forelimb of 6-week-old female BALB/c nude mice (purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd.). Tumor growth was monitored every 3 days using digital calipers. The longest diameter was denoted as D, and the shortest diameter was denoted as d. Tumor volume was calculated using the formula: (D * d2)/2. Mice were euthanized by CO₂ inhalation 25 to 30 days after injection, and tumor weight was measured. All procedures were approved by the Medical Animal Laboratory Ethics Committee of Qingdao University and the Affiliated Hospital of Qingdao University (No. 20240305BALB/cNu1220240430079, AHQU-MAL20240301), and all experiments were performed in accordance with relevant guidelines and regulations.
Statistical analysis
Pearson or Spearman correlation coefficients were utilized to evaluate the correlation between variables. Logrank test and Cox regression analysis were employed to assess the statistical significance of survival differences. For continuous variables, either t-test or Mann-Whitney U test was applied. Categorical variables were analyzed using chi-square test or Fisher’s exact test. All analyses were conducted with a significance threshold set at P < 0.05. Each measurement was repeated at least three times to ensure reliability.
Results
The expression level of CISD3 across pan-cancer
First, we queried the subcellular localization information of CISD3, and the results showed that CISD3 is confidently localized to the mitochondria (Supplementary Fig. 1A). Examination of CISD3 expression levels in human normal tissues through the HPA dataset revealed varying degrees of expression across different tissue types (Supplementary Fig. 1B). To compare the mRNA level differences of CISD3 between normal and tumor tissues, we analyzed CISD3 expression data in pan-cancer obtained from the TCGA database and found upregulation of CISD3 expression across multiple cancer types (Fig. 1A), a trend consistent with the TCGA combined with GTEx data (Supplementary Fig. 1C). Analysis of tumor tissue versus paired normal tissue expression showed significant differences in most cancers (Fig. 1B). We also examined the expression of CISD3 across different clinical stages and grades in pan-cancer; unfortunately, only STAD showed statistically significant differential expression (Fig. 1C, Supplementary Fig. 1D). In addition, the differential expression of CISD3 transcripts was examined to investigate changes in mRNA levels. The CISD3 gene, on chromosome 17, generates three distinct transcripts (Supplementary Fig. 1E). Moreover, an analysis was performed to compare CISD3 protein levels in normal and primary tumor tissues. Surprisingly, contrary to mRNA expression levels, CISD3 protein expression levels exhibited a downward trend in multiple cancer tissues (Fig. 1D). Immunohistochemistry staining results from the HPA database also showed a similar trend (except for LUAD) (Fig. 1E).
Evaluation of CISD3 mRNA and protein expression levels in pan-cancer. (A) Box plot of CISD3 mRNA expression levels between tumor tissues and normal tissues from the TCGA database. (B) CISD3 mRNA expression in tumor tissues and paired adjacent normal tissues from the TCGA database. (C) Box plot showing the relationship between CISD3 expression and cancer staging in pan-cancer. (D) CISD3 protein expression levels in normal and tumor tissues from the UALCAN database. Blue represents normal tissues, and red represents tumor tissues. (E) Representative images of CISD3 immunohistochemical staining in 10 types of normal and tumor tissues from the HPA database. *P < 0.05, **P < 0.01, ***P < 0.001.
CISD3 is mainly expressed by tumor cells in pan-cancer
To further explore the subcellular types expressing CISD3 in pan-cancer, analysis of CISD3 single-cell expression levels based on the TISCH database revealed that CISD3 is primarily expressed in malignant cells (Fig. 2A). We randomly selected common tumor types from the respiratory, digestive, and urogenital systems for specific analysis, and the UMAP plots of NSCLC, CRC, and KIRC datasets intuitively showed that CISD3 is mainly expressed in malignant cells (Fig. 2B–D). Next, we used spatial transcriptome data to further evaluate the spatial organization of CISD3 and malignant cells in LUSC, CRC, and KIRC. Spatial infiltration heatmaps showed that different sequencing spots were annotated as different cell types, such as malignant cells, fibroblasts, and immune cells. CISD3+ cells were mainly clustered in the same areas as malignant cells (Fig. 2E–G). The correlation analysis results were consistent with the localization results mentioned above, showing a significant positive correlation between the expression level of CISD3 and the content of malignant cells in spots. Particularly, in KIRC, the expression level of CISD3 was positively correlated with the proportions of various immune cells including CD8T cells, NK cells, macrophages, DC cells, and neutrophils, suggesting that CISD3 may play an important regulatory role in the immune microenvironment of KIRC (Fig. 2H–J). In conclusion, these results indicate that CISD3 is mainly expressed by tumor cells in pan-cancer, and it is expressed in immune cells in some cancers, necessitating specific analysis for individual cancers.
Subcellular localization of CISD3 in pan-cancer. (A) Heatmap of CISD3 expression in single-cell subpopulations across pan-cancer. UMAP plots showing cell type distribution annotations and CISD3 expression analysis in NSCLC (B), CRC (C), and KIRC (D). Spatial transcriptomic deconvolution maps illustrating cell localization in LUSC (E), CRC (F), and KIRC (G). Each dot represents a spot in spatial transcriptomic sequencing; the darker the color (red), the higher the content of that cell type in the spot. Spearman correlation of CISD3 expression with microenvironment components at spatial transcriptomic resolution in LUSC (H), COAD (I), and KIRC (J). Red lines indicate positive correlations, green lines indicate negative correlations, gray lines indicate non-significant correlations, and the thickness of the lines represents the absolute value of the correlation coefficient. In the triangular areas, red squares represent positive correlations, blue squares represent negative correlations; the more significant the p-value, the darker the color, the larger the absolute value of the correlation coefficient, and the larger the square.
Prognostic and diagnostic efficacy of CISD3 in pan-cancer
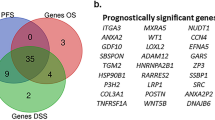
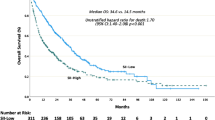
To assess the potential of CISD3 as a prognostic and diagnostic biomarker in cancer, we first evaluated the relationship between CISD3 and prognosis using the GEPIA and Kaplan-Meier Plotter databases. Based on GEPIA data, CISD3 was significantly associated with better OS in KICH, KIRP, and READ, while it was significantly associated with worse OS in KICH, LAML, and LIHC (Fig. 3A). Regarding DFS, CISD3 acted as a protective factor in READ and STAD but as a risk factor in TGCT (Supplementary Fig. 2A). Kaplan-Meier Plotter data showed that high expression of CISD3 was significantly associated with better OS in COAD, KIRC, and KIRP, whereas it was associated with poorer OS in HNSC and UCEC (Fig. 3B). For FP, CISD3 was a risk factor in NSCLC but a protective factor in STAD (Supplementary Fig. 2B). In terms of PPS, CISD3 was protective in COAD and STAD (Supplementary Fig. 2C). Additionally, CISD3 was protective for DFS in LIHC (Supplementary Fig. 2D) and for RFS in COAD, STAD, and UCEC but a risk factor in LUSC and LIHC (Supplementary Fig. 2E). CISD3 plays different roles in various cancer types. Furthermore, we evaluated the diagnostic value of CISD3 using ROC curve analysis in cancers with significant survival outcomes, revealing its efficacy in HNSC, NSCLC, COAD, LIHC, KIRC, and KICH (Fig. 3C). Subsequently, we assessed whether CISD3 served as a prognostic (OS) risk factor in NSCLC, CRC, and KIRC using univariate Cox analysis, which demonstrated significant prognostic value only in KIRC (P < 0.05) (Fig. 3D–F). Further incorporation of statistically significant variables into multivariate Cox regression analysis identified CISD3 as an independent prognostic predictor for KIRC (HR = 0.523, P = 0.004) (Fig. 3G). Finally, a prognostic nomogram was successfully constructed for KIRC using Cox regression algorithm to predict 1-year, 3-year, and 5-year OS (Fig. 3H). These results underscore the potential of CISD3 as a promising prognostic marker for KIRC.
Correlation analysis of CISD3 expression with prognosis and diagnosis in pan-cancer. (A) Overall survival (OS) of CISD3 assessed based on the GEPIA database. (B) OS of CISD3 assessed based on the Kaplan–Meier plotter database. (C) Diagnostic ROC curve of CISD3 in cancer. Univariate COX analysis of CISD3 in NSCLC (D), CRC (E), and KIRC (F). (G) Multivariate COX analysis of CISD3 in KIRC. (H) Nomogram predicting 1-year, 3-year, and 5-year OS in KIRC.
CISD3 expression correlates with genomic instability and epigenetic modifications
To assess CISD3’s involvement in genomic instability across cancer types, we conducted CNV analysis. The most common CISD3 alterations were observed in kidney cancer, with KIRP mainly exhibiting heterozygous amplification and KICH primarily showing heterozygous deletion (Fig. 4A). Significant differences in CISD3 mRNA expression were observed among different CNV alteration types in multiple cancers (Fig. 4B). Survival analysis revealed correlations between CNV and survival across various cancer types (Fig. 4C). Specifically, in UCEC, LGG, GBM, and ACC, wild-type CISD3 was associated with higher OS and DSS (Fig. 4D, Supplementary Fig. 3A). Wild-type CISD3 also exhibited better PFS and DF in UCEC and better PFS in LGG (Supplementary Fig. 3A). Given that HRD status is a critical indicator for treatment selection and prognosis in various tumors, we evaluated the correlation between CISD3 and HRD. CISD3 showed significant positive correlation with HRD across multiple tumor types, with only TGCT and BRCA showing negative correlation (Fig. 4E). Tumor-specific neoantigens are important targets inducing anti-tumor immune responses and are associated with cancer prognosis. CISD3 demonstrated positive correlation with neoantigen occurrence in UCEC, THCA, PRAD, PAAD, LIHC, KIRC, and HNSC (Fig. 4F).
Analysis of CISD3 with genomic instability and epigenetic modifications. (A) Pie chart showing the percentage of different types of CISD3 CNV in pan-cancer. (B) Box plot of CISD3 mRNA expression in different types of CNV alterations in pan-cancer. (C) Bubble chart summarizing the survival differences between CISD3 genomic CNV and wild-type. The color of the bubbles ranges from blue to red, representing hazard ratios from low to high, and the size of the bubbles is positively correlated with the significance of the Logrank P value. Black outline indicates Logrank P value ≤ 0.05. (D) Kaplan-Meier curves evaluating the correlation of CISD3 CNV with OS in four types of cancer. (E) Lollipop chart showing the correlation between CISD3 and HRD in pan-cancer. (F) Radar chart showing the correlation between CISD3 and neoantigens in pan-cancer. (G) Box plot of methylation differences between tumor samples and normal samples for CISD3. (H) Bubble chart showing the correlation between CISD3 mRNA expression and methylation. (I) Kaplan-Meier curves evaluating OS differences between high and low methylation groups of CISD3 in four types of cancer. (J) Heatmap showing the correlation between CISD3 expression and mRNA modification methylation regulatory factors in pan-cancer. *P < 0.05, **P < 0.01, ***P < 0.001.
Epigenetic modifications play a crucial role in the development and progression of cancer30, with methylation and mRNA modification being important components of epigenetic regulation. Differential mRNA expression of CISD3 between various tumor tissues and normal tissues is associated with methylation (Fig. 4G). Correlation analysis reveals a negative correlation between CISD3 mRNA expression and methylation (Fig. 4F). Survival analysis indicates significant survival differences across various cancers (Supplementary Fig. 3B), with KM curve evaluation showing that low methylation of CISD3 in COAD, UVM, KIRC, and BLCA is associated with better OS and DSS (Fig. 4I, Supplementary Fig. 3C), while low methylation in BLCA is associated with better PFS, and high methylation in LIHC is associated with better DFI (Supplementary Fig. 3C). mRNA modification participates in post-transcriptional gene regulation and is also an important component of epigenetics. We further evaluated the correlation between CISD3 expression and mRNA modification regulatory factors (Fig. 4J). These data suggest an important association between CISD3 and genomic instability, potentially playing a role in DNA methylation and mRNA modification across a range of cancer types.
CISD3 is associated with mitochondrial homeostasis and participates in suppressing multiple oncogenic pathways
To further explore the pathways and potential mechanisms of CISD3 action, we conducted GSVA enrichment analysis in pan-cancer. The results indicate a significant negative correlation between CISD3 expression and pathways related to angiogenesis, DNA damage, EMT, and stemness, suggesting its involvement in suppressing multiple cancer-causing pathways (Fig. 5A). Next, we utilized the ComPPI database to identify proteins potentially interacting with CISD3 and constructed a protein-protein interaction network (Fig. 5B). Further identification of the top 20 core genes in the network was performed using the MCC algorithm in cytoHubba (Fig. 5C). Kyoto Encyclopedia of Genes and Genomes (KEGG)31 enrichment analysis of these genes revealed significant enrichment in metabolic pathways and Oxidative phosphorylation pathways (Fig. 5D). Gene Ontology (GO) enrichment analysis results indicated enrichment in mitochondrial ATP synthesis and transport, mitochondrial proton-transporting ATP synthase, and 2 sulfur cluster binding, among others. This suggests that CISD3 plays a biological role by modulating mitochondrial function.
CISD3 protein interaction network and enrichment analysis. (A) Pearson correlation between CISD3 gene expression and GSVA scores evaluated by z-score parameters in pan-cancer tumor status. (B) Protein interaction network with subcellular compartment specificity for CISD3 identified by the ComPPI database. The color of the lines represents different subcellular localization evidence, and the length of the lines has no actual significance. (C) Top 20 hub genes in the CISD3 protein interaction network identified by the MCC algorithm in CytoHubba. KEGG (D) and GO (E) enrichment analysis of the Top 20 hub genes.
CISD3 expression influences immune cell infiltration in pan-cancer
To explore the relationship between CISD3 expression and immune infiltration in cancer, we first assessed the association between CISD3 and immunological characteristics in pan-cancer using the ESTIMATE algorithm. Correlation analysis revealed a negative correlation between CISD3 and immune scores in most cancer types (Fig. 6A). We then specifically analyzed the correlation between CISD3 expression and different immune infiltrating cell types in pan-cancer. The results showed that CISD3 expression was positively correlated with Th1 and NKT cells and negatively correlated with CAFs in most cancer types (Fig. 6B). Since Th1 and NKT cells are associated with anti-tumor immunity, while CAFs are involved in forming an immunosuppressive tumor microenvironment32 CISD3 may promote an inflammatory tumor microenvironment in pan-cancer. However, the correlation analysis between CISD3 expression and immune checkpoint genes showed a positive correlation in cancers such as ACC, KICH, UCEC, and LUAD, while a negative correlation was observed in cancers like THCA and READ (Fig. 6C), indicating different immune regulatory roles of CISD3 in different cancer types. TMB, MSI, and NEO are important predictors of tumor immune therapy response33,34. Correlation analysis results showed that CISD3 expression was positively correlated with TMB and MSI in cancers like KICH, UCEC, and ESCA, and positively correlated with NEO in cancers like UCEC, UCS, and DLBC (Fig. 6D–F), suggesting that high CISD3 expression in certain cancers such as KICH and UCEC predicts sensitivity to immune therapy. In summary, CISD3 may influence anti-tumor immune responses by affecting immune cell infiltration in pan-cancer.
Correlation analysis of CISD3 expression with immune infiltration. (A) Heatmap showing the correlation between CISD3 expression and immune score, stromal score, and ESTIMATE score. (B) Heatmap showing the correlation between CISD3 expression and immune cell infiltration. (C) Correlation analysis between CISD3 expression and immune checkpoint genes. Correlation of CISD3 expression with tumor mutation burden (TMB, D), microsatellite instability (MSI, E), and neoantigen expression (NEO, F).
In vivo and in vitro experiments validated the tumor-suppressive role of CISD3 across various cancer types
Through bioinformatic analysis, we identified a discrepancy between CISD3 mRNA and protein levels. Analysis of renal cancer patient samples revealed a significant downregulation of CISD3 protein in renal cancer tissues (Fig. 7A,B), while mRNA analysis of these samples showed high expression levels (Supplementary Fig. 4A). This suggests that CISD3 undergoes post-translational degradation. To investigate its degradation pathway, 786-O and A498 cells were treated separately with the proteasome inhibitor MG132 and the lysosomal inhibitor CQ. The results showed that CQ, but not MG132, blocked CISD3 protein degradation, indicating a primary degradation route via the lysosomal pathway (Supplementary Fig. 4B,C).
CISD3 inhibits the progression of RCC in vitro and in vivo. (A) Western blot analysis of CISD3 expression in 8 pairs of RCC tumor and adjacent non-tumor tissues. (B,C) Relative expression of CISD3 in the human renal proximal tubular epithelial cell line HK2 and various RCC cell lines. (D) Validation of protein levels after introducing CISD3-overexpressing lentivirus. (E,F) CCK-8 assays assessing RCC cell proliferation with stable CISD3 overexpression. (G) Colony formation assays assessing the proliferation capacity of RCC cells after stable CISD3 overexpression. (H,I) Wound healing assays evaluate the migration ability of RCC cells after CISD3 overexpression. (J,K) Transwell assays assessing the impact of CISD3 overexpression on RCC cell migration and invasion capabilities. (L) Xenograft mouse experiments assessing tumor growth in vivo. Tumor weight (M) and growth curve (N) of xenografted tumors. (O) Flow cytometry analysis of ROS levels in CISD3-overexpressing and control RCC cells. (P) The ratio of GSH/GSSG in CISD3-overexpressing and control RCC cells. (Q) Comparison of mitochondrial membrane potential in CISD3-overexpressing and control A498 cells. The original blots are shown in Supplementary Figs. 5 and 6. *p < 0.05; **p < 0.01; ***p < 0.001.
To validate CISD3’s role in cancer, we conducted in vitro and in vivo experiments. Western blot analysis across various cancer cell lines showed low CISD3 expression in KIRC (Fig. 7B,C), NSCLC (Supplementary Fig. 4D), and CRC (Supplementary Fig. 4E), consistent with our bioinformatic predictions. We then performed a series of functional assays using RCC and CRC cells with stable CISD3 overexpression (Fig. 7D, Supplementary Fig. 4F). CCK-8 and clonogenic assays demonstrated that increased CISD3 protein levels significantly inhibited RCC and CRC cell proliferation (Fig. 7E–G, Supplementary Fig. 4G-H). Additionally, wound healing assays revealed a significant reduction in the migratory capacity of RCC and CRC cells with CISD3 overexpression (Fig. 7H,I, Supplementary Fig. 4I). Transwell assays showed a marked decrease in migrating and invading RCC and CRC cells compared to controls (Fig. 7J,K, Supplementary Fig. 4J). In vivo experiments also confirmed that CISD3 overexpression inhibits tumor growth (Fig. 7L–N, Supplementary Fig. 4K–M).
We further investigated the mechanism underlying CISD3’s tumor-suppressive effects. Given its mitochondrial localization, CISD3 is hypothesized to influence metabolic pathways and redox balance. Functional enrichment analysis also links CISD3 with metabolism and oxidative phosphorylation. Flow cytometry assays indicated increased lipid ROS levels with CISD3 overexpression (Fig. 7O). GSH assays revealed a decreased GSH/GSSG ratio (Fig. 7P), and mitochondrial membrane potential assays showed reduced membrane potential levels (Fig. 7Q).
We developed stable CISD3 knockdown RCC cells in ACHN and Caki-1 lines (Fig. 8A). CCK-8 and clonogenic assays showed enhanced proliferation following CISD3 knockdown (Fig. 8B–D). Wound healing assays demonstrated increased migration in CISD3 knockdown RCC cells (Fig. 8E,F). Transwell assays showed a significant rise in migrating and invading cells post-knockdown (Fig. 8G,H). In vivo experiments revealed that tumors grew faster and were heavier after CISD3 knockdown (Fig. 8I–K). In contrast to overexpression results, CISD3 knockdown decreased ROS levels (Fig. 8L), increased the GSH/GSSG ratio (Fig. 8M), and elevated membrane potential levels (Fig. 8N). These findings suggest that CISD3 suppresses RCC progression by inhibiting cellular antioxidant activity and mitochondrial function.
CISD3 promotes RCC progression in vitro and in vivo. (A) Western blot validation of protein expression after stable transfection with shCISD3. (B,C) CCK-8 assays evaluating RCC cell proliferation after stable transfection with shCISD3. (D) Colony formation assays assessing the proliferation capacity promoted by shCISD3. (E,F) Wound healing assays showing shCISD3 promotes RCC cell migration. (G,H) Transwell assays showing stable expression of shCISD3 enhance RCC cell migration and invasion. (I) Images of xenograft tumors formed by RCC cells with CISD3 knockdown. Tumor growth curve (J) and weight (K) of xenografted tumors. (L) Flow cytometry analysis of ROS levels in CISD3 knockdown and control RCC cells. (M) The ratio of GSH/GSSG in CISD3 knockdown and control RCC cells. (N) Comparison of mitochondrial membrane potential in CISD3 knockdown and control ACHN cells. The original blots are shown in Supplementary Fig. 5. *p < 0.05; **p < 0.01; ***p < 0.001.
In summary, our study validates that CISD3 may play a crucial inhibitory role in the onset and progression of various cancers, meriting further exploration.
Discussion
CISD3 is a monomeric protein located in the mitochondria and plays a crucial role in various metabolic disorders35,36. Recently, the significance of CISD3 in cancer has garnered increasing attention4. However, little is known about its role in cancer. Therefore, it is necessary to conduct a comprehensive study to explore the role of CISD3 in cancer from multiple perspectives.
We first analyzed the expression distribution of CISD3 in human tissues and found that it is widely expressed across various organ systems. Our focus was on its expression in tumor tissues. We discovered that CISD3 mRNA levels are upregulated in multiple cancer types, while protein expression levels tend to decrease. IHC staining results also showed the same trend, suggesting that CISD3 may undergo post-translational modifications. The proteasome and lysosomal system are the two main protein degradation pathways in human cells37. We treated RCC cells with the proteasome inhibitor MG132 and the lysosomal inhibitor CQ individually. The results showed that CQ, but not MG132, blocked the degradation of the CISD3 protein, indicating that CISD3 degradation occurs primarily through the lysosomal pathway rather than the proteasome pathway. With no relevant studies reported so far, extensive experimentation is needed to further investigate the mechanism of CISD3’s role in cancer.
We analyzed single-cell transcriptome sequencing data in pan-cancer and found that CISD3 is primarily expressed by malignant cells, with expression also observed in monocyte macrophages, CD8+T cells, and NK cells. However, the expression patterns vary among different cancer types and require specific analysis for each cancer type. In the respiratory, digestive, and urinary systems, we selected NSCLC, CRC, and KIRC with spatial transcriptome data for specific analysis of CISD3 expression cell subtypes. The results showed that CISD3 is mainly localized in malignant cell regions, consistent with single-cell sequencing results. Cell type correlation analysis showed CISD3 expression is positively linked to both malignant cells and various tumor-killing immune cells. Particularly in KIRC, significant positive correlations were observed with CD8 T cells, NK cells, macrophages, dendritic cells, and neutrophils. Analysis of pan-cancer immune infiltration showed that CISD3 expression is generally positively correlated with Th1 and NKT cells. We know that Th1 cells are pivotal in activating cytotoxic CD8+T cells and promoting their targeted killing of tumor cells38. On the other hand, NKT cells directly kill tumor cells by releasing perforin cytotoxic factors and indirectly initiate immune killer cells to exert indirect killing effects on tumor cells39. Both cell types are essential components of anti-tumor immunity, suggesting that CISD3 may influence anti-tumor immune responses by modulating immune cell infiltration in pan-cancer contexts.
We focused our research on how CISD3 affects cancer cell functions. Our investigation into the mRNA and protein expression levels of CISD3 in cancerous and adjacent normal tissues from RCC patients revealed that CISD3 shows high mRNA expression and low protein expression in cancerous tissues, aligning with our bioinformatic analysis. We also measured CISD3 expression levels in various cancer cell lines, such as RCC, CRC, and NSCLC, and found that CISD3 is generally expressed at low levels in these lines. Moreover, we used RCC and CRC cell lines for in vitro experiments and discovered that overexpressing CISD3 significantly inhibited cell proliferation, migration, and invasion. In vivo tumorigenesis experiments also showed that CISD3 suppresses tumor growth. Our findings suggest that CISD3 may act as a tumor suppressor across different cancer types, requiring further experimental validation to clarify the underlying mechanisms.
We attempted to analyze the molecular mechanisms by which CISD3 functions in cancer cells to clarify its role in pan-cancer pathways. CISD3 is a member of the CDGSH iron-sulfur ___domain protein family, and subcellular localization analysis showed that CISD3 is located in the mitochondria. It is known that mitochondria are involved in essential processes of cellular energy metabolism, and their condition is a critical factor in determining cell survival40. Mitochondrial dysfunction triggers oxidative stress responses, generating excessive ROS, which promote cell damage41. Research data indicate that CISD3 is an important regulator of mitochondrial metabolic activity4,12. We speculate that CISD3 is crucial in metabolic pathways and redox balance. Consistent with this, by constructing a protein interaction network for CISD3 and performing GO enrichment analysis, we found that CISD3 is mainly involved in biological processes such as 2 sulfur cluster binding, ATP synthesis and transport, and mitochondrial proton-transporting ATP synthase. KEGG enrichment analysis showed that CISD3 is primarily involved in Metabolic pathways and Oxidative phosphorylation. Further validation revealed that overexpression of CISD3 leads to elevated ROS levels, decreased GSH/GSSG ratio, and reduced mitochondrial membrane potential. These results suggest that CISD3 inhibits cellular antioxidant activity, impairs mitochondrial function, and promotes cell damage and ferroptosis, thereby hindering cancer progression. Additionally, GSVA enrichment analysis showed that CISD3 is involved in inhibiting angiogenesis, DNA damage, EMT, and cancer stemness, among other oncogenic pathways. These potential tumor-regulating mechanisms mediated by CISD3 have not yet been reported in existing studies. In conclusion, CISD3 offers broad opportunities for further exploration of cancer mechanisms.
This study shows that CISD3 expression is upregulated at the mRNA level and downregulated at the protein level, undergoing post-translational degradation via the lysosomal pathway. Its expression level is a strong prognostic biomarker in human cancers. In pan-cancer analysis, CISD3 is primarily expressed in cancer cells, with minimal expression in tumor-killing immune cells like NKT cells. Various in vivo and in vitro experiments have validated CISD3’s role in inhibiting malignant biological phenotypes. Further mechanistic exploration reveals that CISD3 suppresses cancer progression by inhibiting cellular antioxidant activity, impairing mitochondrial function, and promoting cell damage. Although further experimental validation is needed to elucidate CISD3’s biological functions, our findings enhance the understanding of CISD3’s role in cancer, particularly in RCC development, providing new directions for future research on CISD3.
Data availability
The original datasets used in this study are available in the TCGA (https://portal.gdc.cancer.gov/) and GTEx (https://gtexportal.org/) databases. All the data produced or examined in this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Soerjomataram, I. & Bray, F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 18 (10), 663–672 (2021).
Flaherty, R. L., Falcinelli, M. & Flint, M. S. Stress and drug resistance in cancer. Cancer Drug Resist. 2 (3), 773–786 (2019).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15 (2), 81–94 (2018).
Mittler, R. et al. NEET proteins: a New Link between Iron Metabolism, reactive oxygen species, and Cancer. Antioxid. Redox Signal. 30 (8), 1083–1095 (2019).
Ferecatu, I. et al. The diabetes drug target MitoNEET governs a novel trafficking pathway to rebuild an Fe-S cluster into cytosolic aconitase/iron regulatory protein 1. J. Biol. Chem. 289 (41), 28070–28086 (2014).
Mons, C. et al. The H(2)O(2)-Resistant Fe-S Redox switch MitoNEET acts as a pH Sensor to repair stress-damaged Fe-S protein. Biochemistry. 57 (38), 5616–5628 (2018).
Lin, J. et al. Structure and molecular evolution of CDGSH iron-sulfur domains. PLoS One. 6 (9), e24790 (2011).
Wiley, S. E. et al. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc. Natl. Acad. Sci. U S A. 104 (13), 5318–5323 (2007).
Karmi, O. et al. A VDAC1-mediated NEET protein chain transfers [2Fe-2S] clusters between the mitochondria and the cytosol and impacts mitochondrial dynamics. Proc. Natl. Acad. Sci. U S A 119(7), 1 (2022).
King, S. D. et al. The mitochondrial localized CISD-3.1/CISD-3.2 proteins are required to maintain normal germline structure and function in Caenorhabditis elegans. PLoS One. 16 (2), e0245174 (2021).
Pérez-Ramírez, M. et al. Genomics and epigenetics: a study of ependymomas in pediatric patients. Clin. Neurol. Neurosurg. 144, 53–58 (2016).
Li, Y. et al. CISD3 inhibition drives cystine-deprivation induced ferroptosis. Cell. Death Dis. 12 (9), 839 (2021).
Grifagni, D. et al. Biochemical and cellular characterization of the CISD3 protein: molecular bases of cluster release and destabilizing effects of nitric oxide. J. Biol. Chem. 300 (3), 105745 (2024).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38 (6), 675–678 (2020).
Shen, W. et al. Sangerbox: a comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta. 1 (3), e36 (2022).
Chandrashekar, D. S. et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 25, 18–27 (2022).
Sjöstedt, E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367(6482), 1 (2020).
Martin, F. J. et al. Ensembl 2023. Nucleic Acids Res. 51 (D1), D933–d941 (2023).
Binder, J. X. et al. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database (Oxford). 2014, pbau012 (2014).
Sun, D. et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 49 (D1), D1420–d1430 (2021).
Tang, Z. et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45 (W1), W98–w102 (2017).
Nagy, Á., Munkácsy, G. & Győrffy, B. Pancancer Survival Anal. cancer Hallmark Genes Sci. Rep., 11(1): 6047. (2021).
Mermel, C. H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12 (4), R41 (2011).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48(4), 812-830e14 (2018).
Yuan, H. et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 47 (D1), D900–d908 (2019).
Veres, D. V. et al. ComPPI: a cellular compartment-specific database for protein-protein interaction network analysis. Nucleic Acids Res. 43 (Database issue), D485–D493 (2015).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50 (W1), W216–w221 (2022).
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013).
Li, T. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48 (W1), W509–w514 (2020).
Ushijima, T., Clark, S. J. & Tan, P. Mapping genomic and epigenomic evolution in cancer ecosystems. Science. 373 (6562), 1474–1479 (2021).
Kanehisa, M. et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–d592 (2023).
de Visser, K. E. & Joyce, J. A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 41 (3), 374–403 (2023).
Yi, M. et al. The role of neoantigen in immune checkpoint blockade therapy. Exp. Hematol. Oncol. 7, 28 (2018).
Ma, S. et al. Pre-treatment tumor neo-antigen responses in draining lymph nodes are infrequent but predict checkpoint blockade therapy outcome. Oncoimmunology. 9 (1), 1684714 (2020).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18 (10), 1539–1549 (2012).
Danielpur, L. et al. GLP-1-RA corrects mitochondrial Labile Iron Accumulation and improves β-Cell function in type 2 Wolfram Syndrome. J. Clin. Endocrinol. Metab. 101 (10), 3592–3599 (2016).
Paudel, R. R. et al. Targeted protein degradation via Lysosomes. Biochemistry. 62 (3), 564–579 (2023).
Wu, P. et al. [Advances in Research on Cell metabolic interactions in the Tumor Microenvironment]. Sichuan Da Xue Xue Bao Yi Xue Ban. 55 (2), 482–489 (2024).
Romain, G. et al. Antibody fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood. 124 (22), 3241–3249 (2014).
Sedlackova, L. & Korolchuk, V. I. Mitochondrial quality control as a key determinant of cell survival. Biochim. Biophys. Acta Mol. Cell. Res. 1866 (4), 575–587 (2019).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell. 73(2), 354-363e3 (2019).
Acknowledgements
We are grateful to the developers of the online databases used in this manuscript.
Funding
This work was supported by the Natural Science Foundation of China (NSFC Grant No. 82100557, 82200759), the Large Instruments Open Foundation of Nantong University (Grant No. KFJN2375), the Qingdao Science and Technology Benefiting People Demonstration Project (NO: 24-1-8-smjk-4-nsh).
Author information
Authors and Affiliations
Contributions
Conception and design of this study were contributed by KW, XZM, JL and HY. JL, HY, YXQ and PY were responsible for collecting the public data. The bioinformatical and statistical analysis were performed by JL, HY, YXQ, PY, XHH, ZLZ, KZ and XBY. ZLW, GQZ, XCY, and ZQJ conducted the in vitro and vivo experiments. JL and HY, YXQ, PY, XHH and ZLZ prepared the figures & tables and wrote the first draft of the manuscript. JL, HY, YXQ, KW and XZM revised the manuscript. All authors contributed to manuscript read and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The studies in which human RCC tumor samples were collected were approved by the ethical committees of the Affiliated Hospital of Qingdao University (QYFYWZLL 26556). Written consent was obtained from all patients. For mouse studies, all procedures were approved by the Ethics Committee of the Medical Animal Laboratory of the Affiliated Hospital of Qingdao University (AHQU-MAL20240301). The experimental protocol was conducted in accordance with the relevant guidelines and regulations of the Basel Declaration. This study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Yang, H., Qi, Y. et al. CISD3 is a prognostic biomarker and therapeutic target in pan-cancer. Sci Rep 14, 23494 (2024). https://doi.org/10.1038/s41598-024-74247-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74247-1