Abstract
Extracellular vesicles (EVs) are involved in the progression of various diseases. Tumor cell-derived EVs (TEVs) are a particular concern, as they can induce fatty liver by promoting liver macrophages to secrete tumor necrosis factor (TNF), thus enhancing the toxicity of chemotherapy. Therefore, reducing pathogenic EV production is a potential strategy for treating EV-related diseases. However, there are currently no effective clinical reagents to obtain this purpose. In addition, EVs are also natural and ideal drug-delivery vehicles. Improving the delivery efficiency of EVs remains a challenge. Proton pump inhibitors (PPIs) have been demonstrated to promote cell uptake of EVs by inducing micropinocytosis. Here, we show that PPIs can accelerate TEV clearance, reduce TEV uptake by liver macrophages and decrease the mRNA expression of TNF in liver macrophages of tumor-bearing mice. Correspondingly, the fatty liver phenotypes are alleviated, and the tolerance to chemotherapy is improved in these mice. Furthermore, our findings indicate that PPIs facilitate the uptake of red blood cell-derived EVs (RBC-EVs) loaded with antisense oligonucleotides of Trim21 (Trim21-ASOs) by the liver macrophages of obesity. Consequently, the inhibition of macrophage inflammatory responses in obese mice mediated by RBC-EVs/Trim21-ASOs was further enhanced by PPIs, resulting in a more profound improvement in obesity and related metabolic disorders. In conclusion, our findings demonstrated that PPIs can effectively clear pathogenic EVs and enhance the delivery efficacy of EV vehicles, making them a highly promising clinical prospect.
Similar content being viewed by others
Introduction
Extracellular vesicles (EVs) are mainly divided into exosomes and ectosomes. Ectosomes are formed by budding directly from the plasma membrane (PM) and have a wide range of diameters, from 50 nm to 1 μm. During the formation of exosomes, the indentation of the PM forms early endosomes, and early endosomes bud inward to form multivesicular bodies containing numerous intraluminal vesicles in the maturation process. Multivesicular bodies fuse with the PM and release intraluminal vesicles extracellularly to produce exosomes. The diameter of exosomes is relatively concentrated, mainly ranging from 40 to 160 nm1,2,3. Due to the current lack of specific markers, it is impossible to differentiate exosomes and ectosomes. Therefore, a more scientific approach is to term them EVs collectively4,5. EVs are essential in cell communication since they contain proteins, nucleic acids, lipids, metabolites and other information derived from their parental cells. EVs are reportedly involved in various physiological and pathogenic processes1,6. Lung- and gut-derived EVs are critical in maintaining lung and gut mucosal immune balance7,8. EVs are particularly important for creating pre-metastatic tumor niches9. TEVs determine the organ-specific metastasis of tumors via integrins10. TEVs are also crucial in tumor immunosuppression. TEVs induce systemic immunosuppression via PD-L1, mediating anti-PD-L1 therapy resistance11. Recently, TEVs have been demonstrated to cause fatty liver formation by promoting liver macrophages to secrete TNF, generating a pro-inflammatory microenvironment, thus attenuating chemotherapeutic drug metabolism12. Therefore, TEVs are a potential target for intervening in tumor progression and improving chemotherapeutic tolerance.
Conventional virus vehicles, including adenovirus and lentivirus, can efficiently deliver genes, but the risk of mutation introduction, high immunogenicity and induction of inflammatory responses greatly restrict their applications13. Compared with virus vehicles, EVs have higher biocompatibility and lower immunogenicity14. Therefore, besides their physiological and pathogenic functions, EVs are also excellent drug-delivery vehicles, especially for drug delivery to the liver. RBC-EVs are natural liver tropisms and can treat orthotropic liver cancer after loading with doxorubicin15. In addition, EVs can improve obesity-related inflammation by delivering Trim21-ASOs to the liver, thus relieving obesity and related metabolic diseases16. Even with the inherent advantages, the production of EVs is still challenged. If the EV delivery efficacy can be enhanced, the EV dose for in vivo administration might be reduced, which will benefit EV translational applications.
Proton pump inhibitors (PPIs) are H+/K+-ATPase inhibitors. After PPIs diffuse into gastric parietal cells, they covalently bind to H+/K+-ATPases and irreversibly inactivate the proton pump. Only when new pump molecules are synthesized and inserted into the cell membrane can gastric acid be secreted again. Therefore, PPIs have a solid and long-lasting effect in inhibiting gastric acid. PPIs have become the preferred drug for treating peptic ulcers17,18. Our previous study showed that PPIs (Rabeprazole) enhance macropinocytosis-mediated extracellular vesicle endocytosis. PPIs promoted tumor cells to recycle TEVs, thus accelerating TEV clearance, which led to stimulation of antitumor immunity and elevated anti-PD-1 therapy response19. Since TEVs attenuate chemotherapeutic drug metabolism by inducing fatty liver formation, can PPIs improve chemotherapeutic drug metabolism through the induction of TEV clearance?
Here, we show that PPIs effectively alleviate fatty liver phenotypes of tumor mice by reducing TEVs. In tumor mice with PPI treatment, enhanced tolerance to chemotherapy is observed. In addition, we find that PPIs notably enhance Trim21-ASOs delivery to the liver by RBC-EVs, thus alleviating obesity and related metabolic disorders. Our results indicate that PPIs can be used to improve chemotherapeutic toxicity and boost the drug-delivery of EVs.
Results
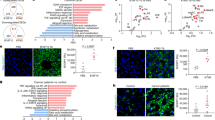
Intratumoral injection of PPIs reduce TEVs uptake by liver macrophages
PPIs were reported to promote TEVs uptake by tumor in a macropinocytosis-dependent manner(), which enhances the recycling of TEVs by tumor cells and accelerates the clearance of TEVs from the tumor microenvironment and systemic circulation. Our previous study verified that Rabeprazole promotes the uptake of EVs by MC38 in vitro, to investigate whether PPI-induced MC38-derived TEVs clearance could reverse TEVs uptake by liver macrophages and hepatic TEVs accumulation in vivo, we first constructed Cre+ MC38 cells that secret EVs with Cre (Cre+ MC38-EVs) (Fig. 1A). Cells from Rosa-LSL-tdTomato mice were reported to express tdTomato after the uptake of Cre+ EVs20. In our study, liver macrophages from Rosa-LSL-tdTomato mice similarly expressed tdTomato after uptake of Cre+ MC38-EVs in vitro(Fig. 1B). We then subcutaneously inoculated Cre+ MC38 cells into Rosa-LSL-tdTomato mice and found that intratumoral injection of Rabe significantly reduced CD63+Cre+ EVs in the blood of Cre+ MC38 tumor-bearing mice (Fig. 1C), suggesting Rabe indeed induced TEVs clearance. Correspondingly, Rabe notably reduced tdTomato-expressing cells in the liver (Fig. 1D), indicating reduced TEVs liver accumulation. TEVs were reported to induce the secretion of TNF by liver macrophages12. Given that EVs mainly accumulate in liver macrophages, intratumoral injection of Rabe may reduce the uptake of TEVs by liver macrophages and thus inhibit TNF expression. As expected, we found that liver macrophages from Rabe-treated Cre+ MC38 tumor-bearing mice indeed had significantly lower Tnfa mRNA expression than those without Rabe treatment (Fig. 1E). Thus, Intratumoral injection of Rabe is effective in reducing TEVs uptake by liver macrophages.
Intratumoral injection of PPIs reduce TEVs uptake by liver macrophages. (A) Cre and EV markers in MC38-EVs or Cre+ MC38-EVs were detected by western blot. (B) Liver macrophages from Rosa-LSL-tdTomato mice were cocultured with 5 µg Cre+ MC38-EVs for 24 h, and the tdTomato expression in isolated F4/80+ liver macrophages was detected by flow cytometry. (C–E) Rosa-LSL-tdTomato mice bearing Cre+ MC38 tumor were intratumorally injected with DMSO or 15 µg Rabe for 3 days. CD63+Cre+ EV levels in the blood of these mice were measured by ELISA (C). Representative tdTomato expression in livers of these mice. Scale bar, 50 μm (D). Tnfa mRNA in liver macrophages from these mice was measured by Real-time PCR (E). *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired two-tailed Student’s t-test; mean ± SD).
PPIs improve chemotherapeutic tolerance of tumor mice
TEVs have been shown to generate a pro-inflammatory microenvironment by promoting TNF secretion from liver macrophages, thereby inducing fatty liver formation and impairing the metabolism of chemotherapeutic agents(). Since intratumoral injection of Rabe reduced Tnfa mRNA expression from liver macrophages, we wondered whether Rabe could improve fatty liver phenotypes and alleviate chemotherapeutic toxicity in tumor mice. Our previous studies have shown that PPIs induce EVs uptake by enhancing micropinocytosis in an ATP6V1A-dependent manner19, and Rabe could also inhibit tumor growth by activating antitumor immunity19. Thus we added Atp6v1a knockdown (Atp6v1aKD) MC38 cells in the following experiments to confirm whether the effect of Rabe was associated with ATP6V1A-dependent macropinocytosis in this study, and used nude mice in the subsequent experiments to exclude the impact of Rabe on antitumor immunity. We first compared the lipid droplet formation in the liver of Rabe-treated MC38 and Atp6v1aKD MC38 tumor-bearing mice. BODIPY staining revealed that intratumoral injection of Rabe obviously reduced lipid droplet formation in the liver of MC38 tumor mice rather than Atp6v1aKD MC38 tumor mice (Fig. 2A), suggesting that Rabe ameliorates the fatty liver phenotype and that the protective effect indeed relies on ATP6V1A. CYP enzymes in hepatocytes metabolized 70–80% of prescribed drugs21. We found that Rabe also enhanced core Cyp genes expression, including Cyp1a2, Cyp2b10 and Cyp2c38 in hepatocytes of MC38 rather than Atp6v1aKD MC38 tumor mice (Fig. 2B). As expected, Rabe decreased the toxicity of the chemotherapeutic drug dacarbazine in MC38 rather than Atp6v1aKD MC38 tumor mice, evidenced by increased RBCs and reticulocytes counts after Rabe combination treatment (Fig. 2C). Ome also reduced dacarbazine toxicity in MC38 tumor mice (Fig. 2D). Therefore, PPIs are expected to reduce the toxicity of chemotherapy and improve the tolerability of chemotherapy in tumor patients.
PPIs improve chemotherapeutic tolerance of tumor mice. (A) Representative BODIPY staining of livers from MC38 and Atp6v1aKD MC38 tumor mice with DMSO or Rabe intratumoral treatment. Scale bar, 50 μm. (B) Real-time PCR analysis of Cyp genes in hepatocytes of MC38 and Atp6v1aKD MC38 tumor mice with DMSO or Rabe intratumoral treatment. (C) Analysis of RBCs and reticulocytes in MC38 and Atp6v1aKD MC38 tumor mice treated with dacarbazine alone or combined with Rabe. (D) Analysis of RBCs and reticulocytes in MC38 tumor mice, treated with dacarbazine alone or combined with Ome. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired two-tailed Student’s t-test in B; one-way ANOVA followed by Tukey test in C,D; mean ± SD).
PPIs promote RBC-EVs/Trim21-ASO-mediated inhibition of macrophage inflammatory responses
In addition to improving fatty liver and chemotherapeutic tolerance by triggering tumor macropinocytosis-mediated clearance of TEVs, we further investigated whether Rabe also improves obesity and related metabolic disorders through enhancing the uptake of drug-loaded EVs by target cells. We have reported that TRIM21 is critical for aggravating obesity-induced inflammation driven by macrophages, and RBC-EVs loaded with Trim21-ASOs (RBC-EVs/Trim21-ASOs) can target TRIM21 in macrophages and alleviate inflammation16. Therefore, we examined the effect of Rabe on RBC-EVs/Trim21-ASOs uptake in vitro. We first loaded Trim21-ASOs into RBC-EVs and confirmed that the loading of Trim21-ASOs did not affect the morphology, size distribution and classical protein markers of RBC-EVs (Fig. 3A-C). Then, we loaded Cy3-conjugated Trim21-ASOs into RBC-EVs and found that RBC-EVs/Trim21-ASOs carrying high levels of Trim21-ASOs (Fig. 3D). RBC-EVs/Trim21-ASOs markedly decreased TRIM21 protein expression in peritoneal macrophages (PEMs) as expected (Fig. 3E). The saturated free fatty acid palmitate (PA), which is increased in obese individuals as a result of excessive dietary fat intake22. We used PA to mimic obesity stimulation and found that RBC-EVs/Trim21-ASOs significantly reduced IL-1β, IL-6 and TNF secretion in PA-stimulated PEMs, and the effect that was further enhanced by Rabe treatment (Fig. 3F). These results indicate that PPIs promote RBC-EVs/Trim21-ASOs uptake by macrophages to suppress inflammatory responses.
PPIs promote RBC-EVs/Trim21-ASO-mediated inhibition of macrophage inflammatory responses. (A–C) The morphology (A), size distribution (B) and EV markers (C) of the RBC-EVs were evaluated by transmission electron microscopy. Scale bar, 500 nm (A), nanoparticle tracking analysis (B) and western blot (C), respectively. (D) Cy3-conjugated cholesterol-modified Trim21-ASOs were loaded into RBC-EVs and analyzed by flow cytometry. (E) PEMs were treated with 10 µg RBC-EVs/Trim21-ASOs for 48 h. TRIM21 protein expression in PEMs was detected by western blot. (F) PEMs were cocultured with 10 µg RBC-EVs/Trim21-ASOs for 48 h together with or without 10 µM Rabe and then stimulated with 50 µM PA for 12 h. IL-1β, IL-6 and TNF secreted from these cells were measured by ELISA. *P < 0.05; **P < 0.01 (one-way ANOVA followed by Tukey test; mean ± SD).
PPIs enhance the therapeutic effects of RBC-EVs/Trim21-ASOs on obesity-related metabolic disorders
We previously reported that RBC-EVs/Trim21-ASOs could relieve obesity and related metabolic disorders16. Since Rabe promotes RBC-EVs/Trim21-ASOs uptake by macrophages and reduces inflammatory responses in vitro, we then test whether Rabe could enhance the effect of RBC-EVs/Trim21-ASOs against obesity by promoting RBC-EVs/Trim21-ASOs uptake by liver macrophages in vivo. First, we found that liver macrophages from Rabe-treated HFD-fed mice took up more RBC-EVs/Trim21-ASOs than those from Rabe-untreated HFD-fed mice (Fig. 4A). As expected, Rabe further enhanced the effects of RBC-EVs/Trim21-ASOs on preventing body weight gain of HFD-fed mice (Fig. 4B). Consistently, Rabe synergized RBC-EVs/Trim21-ASOs to improve glucose tolerance and insulin resistance in HFD-fed mice (Fig. 4C, D). Rabe also strengthened RBC-EVs/Trim21-ASO-mediated decreases in ALT and AST levels (Fig. 4E). Besides, Rabe combined with RBC-EVs/Trim21-ASOs treatment further reduced liver accumulation of lipid droplets in HFD-fed mice (Fig. 4F). Altogether, PPIs effectively strengthen the uptake efficacy of RBC-EVs/Trim21-ASOs and enhance the therapeutic effects on obesity-related metabolic disorders.
PPIs enhance the therapeutic effects of RBC-EVs/Trim21-ASOs on obesity-related metabolic disorders. (A) Mice were intravenously injected with 50 µg PKH26-labeled RBC-EVs/Trim21-ASOs and simultaneously received intraperitoneal injection with 5 mg kg−1 Rabe. RBC-EVs/Trim21-ASOs in isolated F4/80+ liver macrophages were analyzed by flow cytometry. (B–F) Male mice were fed an HFD for 16 weeks and then injected with RBC-EVs/Trim21-ASOs alone or combined with Rabe from a specific time point. Body weights (B), GTT (C), ITT (D), plasma ALT and AST levels (E), representative ORO staining of liver sections (F). Scale bar, 50 μm. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA followed by Tukey test except for unpaired two-tailed Student’s t-test in A; mean ± SD).
Discussion
EVs have been proven to play essential roles in the progression of various diseases. EVs play a role in metabolic and cardiovascular diseases. Adipose tissue EVs mediate the activation of macrophage-induced insulin resistance via the TLR4/TRIF pathway23. Vascular smooth muscle cell-derived EVs induce tissue factor-dependent and phosphatidylserine-dependent thrombogenesis24. EVs are also involved in neurodegenerative disease25. RBC-EVs can effectively transport α-synuclein to the gastrointestinal tract region-dependent, thus probably impacting Parkinson’s disease initiation and progression26. In addition, EVs have extensive effects on tumor metastasis and immunosuppression27,28,29. Therefore, EVs are potential targets for the treatment of the corresponding diseases. Inhibition of pathogenic EV release will probably relieve the symptoms of various diseases.
TEVs are reported to blunt the tolerance to chemotherapeutic drugs by inducing hepatic steatosis12. We previously showed that PPIs can reduce TEVs by inducing cell micropinocytosis-mediated uptake of EVs19. This study found that PPIs effectively reduced TEVs, leading to decreased TEV accumulation in the liver. As a result, TEV-induced fatty liver was apparently attenuated, thus reducing the toxicity of chemotherapeutic drugs. However, our results will cause an issue that PPI-mediated increased EVs uptake is cell nonspecific and that effector cells will also take up more EVs, thus amplifying EVs functions. Actually, we did not observe increased uptake of TEVs by liver macrophages upon Rabe intratumoral injection. These results suggest that even if EVs are naturally liver-accumulated15, Rabe still reduces EVs uptake by liver cells. So, PPIs probably induce EVs clearance in situ by their parent cells due to the spatial and homology advantages, which in turn affects the distant dissemination of EVs and their effects on other tissues or organs.
According to our previous publication, PPIs could induce various types of cells to uptake EVs, relatively reducing the amount of certain EVs in the microenvironment19. From this perspective, PPIs theoretically promote the uptake of most EVs and reduce their levels in the microenvironment, so the effect of PPIs on EVs can theoretically be broadly applicable (although we have not tested all types of EVs). Whether the EVs cleared by PPIs are pathological or not largely depends on the characteristics of the EVs themselves and the particular pathological or physiological state. For example, intestinal epithelial cell-derived EVs (IEC-EVs) that secreted under normal physiological conditions are protective in inflammatory bowel disease (IBD) development, PPIs promote uptake-mediated clearance of IEC-EVs and aggravate IBD symptoms19.
In this study, we also found that PPIs can promote the liver macrophage uptake of RBC-EVs/Trim21-ASOs, thereby improving the therapeutic effect of RBC-EVs/Trim21-ASOs on obesity and related metabolic diseases. Mesenchymal stem cell-derived EVs (MSC-EVs) have been demonstrated to treat various diseases, including improving aging, alleviating inflammation, accelerating skin wound healing and promoting tissue regeneration30,31,32,33. However, the high cost and the low yield of MSC-EVs greatly limit their application prospects. MSC-EVs have also been reported to be helpful in treating acute liver failure34,35. Therefore, PPIs are also considered highly likely to increase the uptake of MSC-EVs and thus improve their disease benefit.
However, our study still has some limitations. In addition to the clinically significant reduction in gastric acid secretion for treating gastritis and gastric ulcers17,18, PPIs can alter gut microbiota by promoting oral microbiota translocation36. Gut microbiota was reported to play roles in both the adverse effects of chemotherapeutic agents and nonalcoholic fatty liver disease37,38,39. Thus, whether gut microbiota was involved in the PPIs-mediated reduction of chemotherapy toxicity and protection against obesity-related metabolic diseases are not clear in this study. Besides, despite the protective effect of PPIs in the above circumstances, careful consideration needs to be given to the appropriate duration and dosage of treatment with PPIs, so that the potential downside of PPIs on organs such as the kidneys can be minimized while maximizing the therapeutic effect of PPIs. In addition, we only verified the protective effect of PPIs using mouse models, and did not carry out relevant clinical trials, which is still a long way from clinical application. The above issues deserve to be further explored in future studies.
Materials and methods
Mice
C57BL/6J, nude mice (6–8 weeks old) were purchased from SLAC Laboratory Animal Co. LTD (Shanghai, China). Rosa-LSL-tdTomato mice were purchased from Cyagen Biosciences (Suzhou, Jiangsu, China). All animal experiments were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020). The research protocol was reviewed and approved by the Animal Care and Use Committee of Zhejiang University School of Medicine. We confirmed that this study strictly followed the ARRIVE guidelines (https://arriveguidelines.org).
Cell culture
MC38 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v v−1) fetal bovine serum (FBS) (Vazyme) and 1% penicillin/streptomycin (P/S). To construct stable Cre+ MC38 cells, MC38 cells were transfected with pcDNA3.1-CMV-CFP; UBC-Cre25nt plasmids and then selected under 200 µg ml−1 Zeocin, Cre expression was verified for Cre+ MC38 cells. To obtain primary mouse peritoneal macrophages (PEMs), mice (6–8 weeks old) were injected intraperitoneally with 3% fluid thioglycollate medium (Merck, Darmstadt, Germany), peritoneal lavage fluid was collected and centrifuged 3 days later. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% P/S, nonadherent cells were removed 2 h later, and the adherent monolayer cells were washed with RPMI-1640 medium and used as PEMs. For liver macrophage isolation, livers were collected and digested with DNAse I and collagenase IV at 37 °C for 1 h. After centrifugation at 500 × g for 20 min at 4 °C, the layer of suspended cells between 25% and 50% Percoll was used for macrophage isolation with MagniSort Mouse F4/80 Positive Selection Kit (Invitrogen). For primary hepatocyte isolation, Livers were collected and digested with DNAse I and collagenase IV at 37 °C for 1 h. After centrifugation at 50 × g for 3 min at 4 °C, the pellet was collected for primary hepatocyte isolation. Then the pellet was washed twice in 30 ml PBS by centrifugation at 50 × g for 2 min at 4 °C. The hepatocytes were pelleted down at 50 × g for 2 min at 4 °C, resuspended in RPMI-1640 medium supplemented with 10% FBS and 1% P/S, and cultured in collagen I-coated plates.
Isolation of EVs
For EV isolation, cell culture supernatants were centrifuged at 300 × g for 10 min, 2000 × g for 20 min and 10,000 × g for 30 min at 4 °C. Then, the supernatants were passed through 0.22 μm syringe filters (Millipore) and collected in 35 ml ultracentrifuge tubes (Beckman Coulter, Brea, CA, USA). The EVs were concentrated using ultracentrifugation with an SW32Ti rotor (L-90 K with SW32Ti rotor, Beckman Coulter) at 100,000 × g for 70 min at 4 °C. The EV protein contents were quantified by a BCA protein assay kit (Thermo Fisher Scientific).
Isolation of RBC-EVs and production of RBC-EVs/Trim21-ASOs
For RBC-EVs isolation, RBCs were separated from plasma by centrifugation at 500 × g for 10 min and passed through a leukodepletion filter (Terumo, Tokyo, Japan). Isolated RBCs were diluted in RPMI-1640 medium and treated with 2 µM calcium ionophore (Merck) for 48 h at 37 °C. Then, the RBCs and cell debris were removed by centrifugation at 600 × g for 20 min, 1,600 × g for 15 min, 3,260 × g for 15 min, and 10,000 × g for 30 min (all steps at 4 °C). RBC-EVs were concentrated using ultracentrifugation with a SW32Ti rotor (L-90 K with an SW32Ti rotor, Beckman Coulter) at 100,000 × g for 70 min at 4 °C. Subsequently, the EV pellets were resuspended in sterile PBS. For the production of RBC-EVs/Trim21-ASOs, RBC-EVs were coincubated with cholesterol-modified Trim21-ASOs at 37 °C for 2 h and washed twice by centrifugation at 10,000 × g for 30 min at 4°C.
EVs labelling
Cy3-conjugated Trim21-ASOs were synthesized by the company and loaded onto RBC-EVs. For PKH26 labelling, PKH26-labelled RBC-EVs were washed and resuspended for 3 times and then intercepted with a 500-KD filter, PKH26-labelled RBC-EVs in the upper chamber were collected for experiments, and the filtrate (which may contain residual PKH26 dye) was incubated with the cells. The uptake of PKH26 dye by cells was analyzed using flow cytometry as a quality control (the PKH26 signal was negative) (Supplementary Fig. 1A).
Western blot
Cell lysates or EV lysates were separated by SDS–PAGE and transferred to PVDF membranes (Millipore, Massachusetts, USA). After blocking with 5% BSA, the membranes were incubated with primary antibodies at 4 °C overnight and then incubated with the corresponding secondary antibodies for 1 h at RT. The membranes were washed three times for 10 min each, incubated with SuperSignal Chemiluminescent Substrate (Pierce, Dallas, Texas, USA) and scanned with a Tanon 4500 Gel Imaging System (Tanon, Shanghai, China). The antibodies are listed in Table S1. In this study, the blots were cut according to the molecular weight of proteins and then incubated with specific antibodies, therefore the full-length blots are not available. 3 replicate blots of each protein and membranes with markers (photographed under bright field) that have clearly visible membrane edges are provided in the Supplementary Information.
Flow cytometric analysis
Livers were collected from Rosa-LSL-tdTomato mice, liver macrophages were isolated for tdTomato fluorescence detection after coculture with 5 µg Cre+ MC38-EVs for 24 h. To analyze the content of Trim21-ASOs in RBC-EVs, RBC-EVs/Trim21-ASOs (Trim21-ASOs conjugated with Cy3) were incubated with 4-µm anti-CD63-coated aldehyde sulfate latex beads (Thermo Fisher Scientific), then were washed and collected for Cy3 fluorescence detection. Livers were collected from mice receiving PKH26-labeled RBC-EVs/Trim21-ASOs, liver macrophages were isolated for PKH26 fluorescence detection. The cells or beads were washed with PBS and analyzed using a ACEA NovoCyteTM system.
ELISA for EVs
Sandwich ELISA measured serum EVs in the individual samples. Briefly, 96-well ELISA plates were coated with 5 µg ml−1 purified anti-CD63 antibodies in coating buffer and incubated overnight at 37 °C. After blocking with assay diluent, serum samples in triplicate were added to individual wells and incubated overnight at 37 °C. The plates were washed, and the bound EVs were incubated with Fixation/Permeabilization buffer for 15 min at room temperature. Then the bound EVs were detected by incubation with anti-Cre and HRP for 2 h at 37 °C. Finally, the signal was developed with tetramethylbenzidine, and the samples were blocked with 2 M H2SO4, after which absorbance at 450 nm was measured with a SpetraMax M5 microplate reader (Molecular Devices, San Jose, CA, USA). The antibodies used are listed in Table S1.
Real-time PCR
According to the manufacturer’s instructions, total RNAs were isolated from cells or tissues using TRIzol reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Single-stranded cDNA was synthesized using Evo M-MLV Mix Kit (AG11706, Accurate Biotechnology, Hunan, China) for reverse transcription of mRNAs. Real-time PCR was performed with the CFX Touch system Bio-Rad using Hieff® qPCR SYBR Green Master Mix (11201ES, Yeasen, Shanghai, China). The primers used are listed in Table S1.
Tumor model
To examine tdTomato expression in the liver, Rosa-LSL-tdTomato mice were injected subcutaneously with 1 × 106 MC38 or Cre+ MC38 tumors on day 0, then mice received an intratumoral injection of DMSO or 15 µg Rabeprazole (Rabe) for 3 days starting on day 9, blood and livers were then collected for subsequent experiments. To examine lipid droplet formation in the liver, nude mice were injected subcutaneously with 1 × 106 MC38 or Atp6v1aKD MC38 tumors on day 0, then mice received an intratumoral injection of DMSO or 15 µg Rabe for 5 days starting on day 11, livers were then collected for subsequent experiments.
Histological analysis
Murine livers were fixed in Tissue-Tek® O.C.T. Compound (SAKURA, USA), and frozen liver sections were prepared and subjected to BODIPY staining or Oil Red O (ORO) staining to visualize lipid droplets. Nuclei were stained with DAPI. Histological changes were examined by Olympus FV3000 laser confocal microscope (Olympus). Images were analyzed with ImageJ software.
Dacarbazine toxicity analysis
To examine the protective effect of PPIs in chemotherapeutic toxicity. MC38 and Atp6v1aKD MC38 tumor mice were intratumoral injected with DMSO or 15 µg Rabe every 2 days for 2 weeks. Then these mice were intraperitoneally injected with 30 mg kg−1 dacarbazine combined with or without intratumoral injection of Rabe every 2 days for 3 injections. Livers were collected for subsequent testing in other experiments. Blood from these mice was collected to examine the number of RBCs and reticulocytes to evaluate toxicity. The above experiment also applies to omeprazole (Ome).
Electron microscopy
For negative EV staining, 200-mesh carbon films were hydrophilized with a glow discharge instrument at 15 mA for 25 s. An EV solution was pipetted onto 200-mesh carbon-coated copper grids and kept at RT for 1 min. After removing any excess suspension with filter paper, the EVs were negatively stained with 2% uranyl acetate at RT for 1 min. Any extra suspension was removed before the grids were air-dried. Images were acquired by electron microscopy (Tecnai G2 Spirit 120 kV, Thermo FEI, Hillsboro, OR, USA).
Nanoparticle tracking analysis
To measure particle sizes and concentrations, purified EVs were analyzed by nanoparticle tracking analysis using a NanoSight NS300 system (Malvern PANalytical) configured with a 488 nm laser and high-sensitivity sCMOS camera and were finally analyzed with NTA 3.3 software.
Measurement of cytokines
ELISA was used to measure the IL-1β, IL-6, TNF levels in murine serum (BioLegend, San Diego, California, USA), alanine aminotransferase (ALT) and aspartate transaminase (AST) (Nanjing Jiancheng, Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
High-fat diet (HFD)-induced obesity model
To establish the HFD-induced obesity model, male mice (6–8 weeks old) were fed an HFD with 60% of the calories from fat (FB-D12492, Fanbo Biotechnology, Wuxi, Jiangsu, China) for 16 weeks, and the body weight was measured weekly. For RBC-EVs/Trim21-ASOs treatment, HFD-fed mice were intraperitoneally injected with RBC-EVs/Trim21-ASOs thrice weekly starting at week 7. For Rabe treatment, HFD-fed mice were intraperitoneally injected with DMSO or 5 mg kg−1 Rabe (Selleck) thrice weekly starting at week 12.
Glucose tolerance test and insulin tolerance test
After 16 h overnight fasting, mice were injected intraperitoneally with glucose (1.5 g kg−1), and blood samples for glucose tolerance test (GTT) were collected from the tail vein at the indicated times. After fasting for 6 h, mice were injected intraperitoneally with regular human insulin (0.75 U kg−1), and blood samples for insulin tolerance test (ITT) were collected from the tail vein at the indicated times. Glucose levels were assessed using Accu-Chek glucose meters (Roche).
Statistical analysis
Statistical differences were compared by students’ t-tests between two groups and one-way ANOVA followed by Tukey tests among multiple groups. All data are expressed as the mean ± SD values and were analyzed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Differences with P < 0.05 were defined as significant.
Data availability
All data needed to evaluate the conclusions in the paper are presented in the main manuscript and supplementary information.
References
Kalluri, R. & Lebleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367 (2020).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19, 213–228 (2018).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 30, 255–289 (2014).
White, R. et al. Special considerations for studies of extracellular vesicles from parasitic helminths: a community-led roadmap to increase rigour and reproducibility. J. Extracell. Vesicles 12, e12298 (2023).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): a position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Marar, C., Starich, B. & Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 22, 560–570 (2021).
Wan, S. et al. Cd8alpha(+)cd11c(+) extracellular vesicles in the lungs control immune homeostasis of the respiratory tract via tgf-beta1 and il-10. J. Immunol. 200, 1651–1660 (2018).
Jiang, L. et al. Epcam-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat. Commun. 7, 13045 (2016).
Dong, Q. et al. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front. Cell. Dev. Biol. 9, 733627 (2021).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Poggio, M. et al. Suppression of exosomal pd-l1 induces systemic anti-tumor immunity and memory. Cell 177, 414–427 (2019).
Wang, G. et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 618, 374–382 (2023).
Arjomandnejad, M., Dasgupta, I., Flotte, T. R. & Keeler, A. M. Immunogenicity of recombinant adeno-associated virus (aav) vectors for gene transfer. BioDrugs 37, 311–329 (2023).
Meng, W. et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 27, 585–598 (2020).
Zhang, G. et al. Extracellular vesicles: natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J. Extracell. Vesicles 10, e12030 (2020).
Lu, X. et al. Ube2m-mediated neddylation of trim21 regulates obesity-induced inflammation and metabolic disorders. Cell. Metab. 35, 1390–1405 (2023).
Li, H., Meng, L., Liu, F., Wei, J. F. & Wang, Y. Q. H+/k+-atpase inhibitors: a patent review. Expert Opin. Ther. Pat. 23, 99–111 (2013).
Yatime, L. et al. P-type atpases as drug targets: tools for medicine and science. Biochim. Biophys. Acta 1787, 207–220 (2009).
Lu, X. et al. Proton pump inhibitors enhance macropinocytosis-mediated extracellular vesicle endocytosis by inducing membrane v-atpase assembly. J. Extracell. Vesicles 13, e12426 (2024).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
Jamwal, R. & Barlock, B. J. Nonalcoholic fatty liver disease (nafld) and hepatic cytochrome p450 (cyp) enzymes. Pharmaceuticals (Basel) 13 (2020).
Carta, G., Murru, E., Banni, S. & Manca, C. Palmitic acid: physiological role, metabolism and nutritional implications. Front. Physiol. 8, 902 (2017).
Deng, Z. B. et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58, 2498–2505 (2009).
Kapustin, A. N. et al. Prothrombin loading of vascular smooth muscle cell-derived exosomes regulates coagulation and calcification. Arterioscler. Thromb. Vasc. Biol. 37, e22–e32 (2017).
Picca, A. et al. Circulating extracellular vesicles: friends and foes in neurodegeneration. Neural Regen. Res. 17, 534–542 (2022).
Yang, Y. et al. Erythrocytic alpha-synuclein and the gut microbiome: kindling of the gut-brain axis in parkinson’s disease. Mov. Disord. 39, 40–52 (2024).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Chen, J., Fei, X., Wang, J. & Cai, Z. Tumor-derived extracellular vesicles: regulators of tumor microenvironment and the enlightenment in tumor therapy. Pharmacol. Res. 159, 105041 (2020).
Asao, T. et al. Extracellular vesicles and particles as mediators of long-range communication in cancer: connecting biological function to clinical applications. Extracell. Vesicles Circ. Nucleic Acids 4, 461–485 (2023).
Ding, J. Y. et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. Mil Med. Res. 10, 36 (2023).
Dorronsoro, A. et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 20, e13337 (2021).
Tsiapalis, D. & O’Driscoll, L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells 9 (2020).
Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N. & Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8 (2019).
Jiang, W. et al. Human umbilical cord msc-derived exosomes suppress the development of ccl(4)-induced liver injury through antioxidant effect. Stem Cells Int. 2018, 6079642 (2018).
Yan, Y. et al. Hucmsc exosome-derived gpx1 is required for the recovery of hepatic oxidant injury. Mol. Ther. 25, 465–479 (2017).
Xiao, X. et al. Proton pump inhibitors alter gut microbiota by promoting oral microbiota translocation: a prospective interventional study. Gut 73, 1098–1109 (2024).
Li, Y. et al. Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating pink1/parkin signaling and intestinal flora. Sci. Rep. 14, 13063 (2024).
Mishra, S. et al. Gut microbiome-derived bacterial extracellular vesicles in patients with solid tumours. J. Adv. Res. (2024).
Cheng, R. et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 13, 2555 (2022).
Acknowledgements
We thank the Key Laboratory of Immunity and Inflammatory Diseases of Zhejiang Province for the support.
Funding
This work was funded by the National Natural Science Foundation of China (82000003, 82402123).
Author information
Authors and Affiliations
Contributions
Mengyu Li, Weiyi Yuan, Xianghui Kong, Hao Wu performed various experiments; Zhijian Cai and Xinliang Lu wrote and discussed the manuscript; Weiguo Zhu and Xinliang Lu designed the project and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, M., Yuan, W., Kong, X. et al. Proton pump inhibitors reduce chemotherapeutic hepatotoxicity and enhance hepatic uptake and accumulation of drug-loaded extracellular vesicles. Sci Rep 14, 28163 (2024). https://doi.org/10.1038/s41598-024-75775-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75775-6