Abstract
Acute rejection (AR) is a significant complication in liver transplantation, impacting graft function and patient survival. Kupffer cells (KCs), liver-specific macrophages, can polarize into pro-inflammatory M1 or anti-inflammatory M2 phenotypes, both of which critically influence AR outcomes. Angiopoietin-like 4 (ANGPTL4), a secretory protein, is recognized for its function in regulating inflammation and macrophage polarization. This study investigates the effects of ANGPTL4 on KC polarization through cellular interactions between hepatocytes (HCs) and KCs. Using a rat orthotopic liver transplantation model, we observed reduced ANGPTL4 expression during AR, whereas increased ANGPTL4 levels were linked to immune tolerance. Administration of ANGPTL4 recombinant protein improved liver function, suppressed inflammation, and promoted M2 polarization of KCs. Co-culture experiments demonstrated that hepatocyte-derived ANGPTL4 significantly modulates KC polarization and inflammatory responses, mainly by inhibiting the NF-κB signaling pathway. The results emphasize the promise of ANGPTL4 as a therapeutic target to reduce AR and improve liver transplant outcomes by influencing hepatocyte-KC interactions.
Similar content being viewed by others
Introduction
Liver transplantation is a crucial intervention for individuals with end-stage liver diseases and certain hepatic cancers1. Despite advancements in surgical techniques and immunosuppressive therapies, acute rejection (AR) remains a significant complication that can compromise graft function and patient survival2. Gaining insights into the molecular and cellular mechanisms of AR is essential for developing targeted therapies to enhance transplant outcomes.
Macrophages are crucial components of both innate and adaptive immunity, differentiating into M1 and M2 phenotypes. M1-type macrophages predominantly secrete pro-inflammatory cytokines like IL-6, IL-1β, and TNF-α, which amplify inflammatory responses. In contrast, M2-type macrophages release IL-10 and TGF-β, anti-inflammatory cytokines that reduce inflammation and support tissue repair3,4.These phenotypic variations enable macrophages to respond flexibly to microenvironmental cues, switching between M1 and M2 states to fulfill distinct immunological functions5. Kupffer cells (KCs), a distinct form of liver-resident macrophages, are essential to the liver’s innate immune defense6. Within liver transplantation scenarios, KCs exhibit dual functionality7. On one hand, their M1 polarization can contribute to AR by promoting inflammation and associated tissue damage8. Conversely, M2 polarization of KCs helps alleviate AR by suppressing inflammation, promoting tissue repair, and inducing tolerance5. Thus, promoting the M2 polarization of KCs could offer an innovative therapeutic approach to reduce AR in liver transplantation.
Angiopoietin-like 4 (ANGPTL4) has attracted notable interest recently because of its involvement in various physiological and pathological functions9. ANGPTL4, a secreted protein from the angiopoietin-like family, is involved in metabolism, tissue repair, and inflammation10,11. By inhibiting lipoprotein lipase, ANGPTL4 regulates lipid metabolism, resulting in higher plasma triglyceride levels. Consequently, it is linked to a range of metabolic disorders, such as obesity, hypertriglyceridemia, and type 2 diabetes. Moreover, its expression is induced by factors such as fasting, hypoxia, and PPAR activation12,13. ANGPTL4 can act as a downstream target of the PPAR signaling pathway to regulate M1/M2 macrophage polarization, thereby alleviating diabetic osteoporosis14. In cancer, ANGPTL4 influences tumor progression and metastasis by enabling anoikis resistance and modulating the tumor microenvironment, exhibiting context-dependent tumor-promoting or tumor-suppressing effects15. One of the most intriguing aspects of ANGPTL4 is its role in inflammation and immune modulation. ANGPTL4 promotes the anti-inflammatory M2 macrophage phenotype, supporting tissue repair and inflammation resolution, particularly in contexts like cardiac repair and wound healing16,17. Given its diverse functions, ANGPTL4 presents a promising target for therapeutic development. However, the involvement of ANGPTL4 in liver transplantation and its effects on KCs, the liver-resident macrophages, remain incompletely understood.
Materials and methods
Animals and model construction
Male BN and Lewis rats (230–260 g) were provided by Beijing Vital River Laboratory Animal Tech (Beijing, China) kept under laminar-flow, specific-pathogen-free conditions at the Chongqing Medical University Experimental Animal Centre (Chongqing, China). The AR model (Lewis as donor, BN as recipient) and the immune tolerance (IT) model (BN as donor, Lewis as recipient) for orthotopic liver transplantation in rats were established using a modified two-cuff technique based on Kamada’s method18. In the pseudo-operation group, rats underwent a laparotomy without liver transplantation. All surgeries were performed under anesthesia with intraperitoneal injection of sodium pentobarbital (30 mg/kg). All rats received humane care according to principle published in the 8th edition of “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health). Euthanasia was performed under anesthesia by intraperitoneal injection of a high dose of sodium pentobarbital (120 mg/kg). The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (IACUC-CQMU-2024-0609). All methods were performed in accordance with the relevant guidelines and regulations, and the study was reported in accordance with the ARRIVE guidelines.
Isolation and culture of primary hepatocytes (HCs) and KCs
Primary rat HCs and KCs were isolated using a two-step collagenase perfusion method19,20. Livers from male Lewis rats (230–260 g) were initially perfused through the hepatic portal vein with HBSS free of calcium and magnesium until the liver appeared pale. The next step included perfusing the liver with HBSS containing calcium, magnesium, and 0.5 mg/mL collagenase IV (C8160, Solarbio, Beijing, China) to soften the tissue. The liver was then carefully dissociated and filtered through a 200 μm strainer to create a suspension of single cells. HCs were further purified by centrifugation with 70% Percoll solution (BS909, Biosharp, China) and cultured in William’s E Medium (Gibco™, USA) with 5% fetal bovine serum (FBS, ExCell Bio, USA), 2 mM L-glutamine, 100 nM insulin, 100 nM dexamethasone, and 1% penicillin-streptomycin. The culture medium was refreshed 4 h after initial seeding and every 2 days thereafter. For KCs, the suspension was separated from HCs and other sinusoidal cells by low-speed centrifugation (50 g, 4 °C). The isolated cells were then cultured in RPMI 1640 Medium (Gibco™, USA), supplemented with 10% FBS and 1% penicillin-streptomycin, and maintained at 37 °C in a 5% CO₂ environment. Non-adherent cells were removed after 2 h by washing with PBS, leaving behind adherent KCs.
Histology and liver function
Liver specimens from each group were collected postoperatively. The liver tissues were preserved in 4% paraformaldehyde, embedded in paraffin, and cut into 5 μm sections. These samples were stained with Hematoxylin-eosin (HE) and examined for histopathological changes using an inverted microscope. The extent of hepatic damage in each group was evaluated based on the Banff scoring system21. According to the rejection activity index (RAI), scores of 0–3 indicated uncertain AR, 3–5 mild AR, 5–7 moderate AR, and 7–9 severe AR. The alanine aminotransferase (ALT) and the aspartate aminotransferase (AST) Assay Kit were obtained from Solarbio (China).
ELISA
The ELISA was conducted using a double-antibody sandwich method following the manufacturer’s instructions. ELISA kits for IL-10 (Cat#: ERC004), TNF-α (Cat#: ERC102), and IL-1β (Cat#: ERC007) were obtained from NeoBioscience (Shenzhen, China). The rat ANGPTL4 (Cat#: LS-F27334) ELISA kit was purchased from LifeSpan BioSciences (Seattle, USA).
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining
Apoptosis in liver tissue was detected using the TUNEL assay with the Colorimetric TUNEL Apoptosis Assay Kit (C1098, Beyotime, China), following the manufacturer’s protocol. Nuclear staining of each group was observed under a fluorescence microscope.
Real-time quantitative PCR analysis (RT-qPCR)
RNA was extracted from liver tissues or cells using Trizol reagent (Invitrogen), and RNA quality was assessed with a NanoDrop spectrophotometer (Thermo Fisher Scientific). cDNA synthesis was performed using the PrimeScript RT Reagent Kit (Takara). RT-qPCR was carried out with 10 µL of 2X SYBR Green PCR Master Mix. Primer sequences are detailed in Table 1. The thermal cycling conditions were set as follows: initial denaturation at 95 °C for 3 min, then 40 cycles of 95 °C for 10 s, and 60 °C for 30 s. Gene expression levels were quantified using the 2^−ΔΔCt method with GAPDH as the reference gene.
Western blot analysis
RIPA lysis buffer (P0013B, Beyotime, China) with added protease and phosphatase inhibitors (P1050, Beyotime, China) was used for protein extraction. Equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, USA). The membranes were blocked with a quick blocking solution (Beyotime) and incubated overnight at 4 °C with primary antibodies as listed in Table 2. The following day, membranes were incubated with secondary antibody and visualized using ECL chemiluminescent substrate (Cat. No. 17047, Zenbio).
Immunohistochemistry (IHC)
The paraffin-embedded sections were deparaffinized with xylene, followed by rehydration through graded ethanol washes. Antigen retrieval was achieved by heating the sections in citrate buffer at over 95 °C for 20 min. Immunohistochemical staining was performed using the PV-9000 detection kit (ZSGB-BIO, Beijing, China) according to the manufacturer’s instructions. The sections were incubated overnight at 4 °C with primary antibodies listed in Table 2. Visualization was done with diaminobenzidine (DAB), and the sections were examined under a light microscope (Olympus, Japan).
Immunofluorescence (IF)
Cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After permeabilization, the cells were blocked with 5% BSA. Primary antibodies (Table 2) were then applied and incubated overnight at 4 °C. After washing, the samples were incubated with secondary antibodies conjugated to Cy3 or FITC at 37 °C for 30 min, followed by nuclear staining using DAPI (Beyotime), and coverslips were mounted using an antifade medium. Images were captured using a fluorescence microscope.
Flow cytometry analysis
KCs isolated from rat liver tissue were prepared as a single-cell suspension in PBS, stained with antibodies at 4 °C in the dark for 1 h, followed by three washes with PBS. The cells were labeled with CD68-FITC and CD86-APC antibodies. Flow cytometry analysis was performed using a CytoFLEX flow cytometer, and the data were processed with CytExpert 2.4.0 software (Beckman Coulter). Details on the antibodies and their dilutions used for flow cytometry are listed in Table 2.
Statistical analysis
Data analysis and visualization were performed using GraphPad Prism version 10 (San Diego, CA, USA). Results are presented as mean ± SD. Comparisons between two groups were made using an unpaired Student’s t-test, while one-way ANOVA followed by Tukey’s multiple comparison test was used for comparisons among three or more groups. Statistical significance was set at P < 0.05, with *P < 0.05, **P < 0.01, and ***P < 0.001 indicating varying levels of significance.
Results
Identification of IT and AR model
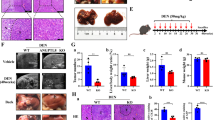
Rat models for the IT and AR groups were established following liver transplantation, followed by comprehensive histological and biochemical analyses of the transplanted liver. HE staining indicated significant hepatocyte edema and necrosis in the AR group, with inflammatory cell infiltration and disrupted hepatic lobule architecture. In contrast, the IT group displayed a milder inflammatory response, with relatively intact tissue architecture (Fig. 1A). The AR group exhibited significantly elevated RAI scores compared to the other groups (Fig. 1B). TUNEL staining further corroborated these findings, as the AR group displayed a substantial rise in apoptotic cells compared to the Sham group, while the IT group showed only a mild increase (Fig. 1C). The AR group had notably higher AST and ALT levels in serum liver function tests, indicating severe liver damage (Fig. 1D). Moreover, Serum analysis showed significantly elevated TNF-α and IL-1β levels, with a marked reduction in IL-10 in the AR group. Conversely, the IT group exhibited higher IL-10 levels and reduced TNF-α and IL-1β, nearing those seen in the Sham group (Fig. 1E). These findings indicate that the immune tolerance mechanism is vital in mitigating post-transplant liver injury, reducing apoptosis, and inhibiting the inflammatory response.
Analyzing AR in rat liver transplant models. (A) Morphological alterations detected 7 days post-liver transplantation (original magnifications: x100 and x400). (B) RAI scoring according to the Banff classification criteria. (C) TUNEL staining at 200x magnification was used to identify apoptotic cells in liver tissues from each group post-transplantation. (D) Serum liver enzyme levels post-transplantation, as measured by microtiter assay. (E) ELISA was employed to quantify serum inflammatory factor levels in rats post-liver transplantation. ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 6.
Significant downregulation of ANGPTL4 expression during AR in Rat Liver Transplantation
To investigate ANGPTL4’s involvement in AR progression post-liver transplantation, we assessed its differential expression. RT-qPCR and ELISA results indicated a significant decrease in ANGPTL4 expression in the AR group compared to the IT group (Fig. 2A, B). IHC analysis supported these findings, highlighting elevated ANGPTL4 expression in the IT group, particularly in hepatocytes, whereas the AR group exhibited reduced expression (Fig. 2C). These findings imply that increased ANGPTL4 expression, primarily secreted by HCs, may critically influence the immune response post-liver transplantation, especially in the context of immune tolerance mechanisms.
ANGPTL4 expression in the liver following transplantation. (A) RT-qPCR evaluation of ANGPTL4 levels in rat liver tissues. (B) ELISA results of serum ANGPTL4 protein levels. (C) Immunohistochemical staining of ANGPTL4 in liver graft sections (200x magnification). ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 6.
ANGPTL4 mitigates AR in rat liver transplantation
To further investigate ANGPTL4’s role in AR in rat liver transplantation, we administered ANGPTL4 recombinant protein in vivo. Compared to the AR and AR + PBS groups, rats treated with ANGPTL4 recombinant protein showed better-preserved liver tissue structure, with notably reduced inflammatory cell infiltration and decreased tissue damage. (Fig. 3A). Correspondingly, The ANGPTL4 recombinant protein group had significantly lower RAI scores (Fig. 3B). TUNEL staining further validated these findings, demonstrating a significant reduction in apoptotic cells in the ANGPTL4 recombinant protein group (Fig. 3C). Moreover, serum AST and ALT levels were markedly decreased in the ANGPTL4-treated group, indicating reduced liver function impairment (Fig. 3D). Inflammatory cytokine analysis showed that rANGPTL4 protein significantly lowered TNF-α and IL-1β levels while boosting IL-10 expression (Fig. 3E). These findings indicate that ANGPTL4 recombinant protein serves an essential protective function in reducing AR in rat liver transplantation.
ANGPTL4 recombinant protein mitigates AR following liver transplantation. (A, B) Representative HE images of liver grafts taken 7 days post-transplantation at original magnifications of ×100 and ×400, along with RAI scoring of the liver. (C) TUNEL staining to identify apoptotic cells in liver tissues across different groups post-transplantation (200× magnification). (D) Measurement of serum liver enzyme levels after transplantation using a microtiter assay. (E) ELISA evaluation of serum inflammatory factors in rats following liver transplantation. ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 6.
ANGPTL4 promotes M2 polarization of KCs in vivo
Since earlier studies have shown the importance of M2 polarization of KCs in reducing AR, we explored the regulatory effects of ANGPTL4 on KC phenotypes. Western blot analysis indicated that administering ANGPTL4 recombinant protein administration significantly upregulated the M2 marker protein Arg1 in KCs, while the M1 marker CD86 expression was notably downregulated (Fig. 4A). IHC staining further validated these findings, showing a significant rise in M2-type KCs and a corresponding reduction in M1-type KCs in ANGPTL4-treated liver tissues (Fig. 4B). These results indicate that ANGPTL4 could be crucial in reducing AR in liver transplantation by promoting KC polarization towards the anti-inflammatory M2 phenotype.
ANGPTL4 alleviates LPS-induced inflammatory response and promotes M2 polarization of KCs in vitro
In this part of the study, we employed an in vitro inflammation model induced by lipopolysaccharide (LPS) in KCs to explore the regulatory effects of ANGPTL4. By adding different concentrations of ANGPTL4 recombinant protein to LPS-induced KC cells, we observed a significant dose-dependent effect. RT-qPCR and ELISA analyses revealed that higher concentrations of ANGPTL4 significantly reduced TNF-α and IL-1β expression while notably increasing IL-10 levels, an anti-inflammatory cytokine (Fig. 5A). Western Blot analysis indicated that as ANGPTL4 concentrations increased, the M2 marker Arg1 expression in KCs gradually rose, while the M1 marker CD86 expression progressively declined (Fig. 5B, C). Flow cytometry confirmed that ANGPTL4 recombinant protein significantly enhanced the polarization of KCs towards the M2 phenotype (Fig. 5D). These findings indicate that ANGPTL4 efficiently regulates the inflammatory response and polarization of KCs in an in vitro inflammation model.
ANGPTL4 recombinant protein promotes M2 polarization of KCs. (A) RT-qPCR and ELISA were used to measure IL-10, IL-1β, and TNF-α expression levels in KCs isolated from rat liver and their corresponding levels in the cell supernatant. (B) WB analysis of polarization-related protein levels in KCs, with quantitative evaluation based on band intensity. (C) Flow cytometry was employed to assess M1-polarized KCs, using FITC-labeled CD68 for KC identification and APC-labeled CD86 to detect M1 polarization. ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 3.
Hepatocyte co-culture reduces inflammatory response in KCs and suppresses NF-κB signaling activation
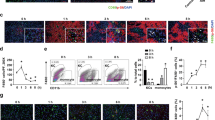
Based on in vivo IHC findings that ANGPTL4 is primarily expressed in HCs, we designed a co-culture experiment involving HCs and KCs (Fig. 6A) to further explore the regulatory role of ANGPTL4 between cells. ELISA results revealed a substantial increase in ANGPTL4 expression in the supernatant of the co-culture system (Fig. 6B). Additionally, RT-qPCR and ELISA analyses showed that co-culture significantly reduced TNF-α and IL-1β levels, while markedly increasing IL-10, an anti-inflammatory cytokine (Fig. 6C). Western blot and flow cytometry results showed a marked enhancement in M2 polarization of KC cells after co-culture (Fig. 6D, E). This suggests that ANGPTL4 may influence the polarization state of KC cells through a paracrine mechanism.
Given that NF-κB is a crucial pathway in regulating KC cell polarization, we investigated the key molecules involved in this pathway. Western blot analysis revealed that LPS treatment significantly elevated the phosphorylation of key NF-κB pathway components, including p65, IκBα, and IKKβ. However, co-culture with LC cells led to a reduction in the phosphorylation levels of these molecules, indicating partial inhibition of the NF-κB pathway (Fig. 6F). Immunofluorescence confirmed these findings, showing a marked decrease in the nuclear translocation of p65 protein in KC cells post-co-culture (Fig. 6G). These results indicate that HCs may interact with KCs by secreting ANGPTL4, enhancing the polarization of KCs toward the anti-inflammatory M2 phenotype and plays a crucial role in regulating the immune response after liver transplantation.
Hepatocyte-derived ANGPTL4 is crucial for suppressing inflammatory activation in KCs. (A) Schematic of the experimental procedure: co-culture of HCs with LPS-stimulated KCs. (B) ELISA analysis of ANGPTL4 levels in the KCs culture medium. (C) RT-qPCR and ELISA analysis of IL-10, IL-1β, and TNF-α expression levels. (D) WB analysis of polarization-related proteins in KCs, with quantitative assessment based on band intensity. (E) Flow cytometry analysis of M1-polarized cells, identifying KCs with FITC-labeled CD68 and M1 markers with APC-labeled CD86. (F) Western blot analysis of proteins related to the NF-κB pathway, with quantification based on band intensity. (G) Immunofluorescence staining to localize phosphorylated p65. ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 3.
siRNA knockdown of ANGPTL4 enhances inflammatory response in KCs and activates NF-κB signaling
To further confirm the role of ANGPTL4 in regulating KC polarization by HCs (HCs), we used siRNA technology to silence ANGPTL4 expression in HC cells (Fig. 7A). RT-qPCR and ELISA analyses indicated that ANGPTL4 knockdown led to a significant increase in TNF-α and IL-1β expression, while IL-10 levels were notably decreased (Fig. 7B). Western blot and flow cytometry analyses indicated that knockdown of ANGPTL4 resulted in a reduction of M2-type KC cells and an increase in M1-type KC cells (Fig. 7C, D). Western blot results further revealed a significant increase in the phosphorylation of key NF-κB pathway molecules after si-ANGPTL4 introduction, indicating enhanced activation of the NF-κB signaling pathway (Fig. 7E). Immunofluorescence results (Fig. 7F) further supported these findings, showing a significant rise in the nuclear translocation of p65 protein in KC cells, indicating intensified NF-κB pathway activation following ANGPTL4 suppression. These results suggest that ANGPTL4 protein secreted by HCs is essential in suppressing the inflammatory response and regulating NF-κB pathway activation in KCs. Disrupting ANGPTL4 expression diminishes this protective effect, leading to a more pronounced inflammatory response.
Knockdown of ANGPTL4 expression in HCs within the co-culture system promotes M1 polarization of KCs. (A) Schematic representation of the experimental procedure: siRNA-mediated knockdown of ANGPTL4 in HCs co-cultured with LPS-stimulated KCs. (B) RT-qPCR and ELISA assessments of IL-10, IL-1β, and TNF-α levels. (C) WB analysis of polarization-related proteins in KCs, with quantification of band intensity. (D) Flow cytometry of M1-type polarized cells, with FITC-labeled CD68 and APC-labeled CD86. (E) Western blot assessment of proteins associated with the NF-κB pathway, including band intensity quantification. (F) Immunofluorescence staining to identify the localization of phosphorylated p65. ns P > 0.05, *P < 0.05; **P < 0.01; ***P < 0.001; n = 3.
Discussion
Liver transplantation continues to be a highly effective treatment for advanced liver disease1. However, AR continues to pose a major challenge to achieving successful outcomes22. Given the critical role of AR in determining the long-term success of liver transplantation, understanding its underlying mechanisms is of paramount importance. This study highlighted the crucial role of ANGPTL4 in regulating the immune response during liver transplantation, particularly by influencing the polarization of KCs. The findings not only enhanced the current understanding of the mechanisms behind AR but also positioned ANGPTL4 as a promising therapeutic target. By promoting the M2 phenotype in KCs, ANGPTL4 appeared to mitigate the inflammatory processes that drive AR, potentially paving the way for new approaches to enhancing graft survival and function.
ANGPTL4, part of the angiopoietin-like family, plays multiple roles in regulating lipid metabolism, angiogenesis, and inflammation23,24. Its role in inflammation is complex, as it can either promote or inhibit inflammatory responses depending on the context25. The immunomodulatory role of ANGPTL4 has been previously documented26,27, and studies have shown that overexpression of ANGPTL4 can inhibit LPS-induced damage in Caco-2 cells, highlighting its protective potential in inflammatory settings28. Our data revealed that ANGPTL4 expression was significantly downregulated during AR. This finding aligns with previous studies that have shown reduced ANGPTL4 levels in inflammatory conditions28,29,30. From these observations, we hypothesized that ANGPTL4 might regulate the immune response in rat liver transplantation. Supporting our hypothesis, we found that overexpression of ANGPTL4 in recipients notably enhanced liver function, minimized hepatic tissue damage, and decreased serum inflammatory factors.
Moreover, in liver transplantation, the dual role of KCs as both promoters of inflammation and agents of immune tolerance was well-documented. M1-polarized KCs exacerbate AR by enhancing inflammatory responses and tissue injury, while M2-polarized KCs contribute to immune tolerance by dampening inflammation and facilitating tissue repair31,32. Our study revealed that ANGPTL4 significantly enhances the M2 polarization, as indicated by the upregulation of Arg1 and downregulation of CD86. The ability of ANGPTL4 to drive M2 polarization was consistent with its known effects in other inflammatory conditions, where it has been shown to shift KCs polarization towards the M2-type, thereby promoting tissue repair and reducing inflammation16,33.
Furthermore, our in vitro study demonstrates that ANGPTL4, secreted by HCs, promotes the shift of KCs from an M1 to M2 phenotype, exerting a dose-dependent effect on KC polarization and cytokine production. This finding highlights a critical regulatory mechanism that differs from previous studies, which primarily focused on various immune cells, with limited attention given to parenchymal cells and their crosstalk with immune cells. Our research emphasizes the significance of this intercellular interaction. Notably, ANGPTL4 significantly decreases TNF-α and IL-1β levels, while increasing IL-10 expression. These findings indicate that ANGPTL4 plays a pivotal role in regulating inflammatory responses within the liver microenvironment.
The NF-κB signaling pathway is widely recognized as a major regulator of inflammation and immune responses34,35. Its activation is closely associated with the M1 phenotype of macrophages, which release pro-inflammatory mediators that amplify inflammatory responses36,37. In liver transplantation, NF-κB activation drives the upregulation of pro-inflammatory genes, perpetuating an inflammatory environment within the graft that can result in tissue damage and graft dysfunction37. Our study demonstrated that ANGPTL4 effectively reduced NF-κB activation in KCs, as evidenced by reduced phosphorylation of key NF-κB pathway components, including p65, IκBα, and IKKβ. Additionally, ANGPTL4 markedly inhibits the nuclear translocation of p65, a crucial marker of NF-κB activation. These findings indicate that ANGPTL4 disrupts the canonical NF-κB signaling pathway, promoting the polarization of KCs toward the M2 phenotype, which is connected to its roles in anti-inflammation and tissue repair.
The inhibitory effect of ANGPTL4 on NF-κB signaling could be a pivotal mechanism through which it promoted a more tolerogenic immune environment in the liver. By suppressing NF-κB mediated pro-inflammatory responses, ANGPTL4 not only mitigated the inflammatory state but also enhanced the anti-inflammatory milieu via IL-10 upregulation, which further supported M2 polarization. This dual action of ANGPTL4 dampening pro-inflammatory signaling while promoting anti-inflammatory pathways underscore its potential as a therapeutic target for modulating immune responses in liver transplantation and possibly other inflammatory conditions.
The observed paracrine interaction between HCs and KCs further underscored the regulatory role of ANGPTL4 in the liver microenvironment. Our co-culture experiments revealed that HCs derived ANGPTL4 significantly suppresses the inflammatory response in KCs, promoting their polarization toward the M2 phenotype. This finding aligns with other studies showing the importance of hepatocyte-macrophage crosstalk in liver disease and regeneration38,39. The reduction in NF-κB pathway activation in KCs following co-culture with HCs highlighted the potential of targeting this axis to control inflammation in liver transplantation.
Moreover, the siRNA-mediated knockdown of ANGPTL4 expression in HCs exacerbated the inflammatory response and enhanced NF-κB pathway activation in KCs. This result underscored the protective role of ANGPTL4, where its deficiency leads to heightened inflammation, as similarly reported in studies of ANGPTL4-deficient models29,30,40. The exacerbation of inflammatory responsed upon ANGPTL4 knockdown suggests that therapeutic strategies aimed at enhancing ANGPTL4 expression or activity could be beneficial in managing AR. Overall, our study presents strong evidence that ANGPTL4 is crucial in regulating immune responses during liver transplantation by enhancing M2 polarization of KCs and suppressing the NF-κB signaling pathway.
There are some limitations to our study. Although we demonstrated the protective effects of ANGPTL4 in a rat liver transplantation model, Further research is required to clarify the precise molecular mechanisms behind its actions. Additionally, the translational potential of these findings to human liver transplantation warrants investigation, particularly in the context of developing ANGPTL4-based therapies for clinical use.
Conclusion
In conclusion, this study highlights ANGPTL4 as a vital regulator of KCs polarization and a protective factor against AR in liver transplantation. Through modulation of the NF-κB signaling pathway and promotion of the M2 phenotype in KCs, ANGPTL4 effectively reduces inflammation and tissue damage, playing a significant role in alleviating AR during liver transplantation. These findings suggest that ANGPTL4 could serve as a potential therapeutic target to enhance outcomes in liver transplantation. Future research should aim to further uncover the molecular mechanisms through which ANGPTL4 regulates immune responses in the liver and investigate its potential for clinical use.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Adam, R. et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 57, 675–688. https://doi.org/10.1016/j.jhep.2012.04.015 (2012).
Loupy, A. et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transpl. 17, 28–41. https://doi.org/10.1111/ajt.14107 (2017).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555. https://doi.org/10.1016/s1471-4906(02)02302-5 (2002).
Xia, T. et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J. Inflamm. 20, 33. https://doi.org/10.1186/s12950-023-00360-z (2023).
Chen, Y. et al. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 14, 823–836. https://doi.org/10.1002/lt.21450 (2008).
Dixon, L. J., Barnes, M., Tang, H., Pritchard, M. T. & Nagy, L. E. Kupffer cells in the liver. Compr. Physiol. 3, 785–797. https://doi.org/10.1002/cphy.c120026 (2013).
Li, J. et al. Knockdown of microRNA-155 in Kupffer cells results in immunosuppressive effects and prolongs survival of mouse liver allografts. Transplantation 97, 626–635. https://doi.org/10.1097/tp.0000000000000061 (2014).
Xu, X. S. et al. SCARF1 promotes M2 polarization of Kupffer cells via calcium-dependent PI3K-AKT-STAT3 signalling to improve liver transplantation. Cell. Prolif. 54, e13022. https://doi.org/10.1111/cpr.13022 (2021).
Aryal, B., Price, N. L., Suarez, Y. & Fernández-Hernando, C. ANGPTL4 in metabolic and cardiovascular disease. Trends Mol. Med. 25, 723–734. https://doi.org/10.1016/j.molmed.2019.05.010 (2019).
Dijk, W. & Kersten, S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 27, 249–256. https://doi.org/10.1097/mol.0000000000000290 (2016).
Hato, T., Tabata, M. & Oike, Y. The role of Angiopoietin-Like proteins in Angiogenesis and Metabolism. Trends Cardiovasc. Med. 18, 6–14. https://doi.org/10.1016/j.tcm.2007.10.003 (2008).
Chugh, S. S., Macé, C., Clement, L. C., Nogal Avila, D., Marshall, C. B. & M. & Angiopoietin-like 4 based therapeutics for proteinuria and kidney disease. Front. Pharmacol. 5, 23. https://doi.org/10.3389/fphar.2014.00023 (2014).
Köster, A. et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology 146, 4943–4950. https://doi.org/10.1210/en.2005-0476 (2005).
Chen, M., Lin, W., Ye, R., Yi, J. & Zhao, Z. PPARβ/δ agonist alleviates diabetic osteoporosis via regulating M1/M2 macrophage polarization. Front. Cell. Dev. Biol. 9, 753194. https://doi.org/10.3389/fcell.2021.753194 (2021).
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77. https://doi.org/10.1016/j.cell.2008.01.046 (2008).
Cho, D. I. et al. Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight 4. https://doi.org/10.1172/jci.insight.125437 (2019).
Chong, H. C. et al. Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol. Ther. 22, 1593–1604 (2014).
Kamada, N. & Calne, R. Y. A surgical experience with five hundred thirty liver transplants in the rat. Surgery 93, 64–69 (1983).
Shen, L., Hillebrand, A., Wang, D. Q. H. & Liu, M. Isolation and primary culture of rat hepatic cells. JoVE e3917. https://doi.org/10.3791/3917 (2012).
Li, P., Li, J., Li, M., Gong, J. & He, K. An efficient method to isolate and culture mouse kupffer cells. Immunol. Lett. 158, 52–56. https://doi.org/10.1016/j.imlet.2013.12.002 (2014).
Racusen, L. C., Halloran, P. F. & Solez, K. Banff 2003 meeting report: New diagnostic insights and standards. Am. J. Transpl. 4, 1562–1566. https://doi.org/10.1111/j.1600-6143.2004.00585.x (2004).
Levitsky, J. et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin. Gastroenterol. Hepatol. 15, 584–593. https://doi.org/10.1016/j.cgh.2016.07.035 (2017). e582.
Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. et Biophys. Acta (BBA) - Mol. Cell. Biology Lipids. 1841, 919–933. https://doi.org/10.1016/j.bbalip.2014.03.013 (2014).
Dijk, W. et al. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 57, 1670–1683. https://doi.org/10.1194/jlr.M067363 (2016).
Aryal, B. et al. ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression. Nat. Commun. 7, 12313. https://doi.org/10.1038/ncomms12313 (2016).
Goh, Y. P. et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. U. S. A. 110, 9914–9919. https://doi.org/10.1073/pnas.1304046110 (2013).
Huang, R. L. et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118, 3990–4002. https://doi.org/10.1182/blood-2011-01-328716 (2011).
Li, K. et al. EZH2 inhibition promotes ANGPTL4/CREB1 to suppress the progression of ulcerative colitis. Life Sci. 250, 117553. https://doi.org/10.1016/j.lfs.2020.117553 (2020).
Lichtenstein, L. et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell. Metab. 12, 580–592. https://doi.org/10.1016/j.cmet.2010.11.002 (2010).
Phua, T. et al. Angiopoietin-like 4 mediates colonic inflammation by regulating chemokine transcript stability via tristetraprolin. Sci. Rep. 7, 44351. https://doi.org/10.1038/srep44351 (2017).
Zhao, Z. et al. IL-34 inhibits acute rejection of rat liver transplantation by inducing Kupffer Cell M2 polarization. Transplantation 102, e265–e274. https://doi.org/10.1097/tp.0000000000002194 (2018).
Pan, G. et al. Soluble fibrinogen-like protein 2 ameliorates acute rejection of liver transplantation in rat via inducing Kupffer cells M2 polarization. Cancer Med. 7, 3168–3177. https://doi.org/10.1002/cam4.1528 (2018).
Zhou, S. et al. High expression of angiopoietin-like protein 4 in advanced colorectal cancer and its association with regulatory T cells and M2 macrophages. Pathol. Oncol. Res. 26, 1269–1278. https://doi.org/10.1007/s12253-019-00695-0 (2020).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2, 17023. https://doi.org/10.1038/sigtrans.2017.23 (2017).
Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect. Biol. 1, a001651. https://doi.org/10.1101/cshperspect.a001651 (2009).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 122, 787–795. https://doi.org/10.1172/jci59643 (2012).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. https://doi.org/10.1038/nri3073 (2011).
Chen, Y. & Tang, L. The crosstalk between parenchymal cells and macrophages: A keeper of tissue homeostasis. Front. Immunol. 13, 1050188. https://doi.org/10.3389/fimmu.2022.1050188 (2022).
Schwabe, R. F., Tabas, I. & Pajvani, U. B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 158, 1913–1928. https://doi.org/10.1053/j.gastro.2019.11.311 (2020).
Guo, L. et al. Role of Angptl4 in vascular permeability and inflammation. Inflamm. Res. 63, 13–22. https://doi.org/10.1007/s00011-013-0678-0 (2014).
Acknowledgements
We express our gratitude to the faculty members at the Experimental Animal Centre of Chongqing Medical University (Chongqing, China) for their assistance.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82400772), Chongqing Natural Science Foundation (CSTB2024NSCQ-MSX0808) and Chongqing Science and Health Joint Project (2024GGXM005).
Author information
Authors and Affiliations
Contributions
W.H. and L.J.: study design, data collection, formal analysis, Writing – original draf. Y.J., S.L. and W.L.: data collection. D.P. and K.Z.: study design, formal analysis. Z.H. and Z.W.: critical revision, funding supply. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (IACUC-CQMU-2024-0609).
Informed consent
No human subjects were used in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, W., Jiang, L., Jiang, Y. et al. ANGPTL4 induces Kupffer cell M2 polarization to mitigate acute rejection in liver transplantation. Sci Rep 15, 986 (2025). https://doi.org/10.1038/s41598-024-81832-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81832-x