Abstract
Insulin receptor substrate (IRS)-1 and IRS-2 are major molecules that transduce signals from insulin and insulin-like growth factor-I receptors. The physiological functions of these proteins have been intensively investigated in mice, while little is known in other animals. Our previous study showed that the disruption of IRS-2 impairs body growth but not glucose tolerance or insulin sensitivity in rats, which led us to hypothesize that IRS-1 plays more pivotal roles in insulin functions than IRS-2. Here, we created IRS-1 knockout (KO) rats to elucidate the physiological roles of IRS-1 in rats. The body weight of IRS-1 KO rats at birth was lower than that of wild-type (WT) littermates, and postnatal growth of IRS-1 KO rats was severely impaired. Compared with WT rats, IRS-1 KO rats displayed insulin resistance but maintained euglycemia because of compensatory hyperinsulinemia. In addition, despite the increased activity of insulin-stimulated IRS-2-associated phosphatidylinositol-3 kinase (PI3K), insulin-induced phosphorylation of the kinases downstream of PI3K was suppressed in the liver and skeletal muscle of IRS-1 KO rats. Taken together, these results indicate that in rats, IRS-1 is essential for normal growth and the glucose-lowering effects of insulin. IRS-1 appears to be more important than IRS-2 for insulin functions in rats.
Similar content being viewed by others
Introduction
Insulin receptor substrate (IRS) proteins mediate a variety of physiological functions of insulin and insulin-like growth factor (IGF)-I, including somatic growth and metabolism1. They have no intrinsic activity but serve as cytoplasmic adaptor proteins to relay signals from insulin and IGF-I receptors to the intracellular signaling molecules. The activation of insulin and IGF-I receptor tyrosine kinase results in phosphorylation of numerous tyrosine residues of IRS proteins. Subsequently, tyrosine-phosphorylated IRS proteins bind to downstream signaling proteins, which mainly leads to activation of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase pathways1,2.
Thus far, at least four IRS isoforms have been identified1,2. Among them, IRS-1 and IRS-2 are widely expressed and recognized as the major proteins for mediating insulin and IGF-I actions in mammalian tissues1. IRS-1 and IRS-2 have similar structures and tissue distributions, but their distinct physiological roles have been demonstrated solely by using knockout (KO) mouse models3,4,5,6. Systemic Irs-1 null mice showed embryonic and postnatal growth retardation and insulin resistance but did not develop type 2 diabetes because of β-cell hyperplasia and sustained insulin secretion3,4. In contrast, systemic Irs-2 null mice exhibited normal growth, insulin resistance, abnormal glucose tolerance, and type 2 diabetes due to the loss of β-cell function and the absence of compensatory insulin secretion5,6. In addition, it has been shown that insulin resistance of Irs-1 null mice derived mainly from impairment of insulin activity in the skeletal muscle, while that of Irs-2 null mice derived from inhibition of insulin activity in the liver7,8,9. However, thus far these observations have been limited to mice because of technical difficulties associated with targeted modification of the genomes of other animals.
The development of the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease (Cas) 9 system has allowed investigators to establish gene-targeting models of multiple species10,11,12,13. Of experimental animal species, laboratory rats have historically been used as models for human diseases in the fields of physiology, pharmacology, and behavioral studies. Rats are larger than mice, which enables investigators to collect adequate blood and tissue samples. In addition, it has been known that rats exhibit more similar symptoms to human diseases in response to the changes of external environmental factors, such as stress and diet14,15. Using CRISPR/Cas 9 system, we previously produced IRS-2 KO rats and showed that the deletion of IRS-2 inhibited body growth but did not affect glucose tolerance or insulin sensitivity in rats16. These results led us to postulate that IRS-1 is the main mediator of the glucose-lowering effects of insulin in rats. Thus, in this study, we generated IRS-1 KO rats using the same technology and investigated the role of IRS-1 in the actions of insulin and IGF-I.
Results
Generation of IRS-1 knockout rats
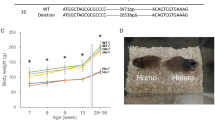
Each obtained F0 rat had 2 or 3 mutations in the targeted loci. To establish the IRS-1 knockout (KO) line, we chose the mutant having a 35-bp deletion located 349–383 bp downstream of the start codon. This deletion was expected to cause a frameshift and premature termination of translation (Fig. 1A).
Generation of IRS-1 knockout rats with CRISPR/Cas9. (A) Structure of the Irs-1 gene in wild-type (WT) and IRS-1 knockout (KO) rats. Representative sequences of WT and KO rats and the wave form data of KO rats are presented below. The gRNA sequence is shown in magenta, and the protospacer adjacent motif (PAM) is in green. (B, C) The IRS-1 protein level in the brains of WT and KO rats. (B) Representative immunoblots. Total brain homogenates from WT (n = 5) and KO (n = 4) rats were analyzed by immunoblotting with antibodies against IRS-1, IRS-2, and actin as an internal control. (C) A representative immunoblot. The immunoprecipitated IRS-1 from the brain homogenates from WT (n = 5) and KO (n = 4) rats were analyzed by immunoblotting with the anti-IRS-1 antibody. (D, E) The IRS-1 protein level in the skeletal muscles of WT and KO rats. (D) Representative immunoblots. Total muscle homogenates from WT (n = 6) and KO (n = 6) rats were analyzed by immunoblotting with antibodies against IRS-1, IRS-2, and β-tubulin as an internal control. (E) A representative immunoblot. The immunoprecipitated IRS-1 from the muscle homogenates from WT (n = 5) and KO (n = 5) rats were analyzed by immunoblotting with the anti-IRS-1 antibody.
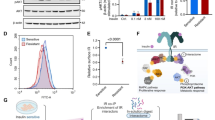
We then performed immunoblot analysis, and the results showed that no IRS-1 protein was detected in either total protein extracts or anti-IRS-1 immunoprecipitates from the brains and skeletal muscles of KO rats (Fig. 1B-E). The IRS-2 protein levels in the brain and skeletal muscle were similar between wild-type (WT) and KO rats (Fig. 1B and D).
Disruption of IRS-1 impaired growth and reduced skeletal muscle mass in the rats
The KO rats were born with the expected Mendelian inheritance by intercrossing heterozygotes. The body weights (BWs) of neonates at 1 − 2 days after birth were about 35% lower in KO rats than in WT and heterozygous (hetero) rats (WT: 5.8 ± 0.5 g; hetero: 5.5 ± 0.5 g; KO: 3.7 ± 0.3 g). Among both males and females, the lower BWs of KO rats persisted throughout the postnatal period compared with WT and hetero rats, and the hetero rats also had lower BWs compared with WT rats from 4 weeks of age (Fig. 2A and D).
Body weight (BW), body length, and organ weights in WT and IRS-1 KO rats. (A) Changes of BW in 3- to 11-week-old male WT (n = 9), heterozygous (hetero; n = 16), and KO (n = 8) rats. (B) Body length in male WT (n = 8) and KO (n = 12) rats at 15 weeks of age. (C) Weights of the liver, gastrocnemius muscle, kidney, brain, and epidydimal white adipose tissue (epiWAT) per BW in male WT (n = 8) and KO (n = 12) rats at 15 weeks of age. (D) Changes of BW in 3- to 11-week-old female WT (n = 9), hetero (n = 21), and KO (n = 8) rats. (E) Body length in female WT (n = 10) and KO (n = 11) rats at 15 weeks of age. (F) Weights of the liver, gastrocnemius muscle, kidney, and brain per BW in female WT (n = 10) and KO (n = 11) rats. Values are means ± SDs. *p < 0.05 vs. WT rats. †p < 0.05 vs. hetero rats.
At 15 weeks of age, male KO rats had 60% lower BWs and 25% shorter body lengths compared with male WT rats (Supplemental Table S1, Fig. 2B). The weights of the liver, gastrocnemius muscle, kidney, brain, and epidydimal white adipose tissue (epiWAT) were significantly lower in KO males than in WT males (Supplemental Table S1). The ratios of the weights of liver, kidney, brain, and epiWAT to the BW (%) were significantly higher, while the ratio of the gastrocnemius muscle weight to the BW (%) was significantly lower in KO males than in WT males (Fig. 2C). The serum growth hormone (GH) levels tended to be higher, whereas the serum IGF-I levels were significantly lower in male KO rats compared with male WT rats (Fig. 3A and B).
Serum growth hormone (GH) and insulin-like growth factor (IGF)-I in WT and IRS-1 KO rats. (A, B) Serum GH (A) and IGF-I (B) levels in male WT (n = 8) and KO (n = 13) rats at 15 weeks of age. (C, D) Serum GH (C) and IGF-I (D) levels in female WT (n = 12) and KO (n = 13) rats at 15 weeks of age. Values are means with all data points. *p < 0.05 vs. WT rats.
Like their male counterparts, female KO rats had 50% lower BWs and 20% shorter body lengths compared with female WT rats (Supplemental Table S1, Fig. 2E). The weights of the liver, gastrocnemius muscle, kidney, and brain were significantly lower in female KO rats than in female WT rats (Supplemental Table S1). The ratios of the weights of these tissues to the BW (%) were significantly higher—except in the case of the gastrocnemius muscle weight, for which the ratio was significantly lower—in KO females than in WT females (Fig. 2F). Serum GH levels were significantly higher, while serum IGF-I levels were significantly lower in female KO rats compared with female WT rats (Fig. 3C and D). Collectively, these data showed that regardless of sex, IRS-1 KO rats had smaller body size, higher levels of circulating GH and lower levels of circulating IGF-I compared with WT rats.
Since GH stimulates lipolysis in the adipose tissue17, serum free fatty acids (FFA) and triglycerides (TG) were measured in 15-week-old WT and KO rats. Among both sexes, the serum FFA level was significantly higher in KO rats than WT rats, while their serum TG levels were comparable (Supplemental Table S2).
Disruption of IRS-1 caused glucose intolerance and insulin resistance in rats
Thus far, two research groups independently developed IRS-1 KO mice, and both lines of IRS-1 KO mice showed insulin resistance and hyperinsulinemia3,4. However, the effect of IRS-1 knock-out on glucose tolerance was different between both lines of mice. One of the lines displayed abnormal glucose tolerance at 6 to 16 weeks of age, while the other had normal glucose tolerance3,4,18. Hence, glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed on male and female IRS-1 KO rats at 12 to 13 weeks of age.
During GTT, the blood glucose levels were not different between WT and KO males at any time point after glucose injection (Fig. 4A) although the incremental area under the curve (AUC) for the changes of glucose levels during GTT was significantly higher in KO males than in WT males (Fig. 4B). These results indicate that the deletion of IRS-1 did not greatly affect glucose tolerance in male rats. In addition, the KO males had significantly higher plasma insulin levels after glucose injection compared with WT males (Fig. 4C). The incremental AUC for the changes of plasma insulin levels during GTT was significantly higher in KO males than in WT males (Fig. 4D).
Glucose tolerance test (GTT) and insulin tolerance test (ITT) in male WT and IRS-1 KO rats. (A–D) The GTT results for the male WT (n = 8) and KO (n = 9) rats at 12 weeks of age. (A) Changes in the blood glucose levels during the GTT. B The incremental area under the curve (AUC) for the blood glucose level during the GTT. (C) Changes in plasma insulin levels during the GTT. (D) The incremental AUC for the plasma insulin level during the GTT. (E, F) The ITT results of the male WT (n = 7) and KO (n = 8) rats at 13 weeks of age. (E) Changes in the blood glucose levels during the ITT. (F) The area above the curve (AAC) for the blood glucose level during the ITT. Values are means ± SDs. *p < 0.05 vs. WT rats.
In ITT, before insulin injection, the blood glucose levels were slightly lower in male KO rats than in male WT rats. At 60 min after insulin injection, however, KO males had significantly higher blood glucose levels as compared with WT males (Fig. 4E). The area above the curve (AAC) for the changes of blood glucose levels during ITT was significantly lower in KO males than in WT males (Fig. 4F), indicating that IRS-1 KO males were insulin resistant.
Like males, the absence of IRS-1 caused hyperinsulinemia and insulin resistance but had small impact on glucose tolerance in females (Supplemental Fig. S1).
At 15 weeks of age, after 4–5 h fasting, among both sexes of rats, the plasma insulin level was significantly higher in KO rats compared with WT rats, but the blood glucose level was comparable between WT and KO rats (Supplemental Table S2). These results imply that high levels of circulating insulin enabled IRS-1 KO rats to maintain normal blood glucose levels.
Disruption of IRS-1 enhanced insulin-induced IRS-2-associated PI3K activity in the liver and skeletal muscle
The deletion of IRS-1 was similarly associated with glucose tolerance and insulin resistance among both sexes of rats, suggesting that unlike IRS-2 KO rats16, there was no sexual dimorphism for glucose metabolism or insulin action in IRS-1 KO rats. Thereafter, we examined the effects of IRS-1 KO on insulin signaling using males only.
To confirm no activation of IRS-1-mediated signaling in response to insulin in the liver and muscle of IRS-1 KO rats, we firstly performed the immunoblot analysis of the anti-IRS-1 immunoprecipitates from the liver and skeletal muscle homogenates. The results revealed that there was no increase in the tyrosine phosphorylation of IRS-1 or in the IRS-1-associated PI3K activity in response to insulin stimulation in the liver and skeletal muscle of IRS-1 KO rats (Fig. 5).
The insulin-stimulated tyrosine phosphorylation of IRS-1 and activation of IRS-1-associated PI3K in the liver and skeletal muscle of WT and IRS-1 KO rats. Eight-week-old male WT and KO rats were fasted overnight, and insulin or vehicle was subsequently injected into the inferior vena cava. The liver was dissected at 1 min and skeletal muscle was dissected at 2 min after the insulin injection. (A) Representative immunoblots of the insulin-stimulated tyrosine phosphorylation of IRS-1 in the liver. The immunoprecipitated IRS-1 from the liver homogenates was analyzed by immunoblotting with anti-phosphotyrosine (pTyr) and anti-IRS-1 antibodies. (B, C) The IRS-1-associated PI3K activity in the liver. (B) A representative autoradiograph. (C) The quantification of radioactivity incorporated into phosphatidylinositol32P-PIP). (D) Representative immunoblots of the insulin-stimulated tyrosine phosphorylation of IRS-1 in the skeletal muscle. The immunoprecipitated IRS-1 from the skeletal muscle homogenates was analyzed by immunoblotting with anti-pTyr and anti-IRS-1 antibodies. (E, F) The IRS-1-associated PI3K activity in the skeletal muscle. (E) A representative autoradiograph. (F) The quantification of32P-PIP. Values are means ± SDs. WT with − insulin group, n = 5. WT with + insulin group, n = 5. KO with − insulin group, n = 6. KO with + insulin group, n = 7. The results of two-way ANOVA are shown beside the graph (*p < 0.05; NS, not significant). The results of Tukey-Kramer post-hoc test are shown in the graph when there was a significant interaction of genotype and insulin stimulation (†p < 0.05).
In the liver, insulin significantly increased tyrosine phosphorylation of β-subunits of the insulin receptor (IRβ) in both WT and KO rats with no significant difference between genotypes (Fig. 6A and B). Insulin also significantly increased tyrosine phosphorylation of IRS-2 in both genotypes. In KO rats, the levels of insulin-induced IRS-2 tyrosine phosphorylation appeared to be increased because of the higher level of IRS-2 in the anti-IRS-2 immunoprecipitates compared with WT rats (Fig. 6C). Consequently, the levels of insulin-induced tyrosine phosphorylation of IRS-2 normalized to the amount of IRS-2 protein were similar in both WT and KO rats (Fig. 6D). Insulin increased the IRS-2-associated PI3K activity in WT and KO rats, and the loss of IRS-1 affected IRS-2-associated PI3K activity (Fig. 6E and F). Although there was no significant interaction between genotype and insulin stimulation, the insulin-induced activation appeared to be enhanced in KO rats relative to WT rats (Fig. 6E and F).
The insulin-simulated tyrosine phosphorylation of IRβ and IRS-2 and activation of IRS-2-associated PI3K in the liver and skeletal muscle of WT and IRS-1 KO rats. Eight-week-old male WT and KO rats were fasted overnight, and insulin or vehicle was subsequently injected into the inferior vena cava. The liver was dissected at 1 min and skeletal muscle was dissected at 2 min after the insulin injection. (A, B) Insulin-stimulated tyrosine phosphorylation of IRβ in the liver. The immunoprecipitated IRβ from the liver homogenates was analyzed by immunoblotting with anti-phosphotyrosine (pTyr) and anti-IRβ antibodies. (A) Representative immunoblots. (B) The ratio of pTyr to the amount of immunoprecipitated IRβ. The immunoreactivity of pTyr was quantified and divided by the immunoreactivity of IRβ. (C, D) Insulin-stimulated tyrosine phosphorylation of IRS-2 in the liver. The immunoprecipitated IRS-2 from the liver homogenates was analyzed by immunoblotting with anti-pTyr and anti-IRS-2 antibodies. (C) Representative immunoblots. (D) The ratio of pTyr to the amount of immunoprecipitated IRS-2. The immunoreactivity of pTyr was quantified and divided by the immunoreactivity of IRS-2. (E, F) Insulin-stimulated IRS-2-associated PI3K activity in the liver. (E) A representative autoradiograph. (F) The quantification of radioactivity incorporated into phosphatidylinositol32P-PIP). (G, H) Insulin-stimulated tyrosine phosphorylation of IRβ in skeletal muscle. (G) Representative immunoblots. (H) The ratio of pTyr to the amount of immunoprecipitated IRβ. (I, J) Insulin-stimulated tyrosine phosphorylation of IRS-2 in skeletal muscle. (I) Representative immunoblots. (J) The ratio of pTyr to the amount of immunoprecipitated IRS-2. (K, L) Insulin-stimulated IRS-2-associated PI3K activity in skeletal muscle. (K) A representative autoradiograph. (L) The quantification of32P-PIP. Values are means ± SDs. WT with − insulin group, n = 5. WT with + insulin group, n = 5. KO with − insulin group, n = 6. KO with + insulin group, n = 7. The results of two-way ANOVA are shown beside the graph (*p < 0.05, NS; not significant).
In skeletal muscle, insulin significantly increased tyrosine phosphorylation of IRβ, and the levels of its insulin-induced tyrosine phosphorylation were comparable between WT and KO rats (Fig. 6G and H). Insulin significantly increased and the absence of IRS-1 affected tyrosine phosphorylation of IRS-2 and its associated PI3K activity, and these levels after insulin stimulation tended to be higher in KO rats relative to WT rats (Fig. 6I-L). Unlike in the liver, the IRS-2 level in the anti-IRS-2 immunoprecipitates from the homogenate of skeletal muscle was comparable between WT and KO rats (Fig. 6I).
The loss of IRS-1 increased the IRS-2 level in anti-IRS-2 immunoprecipitates from the liver and did not change its level in anti-IRS-2 immunoprecipitates from the skeletal muscle, suggesting that the absence of IRS-1 increased the amount of IRS-2 in the liver but not in skeletal muscle. To verify these, we then analyzed IRS-2 protein levels in total homogenates from the liver and skeletal muscle by direct immunoblotting. The results of immunoblotting showed that the loss of IRS-1 increased IRS-2 protein levels in the liver but not in the skeletal muscle (Fig. 7). These results suggest that insulin-induced IRS-2-associated PI3K activity was enhanced by increasing the IRS-2 protein level in the liver and by increasing the efficiency of IRS-2 tyrosine phosphorylation in the skeletal muscle.
The IRS-2 protein level in the liver and skeletal muscle of WT and IRS-1 KO rats. Total liver and skeletal muscle homogenates were prepared from the vehicle-injected male WT and KO rats that were the same as used in Fig. 8. (A–D) The IRS-2 protein level in the liver (A, B) and skeletal muscle (C, D) of 8-week-old WT (n = 7) and KO (n = 7) rats with − insulin. Total liver and muscle homogenates were analyzed by immunoblotting with antibodies against IRS-2 and β-tubulin. β-tubulin was used as an internal control. (A, C) Representative immunoblots. (B, D) The immunoreactivity of IRS-2 was quantified and divided by the immunoreactivity of β-tubulin. Values are means ± SDs. *p < 0.05 vs. WT rats. Similar results were obtained using the liver and skeletal muscle homogenates from the vehicle-injected male WT and KO rats that were the same as used in Figs. 5 and 6.
The disruption of IRS-2 attenuated insulin-induced phosphorylation of Akt and S6K in the liver and skeletal muscle
Subsequently, the effect of IRS-1 KO on insulin signaling downstream of PI3K in the liver and skeletal muscle was examined.
In the liver, insulin significantly increased phosphorylation of Akt, GSK3β, and S6K in both WT and KO rats (Fig. 8A-E). The absence of IRS-1 affected Akt and S6K phosphorylation but not GSK3β phosphorylation (Fig. 8B-E). In addition, insulin-induced phosphorylation of Akt was significantly suppressed in KO rats (Fig. 8B and C), and the levels of insulin-induced phosphorylation of S6K tended to be lower in KO rats than in WT rats (Fig. 8E).
The insulin-stimulated phosphorylation of Akt, GSK3β, and S6K in the liver and skeletal muscle of WT and IRS-1 KO rats. Eight-week-old male WT and KO rats were fasted overnight, and insulin or vehicle was subsequently injected into the inferior vena cava. The liver and gastrocnemius muscle were excised at 15 min after the insulin injection. (A–E) Insulin-stimulated phosphorylation of Akt, GSK3β, and S6K in the liver. (A) Representative immunoblots. (B–E) The immunoreactivities of Thr308 phosphorylation of Akt (B), Ser473 phosphorylation of Akt (C), Ser9 phosphorylation of GSK3β (D), and Thr389 phosphorylation of S6K (E) were quantified and divided by the immunoreactivity of β-tubulin. (F–J) Insulin-stimulated phosphorylations of Akt, GSK3β, and S6K in the gastrocnemius muscle. (F) Representative immunoblots. (G–J) The immunoreactivities of Thr308 phosphorylation of Akt (G), Ser473 phosphorylation of Akt (H), Ser9 phosphorylation of GSK3β (I), and Thr389 phosphorylation of S6K (J) were quantified and divided by the immunoreactivity of β-tubulin. Values are means ± SDs (n = 7). The two-way ANOVA results are shown below the graph (*p < 0.05, NS; not significant). The results of Tukey-Kramer post-hoc test are shown in the graph when there was a significant interaction of genotype and insulin stimulation (†p < 0.05).
In skeletal muscle, insulin significantly increased phosphorylation of Akt, GSK3β, and S6K, and the loss of IRS-1 affected these phosphorylations (Fig. 8F-J). Additionally, the insulin-induced phosphorylations of Akt and S6K were significantly attenuated in KO rats (Fig. 8G, H, and J).
Besides the liver and skeletal muscle, insulin signaling in epiWAT was analyzed. In epiWAT, insulin and the absence of IRS-1 affected tyrosine phosphorylation of IRβ, and the levels of its insulin-induced tyrosine phosphorylation were significantly suppressed in KO rats (Supplemental Fig. S2A and B). Insulin significantly increased and the absence of IRS-1 affected tyrosine phosphorylation of IRS-2 and its association with p85 regulatory subunits of PI3K (p85 of PI3K), an activation step of IRS-2-associated PI3K. Despite no significant interaction between genotype and insulin stimulation, these levels with or without insulin stimulation tended to be higher in KO rats compared to WT rats (Supplemental Fig. S2C-E). The IRS-2 protein levels in total homogenates from epiWAT were comparable between WT and KO rats (Supplemental Fig. S2F and G). Thus, these data suggest that the efficiency of IRS-2 tyrosine phosphorylation was markedly increased in epiWAT of KO rats compared to WT rats. In addition, insulin signaling downstream of PI3K in epiWAT was examined, and the results showed that insulin significantly increased phosphorylation of Akt, S6K, and GSK3β. Unlike in the liver and skeletal muscle, the absence of IRS-1 did not affect these insulin-induced phosphorylations (Supplemental Fig. S3).
Discussion
In the present study, we generated IRS-1 KO rats and examined their phenotypes. The deletion of IRS-1 led to growth inhibition and insulin resistance but did not cause hyperglycemia up to 15 weeks of age in both males and females. In addition, in the liver and muscle of IRS-1 KO rats, the IRS-2-associated PI3K activity in response to insulin was increased, whereas insulin-induced phosphorylation of signaling molecules downstream of PI3K was suppressed. Down-regulation of liver and muscle insulin signaling was implicated in the development of insulin resistance in IRS-1 KO rats. These results indicate that IRS-1 is an important molecule for normal growth and the blood glucose-lowering effects of insulin in rats.
The IRS-1 KO rats exhibited lower BWs and shorter body lengths compared with WT rats from the postnatal period until 15 weeks of age. Since GH and IGF-I are critical regulators of somatic growth19, these hormone levels were measured in WT and IRS-1 KO rats. The serum GH level tended to be higher in male IRS-1 KO rats and was significantly higher in female IRS-1 KO rats, while the serum IGF-I level was significantly lower in both male and female IRS-1 KO rats than in WT rats. Besides the impairment of insulin and IGF-I signaling by the lack of IRS-1, the decrease in the serum IGF-I level could be related to the inhibition of growth in IRS-1 KO rats.
The IRS-1 KO rats had an elevated serum GH level and a reduced serum IGF-I level, which could be explained by GH resistance20. In general, GH stimulates IGF-I synthesis mainly in the liver and increases the circulating IGF-I level. The circulating IGF-I then represses GH production by a negative feedback effect19. Accordingly, the present data suggest that the lack of IRS-1 causes GH resistance, leading to impairment of IGF-I production in the liver and reduction of the level of circulating IGF-I. The reduced serum IGF-I could not adequately inhibit GH secretion, resulting in higher serum GH levels in IRS-1 KO rats. GH has been shown to induce tyrosine phosphorylation of IRS-1 and association of the p85 regulatory subunit of PI3K with IRS-1 in cells and rodent tissues21,22. It is likely that IRS-1 plays a role in the GH-mediated IGF-I expression, in addition to insulin action, in rat liver. Since IRS-1 KO mice had a normal circulating IGF-I level23, the role of IRS-1 in hepatic GH signaling could be different between rats and mice. Further studies are necessary to elucidate the role of IRS-1 in GH activity including the induction of IGF-1 production in the liver.
The IRS-1 KO rats, as well as the IRS-2 KO rats, exhibited lower BWs and lower weights of liver, gastrocnemius muscle, kidney, and brain compared with WT rats. However, the absence of each molecule affected the relative size of these organs (the ratio of organ weight to body weight) differently. In IRS-2 KO rats, the weights of kidney and brain normalized to the BW were much lower than those in WT rats, while there was small alteration in the normalized liver weight and no change in the normalized gastrocnemius muscle weight16. In contrast, in IRS-1 KO rats, the weights of kidney, brain, and liver normalized to the BW were higher than in WT rats, implying that the sizes of these organs were preserved. Furthermore, significant reduction in the normalized gastrocnemius muscle weight was observed in IRS-1 KO rats. The previous study in mice also showed reduced gastrocnemius muscle weight for the IRS-1 KO genotype, and this reduction was proportionately greater than the reduction in BW23. These findings support the idea that IRS-1 is required for the growth of skeletal muscle in animals. Taken together with the data of the IRS-2 KO rats16, the present results indicate that IRS-1 and IRS-2 regulate the growth of target organs differently. The different contributions for organ growth between IRS-1 and IRS-2 in rats might be attributed to their different tissue expression patterns.
In our previous study, the lack of IRS-2 did not impair insulin function in rats16. This result led us to speculate that the disruption of IRS-1 would cause type 2 diabetes in rats. In this study, the IRS-1 KO rats displayed insulin resistance but did not develop type 2 diabetes up to 15 weeks of age. Furthermore, the results showing the changes in the plasma insulin levels during GTT suggest that insulin secretion was augmented in the IRS-1 KO rats. Since IRS-1 KO mice exhibited hyperinsulinemia with hyperplastic islets5,24, we also examined the islet morphology in IRS-1 KO rats. Despite small impacts of IRS-1 deletion on the size and total number of islets in females, these were larger in KO males than in WT males at 15 weeks of ages (Supplemental Fig. S4). The increased number and mass of islets could be related to the augmented insulin secretion and hyperinsulinemia in male IRS-1 KO rats but not in female IRS-1 KO rats. Collectively, these results suggest the likelihood that increased insulin secretion largely compensated for the insulin resistance in IRS-1 KO rats. Although the insulin signaling downstream of PI3K was attenuated in the liver and skeletal muscle, it was comparable between the white adipose tissue (WAT) of WT and IRS-1 KO rats. The normal insulin sensitivity in WAT may also have contributed to the maintenance of normoglycemia in the IRS-1 KO rats.
Previous studies have shown that in mice, knock-out of IRS-1 resulted in smaller bodies and insulin resistance3,4. Although the smaller phenotype was also observed in IRS-1 KO rats, the organs involved in insulin resistance in rats are different from those in mice. The insulin resistance of IRS-1 KO mice was considered to be driven by impaired insulin action in the muscle7,8,9. The insulin-induced tyrosine phosphorylation of IRS-2 and its associated PI3K activity in response to insulin were enhanced in both liver and muscle of IRS-1 KO mice, and this enhancement of IRS-2-mediated signaling sufficiently compensated for the absence of IRS-1-mediated signaling in the liver but not in the muscle9,25. Similarly, the IRS-1 KO rats showed an enhancement of insulin-induced activation of IRS-2-associated PI3K, which was associated with an increase in IRS-2 protein levels in the liver and the potentiation of tyrosine phosphorylation in the muscle. Nevertheless, the insulin-induced phosphorylation of Akt, a downstream signaling molecule of PI3K, was suppressed in the liver and muscle of the IRS-1 KO rats, suggesting that enhancement of IRS-2-mediated signaling could not prevent attenuation of the downstream signaling of PI3K in either the liver or muscle. Therefore, the insulin resistance of IRS-1 KO rats seems to be due to resistance in both tissues. In WAT, insulin-induced activation of IRS-2-associated PI3K appeared to be enhanced in KO rats, and insulin-induced phosphorylation of downstream signaling molecules of PI3K was similar between WT and KO rats. In contrast to the cases in the liver and muscle, the enhancement of IRS-2-mediated signaling could be sufficient to compensate for the lack of IRS-1-mediated signaling in WAT.
Besides the defect of IRS-1-mediated signaling, higher serum GH levels may have been involved in the development of insulin resistance of IRS-1 KO rats. Previous studies have shown that the chronic elevation of circulating GH levels induces insulin resistance26,27,28,29. Further, GH has anti-insulin effects including the facilitation of lipolysis in the adipose tissues and a resultant increase in circulating levels of FFA, which promote lipotoxicity and insulin resistance17,30. The IRS-1 KO rats had higher GH and higher FFA levels in their circulation compared with WT rats. These data suggest that an increase in serum FFA level might be attributed to the high level of serum GH, leading to an increase in lipotoxicity and the development of insulin resistance in IRS-1 KO rats.
In conclusion, our findings demonstrate that knock-out of IRS-1 in rats impaired body growthand insulin sensitivity. These data indicate that IRS-1 is required for normal growth and the blood glucose-lowering effects of insulin in rats. While neither deletion of IRS-1 nor deletion of IRS-2 led to type 2 diabetes, IRS-1 signaling was shown to be more important for insulin action and glucose homeostasis in rats. The phenotypes of IRS-1 knock-out rats and mice were similar; however, this rat model, along with IRS-2 knock-out rats, will be useful in future studies to uncover novel functions of IRS proteins because the knowledge of relevance to human diseases has been accumulated in rats14,15,31,32.
Methods
Animals
For all animal experiments, rats were kept in a room maintained at 22 ± 2 °C with relative humidity at 40–60% and a 12-h light (8:00–20:00) and 12-h darkness (20:00–8:00) cycle. They were allowed free access to water and a standard chow (MF; Oriental Yeast, Tokyo). All experimental protocols were deemed to be in accordance with the guidelines of the Animal Usage Committee of Nippon Medical School and were approved by the Animal Usage Committee of Nippon Medical School (approval numbers: 29-006 and 2019-004). This study complied with the ARRIVE guidelines.
The generation of IRS-1 KO rats with CRISPR/Cas9
Wistar-Imamichi rats were purchased from Institute for Animal Production (Ibaraki, Japan). The design of single guide (sg) RNA for targeting the rat Irs-1 gene and the preparation of sgRNA and Cas9 mRNA were conducted as previously described16,33,34. The sgRNA sequence and protospacer adjacent motif is 5’-GCAGCTGCATAATCGGGCAAAGG-3’, which is the site from 333-bp to 355-bp downstream from the start codon. The injection of Cas9 mRNA and sgRNA was performed as previously described16,34. Briefly, 25 IU of equine chronic gonadotropin (eCG) was intraperitoneally injected into 4- to 5-week-old sexually immature female Wistar-Imamichi rats (Institute for Animal Production, Ibaraki, Japan). At 48 h after the eCG injection, these rats were intraperitoneally injected with 25 IU of human chronic gonadotropin and were mated with male Wistar-Imamichi rats (Institute for Animal Production) overnight. The next day, superovulated zygotes were collected from the female rats’ oviducts, treated with hyaluronidase (Sigma-Aldrich, St. Louis, MO), washed, and cultured in M2 medium (Sigma-Aldrich) until the micro-injection, when approx. 4 pL of a mixture of 50 µg/mL sgRNA and 50 µg/mL Cas9 mRNA were injected into the zygotic cytosol with a micro-injector (Narishige, Tokyo). After injection, the zygotes were recovered in M16 medium (Sigma-Aldrich) for 1 h and were transferred to the oviductal ampullas of the pseudopregnant Wistar-Imamichi rats.
The obtained F0 rats were analyzed for mutations in the Irs-1 gene and 10 potential off-target sites. We confirmed that all F0 rats had mutations in the target loci with no mutation in the potential off-target sites. One female F0 rat that had a 35-bp deletion in exon 1 of the Irs-1 gene (Fig. 1A) was selected and crossed with a male Wistar-Imamichi rat to obtain F1 heterozygous males and females that were subsequently intercrossed to obtain homozygous littermates. Genotyping was performed by PCR with the genomic DNA extracted from the rats’ tail tips. The primer sequences used were 5’- CGATGGCTTCTCAGACGTGC − 3’ and 5’-CACCACGGAGTCATCCACTTGC-3’. To perform each experiment with adequate numbers of rats, over 10 mating pairs were simultaneously set up, and the pooled littermates were used. All rats used in this study were maintained on a Wistar-Imamichi background. The homozygous rats carrying a 35-bp deletion mutation in the Irs-1 gene are referred to as IRS-1 knockout (KO) rats, and the littermates with no mutation as WT rats.
At 15 weeks of age, after 4–5 h fasting, WT and KO rats were anesthetized with isoflurane (Fujifilm Wako Pure Chemical Corp., Osaka, Japan), blood samples were obtained from the carotid artery. The rats were sacrificed by exsanguination and decapitation under anesthesia, and various tissues were collected. The blood was allowed to clot overnight at 4 °C and then subjected to centrifugation at 2,700 g for 10 min at 4 °C. Serum samples were stored at − 80 °C until analysis. The excised tissues were frozen immediately in liquid nitrogen and stored at − 80 °C until use.
The measurement of serum parameters
The serum GH level was measured by a rat/mouse GH ELISA kit (EMD Millipore, Billerica, MA). The serum IGF-I level was measured by a mouse/rat IGF-I Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The serum triglyceride (TG) level was measured using a Triglyceride E-Test Wako kit (Fujifilm Wako Pure Chemical). The serum nonesterified fatty acid (NEFA) level was measured using a NEFA C-Test Wako kit (Fujifilm Wako Pure Chemical).
Glucose and insulin tolerance tests
A GTT and ITT were performed as previously described16. For the GTT, 12-week-old rats were fasted for 4–5 h and then intraperitoneally injected with glucose solution (1.2 g/kg body weight [BW]). Blood was obtained from the tail vein at 0, 15, 30, 60, and 120 min after the glucose injection. For the ITT, 13-week-old rats were fasted for 3–4 h and then intraperitoneally injected with bovine insulin (0.75 U/kg BW; Sigma-Aldrich). Blood was obtained from the tail vein at 0, 30, 60, 90, and 120 min after the insulin injection.
Blood glucose levels were measured with a glucometer (Ascensia Breeze; Bayer Medical, Leverkusen, Germany). Plasma insulin levels were measured by an ultra-sensitive mouse/rat insulin ELISA kit (Morinaga, Yokohama, Japan). We used the trapezoidal rule to calculate the incremental AUC for the changes in both blood glucose and plasma insulin levels during the GTT and AAC for the changes in blood glucose levels during the ITT.
In vivo insulin stimulation
The experiment of in vivo insulin stimulation was performed as described previously16. After overnight (15–18 h) fasting, 8-week-old male rats were anesthetized with isoflurane (Fujifilm Wako), and their abdominal cavities were then opened. PBS or bovine insulin (0.5 U/kg BW; Sigma-Aldrich) diluted in PBS was then injected into the inferior vena cava. To analyze the early steps of insulin signaling, the liver was dissected at 1 min after insulin injection, and the gastrocnemius muscle was dissected at 2 min after insulin injection. To analyze the downstream of insulin signaling, we prepared a separate set of rats, and the liver and skeletal muscle were dissected at 15 min after insulin injection. The rats were sacrificed by exsanguination under anesthesia. The dissected tissues were immediately frozen in liquid nitrogen and stored at − 80 °C until analysis.
The preparation of protein extracts from tissues
The frozen tissues were homogenized with a polytron homogenizer in ice-cold homogenizing buffer (50 mM HEPES-NaOH; pH 7.6, 10 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 2 mM phenylmethylsulfonyl fluoride, 100 KIU/mL aprotinin, 2 mM EDTA, and 2% Triton X-100). The homogenates were centrifuged at 100,000 g for 1 h at 4 °C, and the supernatants were collected as protein extracts.
Immunoprecipitation
The protein extracts were immunoprecipitated with 1 µg of anti-IRβ (C-19; Santa Cruz Biochemistry, Dallas, TX), IRS-1 (#06-248; EMD Millipore, Temecula, CA), or IRS-2 (H-205; Santa Cruz Biochemistry) antibodies bound to protein-G Sepharose 4FF beads (GE Healthcare, Piscataway, NJ) as previously described35,36. Each immunoprecipitated sample was subjected to SDS-PAGE, followed by immunoblotting.
Immunoblotting
Equal amounts of protein sample were subjected to SDS-PAGE, and immunoblotting was performed as previously described16,36. Bands of immunoreactive proteins were detected with an ECL kit (PerkinElmer Life Sciences, Boston, MA) and quantified using a cooled CCD camera system, the LAS-3000 mini (Fujifilm, Kanagawa, Japan). Anti-IRβ (C-19), anti-IRS-1 (C-20), and anti-IRS-2 (H-205) were purchased from Santa Cruz Biochemistry. Anti-phosphotyrosine 4G10 (#05-321), anti-IRS-2 (#MABS15), and anti-p85 of PI3K (#06-195) antibodies were purchased from EMD Millipore. Anti-actin was purchased from Sigma. Anti-phospho-Akt (Thr308: #4056, Ser473: #4060), anti-Akt (#9272), anti-phospho-GSK3β (Ser9: #9323), anti-GSK3β (#9315), anti-phospho-S6K (Thr389: #9205), anti-S6K (#2708), and anti-β-tubulin (#2128) antibodies were purchased from Cell Signaling Technology (Danvers, MA).
The band of IRS-2 in total tissue homogenates was detected by a polyclonal anti-IRS-2 (H-205) antibody, and the band of IRS-2 in the immunoprecipitates with a polyclonal anti-IRS-2 (H-205) was detected by a monoclonal anti-IRS-2 (#MABS15) antibody.
PI3K activity assay
The PI3K activity assay was conducted with immunoprecipitated samples using anti-IRS-1 or IRS-2 antibody as described previously37 with slight modifications. Briefly, the immunocomplex was incubated at 25 °C in 50 µL of reaction buffer (20 mM Tris-HCl; pH7.5, 100 mM NaCl, 0.5 mM EGTA) containing 20 µg phosphatidylinositol, 20 mM MgCl2, 1 mM dithiothreitol, 30 µM ATP, and 3 µCi [γ-32P] ATP (3,000 Ci/mmol; PerkinElmer). After the incubation for 15 min, the reaction was stopped by adding 100 µL of chloroform-methanol-HCl (10:20:1). Lipid products were then extracted, spotted onto a silica gel plate (Merck KGaA, Darmstadt, Germany), and developed with chloroform-methanol-25% ammonia solution-water (43:38:6:6). The32P radioactivity incorporated into phosphatidylinositol was measured as the PI3K activity using a BAS-2500 image analyzer (Fujifilm).
Histology
The pancreases were excised, fixed in 4% paraformaldehyde in PBS, and embedded in paraffine. They were processed for 5-µm thick sections and then stained with hematoxylin and eosin (Genostaff Co., Ltd., Tokyo, Japan). The islet area was quantified from two different sections per each rat using by Image J software (U.S. National Institutes of Health, MD).
Statistical analyses
All values are given as means ± standard deviation (SDs). When two groups were compared, the results were analyzed using unpaired Student’s t-test. The results for the changes of body weight, blood glucose level, and insulin level were analyzed by a two-way repeated measure analysis of variance (ANOVA). The results for insulin signaling were analyzed by a two-way factorial ANOVA. The Tukey-Kramer post-hoc test was performed when a significant interaction between factors was revealed by the two-way ANOVA. Differences were considered significant at p < 0.05.
Data availability
All data shown in this manuscript are available from the corresponding author on reasonable request.
References
White, M. F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 283, E413–422. https://doi.org/10.1152/ajpendo.00514.2001 (2002).
Thirone, A. C., Huang, C. & Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol. Metab. 17, 72–78. https://doi.org/10.1016/j.tem.2006.01.005 (2006).
Tamemoto, H. et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372, 182–186. https://doi.org/10.1038/372182a0 (1994).
Araki, E. et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372, 186–190. https://doi.org/10.1038/372186a0 (1994).
Withers, D. J. et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904. https://doi.org/10.1038/36116 (1998).
Kubota, N. et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49, 1880–1889 (2000).
Kido, Y. et al. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Investig. 105, 199–205. https://doi.org/10.1172/JCI7917 (2000).
Previs, S. F., Withers, D. J., Ren, J. M., White, M. F. & Shulman, G. I. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J. Biol. Chem. 275, 38990–38994. https://doi.org/10.1074/jbc.M006490200 (2000).
Yamauchi, T. et al. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol. Cell. Biol. 16, 3074–3084. https://doi.org/10.1128/MCB.16.6.3074 (1996).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. https://doi.org/10.1126/science.1231143 (2013).
Li, D. et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31, 681–683. https://doi.org/10.1038/nbt.2661 (2013).
Li, W., Teng, F., Li, T. & Zhou, Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 31, 684–686. https://doi.org/10.1038/nbt.2652 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826. https://doi.org/10.1126/science.1232033 (2013).
Abbott, A. Laboratory animals: The Renaissance rat. Nature 428, 464–466. https://doi.org/10.1038/428464a (2004).
Iannaccone, P. M., Jacob, H. J. & Rats! Dis. Model. Mech. 2, 206–210. https://doi.org/10.1242/dmm.002733 (2009).
Toyoshima, Y. et al. Disruption of insulin receptor substrate-2 impairs growth but not insulin function in rats. J. Biol. Chem. 295, 11914–11927. https://doi.org/10.1074/jbc.RA120.013095 (2020).
Vijayakumar, A., Yakar, S. & Leroith, D. The intricate role of growth hormone in metabolism. Front. Endocrinol. (Lausanne). 2, 32. https://doi.org/10.3389/fendo.2011.00032 (2011).
Bunner, A. E., Chandrasekera, P. C. & Barnard, N. D. Knockout mouse models of insulin signaling: Relevance past and future. World J. Diabetes. 5, 146–159. https://doi.org/10.4239/wjd.v5.i2.146 (2014).
Le Roith, D., Bondy, C., Yakar, S., Liu, J. L. & Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 22, 53–74. https://doi.org/10.1210/edrv.22.1.0419 (2001).
Rosenfeld, R. G. & Hwa, V. New molecular mechanisms of GH resistance. Eur. J. Endocrinol. 151 (Suppl 1), 11–15. https://doi.org/10.1530/eje.0.151s011 (2004).
Argetsinger, L. S. et al. Growth hormone, interferon-gamma, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J. Biol. Chem. 270, 14685–14692. https://doi.org/10.1074/jbc.270.24.14685 (1995).
Thirone, A. C., Carvalho, C. R. & Saad, M. J. Growth hormone stimulates the tyrosine kinase activity of JAK2 and induces tyrosine phosphorylation of insulin receptor substrates and shc in rat tissues. Endocrinology 140, 55–62. https://doi.org/10.1210/endo.140.1.6417 (1999).
Pete, G. et al. Postnatal growth responses to insulin-like growth factor I in insulin receptor substrate-1-deficient mice. Endocrinology 140, 5478–5487. https://doi.org/10.1210/endo.140.12.7219 (1999).
Kulkarni, R. N. et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J. Clin. Investig. 104, R69–75. https://doi.org/10.1172/JCI8339 (1999).
Patti, M. E. et al. 4PS/insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice. J. Biol. Chem. 270, 24670–24673 (1995).
Barbour, L. A. et al. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J. Biol. Chem. 280, 37489–37494. https://doi.org/10.1074/jbc.M506967200 (2005).
Dominici, F. P., Cifone, D., Bartke, A. & Turyn, D. Alterations in the early steps of the insulin-signaling system in skeletal muscle of GH-transgenic mice. Am. J. Physiol. 277, E447–454. https://doi.org/10.1152/ajpendo.1999.277.3.E447 (1999).
Dominici, F. P., Cifone, D., Bartke, A. & Turyn, D. Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J. Endocrinol. 161, 383–392. https://doi.org/10.1677/joe.0.1610383 (1999).
Piatti, P. M. et al. Mediation of the hepatic effects of growth hormone by its lipolytic activity. J. Clin. Endocrinol. Metab. 84, 1658–1663. https://doi.org/10.1210/jcem.84.5.5685 (1999).
Sharma, R., Kopchick, J. J., Puri, V. & Sharma, V. M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 518, 111038. https://doi.org/10.1016/j.mce.2020.111038 (2020).
Aitman, T., Dhillon, P. & Geurts, A. M. A RATional choice for translational research? Dis. Model. Mech. 9, 1069–1072. https://doi.org/10.1242/dmm.027706 (2016).
Szpirer, C. Rat models of human diseases and related phenotypes: A systematic inventory of the causative genes. J. Biomed. Sci. 27, 84. https://doi.org/10.1186/s12929-020-00673-8 (2020).
Fujii, W., Kawasaki, K., Sugiura, K. & Naito, K. Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res. 41, e187. https://doi.org/10.1093/nar/gkt772 (2013).
Nakamura, K. et al. Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci. Rep. 4, 5635. https://doi.org/10.1038/srep05635 (2014).
Toyoshima, Y., Ohne, Y., Takahashi, S. I., Noguchi, T. & Kato, H. Dietary protein deprivation decreases the serine phosphorylation of insulin receptor substrate-1 in rat skeletal muscle. J. Mol. Endocrinol. 32, 519–531 (2004).
Toyoshima, Y. et al. Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J. Mol. Endocrinol. 45, 329–340. https://doi.org/10.1677/JME-10-0102 (2010).
Shibata, M. et al. Paraquat-induced oxidative stress represses phosphatidylinositol 3-kinase activities leading to impaired glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 285, 20915–20925. https://doi.org/10.1074/jbc.M110.126482 (2010).
Acknowledgements
We thank Dr. Takashi Yagi and Ms. Kanako Oki for their help with the animal care, and we appreciate the helpful discussions with Dr. Oksana Gavrilova (NIDDK, NIH) during the preparation of this manuscript. This work was partially supported by a Grant-in-Aid for Scientific Research to Y. T. (no. 22K05488) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Y.Toyoshima, K.N., and K.Y. designed the research. Y.Toyoshima, K.N., Y.Taguchi, R.T., S.T., H.O., N.T., and H.S. performed the experiments and analyzed the data. Y.Toyoshima, K.N., F.Y., K.Y., and S.M. interpreted the results of experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Toyoshima, Y., Nakamura, K., Taguchi, Y. et al. Deletion of IRS-1 leads to growth failure and insulin resistance with downregulation of liver and muscle insulin signaling in rats. Sci Rep 15, 649 (2025). https://doi.org/10.1038/s41598-024-84234-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84234-1