Abstract
Urinary tract infections are a common condition affecting people globally, with multidrug-resistant (MDR) Escherichia coli (E. coli) being a major causative agent. Antimicrobial susceptibility profiling was performed using the VITEK 2 automated system for 1254 E. coli isolates, revealing that 831(66.2%) isolates were determined as MDR E. coli. A significant resistance pattern was observed for nalidixic acid (86.04%), ampicillin (74.16%), ticarcillin (70.73%), cefalotin (65.23%), cefixime (62.68%), ciprofloxacin (55.18%), ceftriaxone (53.75%), amoxicillin-clavulanic acid (22.81%), ertapenem (7.18%), and fosfomycin (2.23%). Whole Genome Sequencing of Carbapenem-resistant E. coli (CREC)—CREC 3 (ST405), CREC 4 (ST448), and CREC 5 (ST167) was performed to determine genomic characteristics. CREC 3, CREC 4, and CREC 5 belong to the phylogroup D, B1, and A, respectively. The NDM-5 gene was common in all three isolates, with CTX-M-15 being present in CREC 3 and CREC 4. Virulence factors of CREC 3 (fliC, shuA), CREC 4 (spaS), CREC 5 (iucA, papH, papG, iucB, yigF), and plasmids (IncFIA, IncFIB) were identified to be significant. The use of pangenome analysis enhances our understanding of resistance traits of isolates ST167, ST405, and ST448, offering valuable insights into comparative genomics of uropathogenic MDR E. coli.
Similar content being viewed by others
Introduction
Antimicrobial resistance is a serious public concern, especially in developing countries such as India, where easy access to and usage of antibiotics has increased the prevalence of inappropriate antibiotic use and has raised resistance levels. According to the World Health Organization (WHO), antimicrobial resistance (AMR) stands out as a paramount global health and development challenge. The prevalence of bacterial AMR has directly led to approximately 1.27 million deaths globally out of 4.95 million deaths associated with infection1. One of the most frequent infectious diseases affecting humans is urinary tract infection (UTI), the second most common reason, after respiratory tract infections, for which physicians prescribe antibiotics2. In India, it was reported the Enterobacterales group was a commonly isolated species in urine (85%), with E. coli as the predominant organism (72%)3. Due to the widespread prevalence of E. coli and its ongoing development of antibiotic resistance, humans and animals are seriously at risk of infection, expecting the likelihood of antibiotic use to surge by 67% by 2030, placing a financial burden on healthcare systems4. Extended-Spectrum Beta-Lactamase (ESBL) E. coli, a major multidrug-resistant bacterium, is accountable for causing severe infections in both hospital and community settings, especially in lower-middle-income countries with poor hygiene and sanitation. Consequently, there is a rise in hospital stays, death, morbidity, and medical expenses5. Global health is challenged by increasing antimicrobial resistance, particularly to broad-spectrum antibiotics. Beta-lactam antibiotics, frequently used as a primary treatment, are of significant concern. The WHO states that third-generation cephalosporins (3GCs), including ceftriaxone and cefotaxime, are essential antimicrobials in human medicine and are used to treat Gram-negative infections in humans. The increasing use of these antibiotics, especially in developing countries where they are more readily available and affordable than other classes of antibiotics, emphasizes an urgent need for strategies to address this practice, which poses a danger to health care6. Despite the efficacy of carbapenem antibiotics for treating Enterobacterial infections, resistance to these drugs is increasing. With high rates of illness and mortality, Carbapenem-Resistant Enterobacteriaceae (CRE) represent a significant public health concern. The rapid global dissemination of CREs can be attributed to the carriage of the β-lactamase gene on mobile genetic elements. Thus, the selection of antimicrobials for infections has become complex because of the emergence of Carbapenem-Resistant E. coli (CREC)7.
As bacterial resistance to cephalosporins rises, the reliance on carbapenems for treatment grows. The objective of this research focuses on addressing the increasing resistance of E. coli to carbapenem, often referred to as a “last-resort antibiotic”, known for its broad-spectrum activity. Unlike previous studies, which have largely concentrated on sequence types of E. coli from India without exploring the genomic aspects, this study provides a genomic perspective on carbapenem resistance in E. coli strains. We aimed to evaluate the occurrence of antimicrobial resistance, by phenotypic and genomic analysis, with a focus on AMR genes, virulence factor genes, and pangenome analysis to compare Indian isolates with global ST types (Fig. 1).
Experimental design of the study: This figure illustrates the study workflow, including the isolation and identification of E. coli from UTI samples, followed by antibiotic susceptibility testing (VITEK-2), MDR profiling, and MAR index. ESBL-producing carbapenem-resistant E. coli were subjected to WGS and bioinformatics analysis (BAcWGSTdb, CARD, PLasmidFinder, IS Finder). Pangenome analysis was conducted using the Roary pipeline.
Results
Phenotypic analysis
The antibiotic resistance pattern of the isolates (n = 1254) for the 18 antibiotics was as follows: ampicillin (74.16%), amoxicillin-clavulanic acid (22.81%), ticarcillin (70.73%), piperacillin/tazobactam (18.90%), cefalotin (65.23%), cefoxitin (21.29%), cefixime (62.68%), ceftazidime (32.85%), ceftriaxone (53.75%), ertapenem (7.18%), amikacin (2.23%), gentamicin (17.46%), nalidixic acid (86.04%), ciprofloxacin (55.18%), norfloxacin (43.94%), ofloxacin (47.21%), fosfomycin (2.23%), and nitrofurantoin (3.59%), represented in (Fig. 2).
Antibiotic resistance graph of 1254 isolates as a result of AST from VITEK 2 data: The graph provides an overview of resistance pattern across a diverse set of antibiotics. The X-axis represents the list of antibiotics tested, while the Y-axis represents the percentage of isolates sensitive (yellow), resistant (blue), and intermediate (red) to the specific antibiotics. Higher resistance was observed for nalidixic acid (86.04%) and higher sensitivity to fosfomycin (2.23%).
Statistical analysis
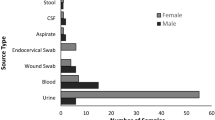
The data revealed 831 (66.26%) isolates to be MDR, of which 617 (74.24%) were females and 214 (25.75%) were males (Fig. 3a, Supplementary Figure 1 and Supplementary Figure 2). The statistical analysis revealed significant differences (P < 0.05) using the paired t-test (two-tailed). Multi-drug resistance (MDR) in male and female samples was analyzed using the Chi-square test (GraphPad Prism). On the outcome, Chi-square, df was found to be 12.83, 1 with a statistically significant P value of 0.0003*** (two-sided) (P < 0.05). The test was measured with sensitivity, specificity, and predictive values categorized for diagnostic tests. The sensitivity & specificity test (SS) was measured using the Wilson-Brown method. Data were analyzed for MDR (214—Male, 617—Female) and Non-MDR (71—Male, 352—Female) populations. SS (represented in percentage) was found to be MDR (17.07%—Male, 49.20%- Female); and Non-MDR (5.66%—Male, 28.07%—Female).
(a) MDR Bar graph represents the occurrence of UTI in males and females: The graph provides details on MDR profiling.The bar graph depicts the number of MDR and NON-MDR isolates among males (red) and females (blue). A higher incidence of MDR isolates were observed in females compared to males. (b) Age-Gender-ESBL Bar graph represents the occurrence of UTI in various age groups: The bar graph shows the distribution of the ESBL-producing isolates among the groups of different ages and genders. In the majority of age groups, the female patients had higher numbers of ESBL-positive isolates compared with the male patients. A significant peak in the prevalence of ESBL was observed for both genders in the 61–70 age group; at the same time, females uniformly showed higher rates in all age groups. Such a trend points toward a number of age- and gender-related dynamics in the spread of ESBL.
The mean multiple antibiotic resistance index (MAR index) of the isolates was 0.38. A total of 505 (40.27%) isolates were categorized as Extended Spectrum Beta Lactamase producing (ESBL) isolates. The isolates showed a higher prevalence of UTI in females 969 (77.27%), than in males 285 (22.72%). The MAR index in male and female samples was analyzed using the Chi-square test (GraphPad Prism). On the outcome, Chi-square, df was found to be 14.70, 1 with a statistically significant P value of 0.0001*** (two-sided) (P < 0.05). The test was measured with sensitivity, specificity, and predictive values categorized for diagnostic tests. The SS test was measured using the Wilson-Brown method. Data were analyzed for MAR > 0.2 (232—Male, 677—Female) and MAR < 0.2 (53—Male, 292—Female) populations. The SS (represented in percentage) was found to be MAR > 0.2 (18.50%—Male, 53.99%—Female); MAR < 0.2 (4.23%—Male, 23.29%—Female) (Supplementary Figure 3).
The Age-Gender-ESBL graph (Fig. 3b) shows the occurrence of UTI in the isolates of various age groups from 0 to 100 years, where females are affected more in the 61–70, 0–10, and 21–30 years of age groups and males in the 0–10 and 61–70 years of age groups. Men are least affected in the age groups of 11–20 and 91–100 years of age, and women in the 91–100 and 81–90 age groups. This finding was found to be statistically significant (P < 0.05) using the paired t-test (two-tailed).
Genomic analysis
From the 1254 isolates that were evaluated phenotypically using the VITEK 2 instrument (Supplementary information in excel file) for their Antibiotic susceptibility pattern against 18 different antibiotics, three isolates that were resistant to carbapenem drugs along with coresistance to cephalosporins, fluoroquinolones, and penicillins were further evaluated using whole genome sequencing (WGS). Table 1 summarizes the genomic analysis of the three isolates CREC 3, CREC 4, and CREC 5.
Carbapenem-resistant Escherichia coli (CREC 3)
The isolate CREC 3 was identified to belong to the sequence type ST405, using BacWGSTdb. The isolate belongs to the Serotype O102:H6, with fliC, wzy, and wzx genes identified by SerotypeFinder. The isolate CREC 3 belongs to the phylogroup D, was evaluated using EzClermont. Antibiotic resistance genes such as evgA, emrK, emrY, TolC, msbA, CTX-M-15, emrR, emrB, marA, mdtH, mdtG, H-NS, sul2, NDM-5, BRP(MBL), sul1, qacEdelta1, aadA2, dfrA12, rmtB, TEM-1, and Mrx against various classes of antibiotics such as Fluoroquinolone, Tetracycline, Beta lactam, Macrolide, Sulfonamide, and Aminoglycoside, respectively, were determined using CARD Resistance. Virulence factors (fliC, shuA, udg, rfbA, rfbD, rfbB, KpsC) were determined using PATRIC. Plasmids (ColpEC648, IncB/O/K/Z, IncFIA, IncFIB(pB171), IncX4) were determined using PlasmidFinder. Insertion sequences (IS630, IS110) were determined using IS Finder (BLAST).
Carbapenem-resistant Escherichia coli (CREC 4)
The isolate CREC 4 was identified to belong to the sequence type ST448 using BacWGSTdb. The CREC 4 isolate belongs to the Serotype O188:H19, with fliC, wzy, and wzx genes identified by SerotypeFinder. The isolate CREC 4 belongs to the phylogroup B1, was evaluated using EzClermont. Antibiotic resistance genes such as mdtG, mdtH, H-NS, acrB, TolC, AcrE, evgA, PmrF, emrB, emrR, msbA, cpxA, marA, mdtE, CTX-M-15, dfrA12, aadA2, qacEdelta1, sul1, BRP(MBL), NDM-5, OXA-181, QnrS1, catA1, Mrx, TEM-1, and rmtB against various classes of antibiotics such as Tetracycline, Fluoroquinolone, Aminoglycoside, Beta-lactam, Sulfonamide, Phenicol, respectively, were determined using CARD Resistance. Virulence factors (spaS) were determined using PATRIC. Plasmids (Col(pHAD28), Col(pHAD28), ColKP3, ColRNAI, IncFIA, IncFIB(AP001918), IncFIB(H89-PhagePlasmid), IncX3) were determined using PlasmidFinder. Insertion sequences (IS110, IS256, IS4, IS630) were determined using IS Finder (BLAST).
Carbapenem-resistant Escherichia coli (CREC 5)
The isolate CREC 5 was identified to be the sequence type ST167, using BacWGSTdb. The CREC 5 isolate belongs to the Serotype O101:H9, with fliC, wzt, and wzm genes identified by SerotypeFinder. The isolate CREC 5 belongs to the phylogroup A, was evaluated using EzClermont. Antibiotic resistance genes such as marA, Pmrf, mdtB, acrD, mdtN, mdtP, acrB, H-NS, emrA, emrB, TolC, cpxA, bacA, mdtG, mdtH, gadX, mdtF, mdtE, evgA, emrK, emrY, kdpE, NDM-5, BRP(MBL), sul1, qacEdelta1, aadA2, dfrA12, AcrF, rmtB, and CMY-145 against various classes of antibiotics such as Beta-lactam, Aminoglycoside, Tetracycline, Fluoroquinolone, and Sulfonamide, respectively, were determined using CARD Resistance. Virulence factors (iucA, papH, iucC, wcaM, papG, iucB, iutA, papF, papD, iucD, yigF) were determined using PATRIC. Plasmids (IncFIA, IncFIB(AP001918), IncFII, IncI(Gamma)) were determined using PlasmidFinder. Insertion sequences (IS200, IS605, IS630, IS110) were determined using IS Finder (BLAST).
Mobile genetic elements were reported using Proksee genome viewer for the three isolates; CREC 3, CREC 4, and CREC5 (bamA, clpA, clpB, dnaK, fhuE, gyrB, mukB, mutS, parC, polA, polB, recB, recC, recE_1, recG, rep, rnr, sbcC, topA, uvrA, uvrB, uvrD, virB4).
Phylogenetic tree
The most common AMR genes detected in the isolates were NDM-1, NDM-5, CTX-M-15, TEM-1, TolC marking carbapenem, cephalosporin, beta-lactam, efflux pump related resistance, respectively, which are amongst all the closely related isolates across the world. Because the isolates harbour resistance genes of three different classes of antibiotics, the isolates are termed MDR. The phylogenetic trees generated for the three sequence types revealed the spread of antibiotic resistance worldwide and the impact of superbugs on humans. These images (Figs. 4a, 5a, 6a) represent phylogenetic trees of the respective CREC 3, CREC 4, and CREC 5 generated using iTOL.
(a) Phylogenetic tree of ST405 representing global scenario: The phylogenetic tree of ST405 depicts the distribution of genes across different countries. The presence of specific genes like NDM-1, CTX-M-15, TEM-1B are represented by distinct shapes across different branches. (b) Pangenome analysis of ST405: A Pangenome pie chart presentation for the strains selected globally for ST405. The pie chart represents the percentage of gene families from the total pangenome. The left side indicates the phylogenetic tree and the metadata represents the significant AMR genes for MDR E. coli in the middle. The right side represents the matrix of the core and accessory genes of the isolates, where blue indicates presence and white indicates absence of genes.
(a) Phylogenetic tree of ST448 representing global scenario: The phylogenetic tree of ST448 depicts the distribution of genes across different countries. The presence of specific genes like NDM-1, CTX-M-15, TEM-1, mcr-1 are represented by distinct shapes across different branches. (b) Pangenome analysis of ST448: A Pangenome pie chart presentation for the strains selected globally for ST448. The pie chart represents the percentage of gene families from the total pangenome. The left side indicates the phylogenetic tree and the metadata represents the significant AMR genes for MDR E. coli in the middle. The right side represents the matrix of the core and accessory genes of the isolates, where blue indicates presence and white indicates absence of genes.
(a) Phylogenetic tree of ST167 representing global scenario: The phylogenetic tree of ST167 depicts the distribution of genes across different countries. The presence of specific genes like NDM-1, CTX-M-15, TEM-1 are represented by distinct shapes across different branches. (b) Pangenome analysis of ST167: A Pangenome pie chart presentation for the strains selected globally for ST167. The pie chart represents the percentage of gene families from the total pangenome. The left side indicates the phylogenetic tree and the metadata represents the significant AMR genes for MDR E. coli in the middle. The right side represents the matrix of the core and accessory genes of the isolates, where blue indicates presence and white indicates absence of genes.
Virulence genes
Around 173 common genes across CREC 3, CREC 4, and CREC 5 were identified. This shows that there is a genetic similarity among these CREC isolates, which is critical for understanding their biological functions and aiding in the development of targeted therapeutics. Virulence factors of CREC 3 (fliC, shuA, udg, rfbA, rfbD, rfbB, KpsC), CREC4 (spaS), and CREC 5 (iucA, papH, iucC, wcaM, papG, iucB, iutA, papF, papD, iucD, yigF) were specifically found to be present in the corresponding ST types, along with information about the product function and classification from the PATRIC Database.
Pangenome analysis
Pangenome analysis was visualized using Phandango for ST 448, ST 167, and ST 405. It illustrates multiple strains from various countries and shows the presence and absence of major antimicrobial resistance (AMR) genes, such as CTX-M-15, blaNDM-1, TEM-1B, and tetR, in MDR E. coli. The increased number of AMR genes can be attributed to the increasing global use of antibiotics and the dissemination of resistance genes associated with plasmids. In ST 405, Fig 4b represents the percentage of gene families within the pangenome, comprising 20,975 genes. First, core genes account for 13%, consisting of 2,816 genes that are present in 99–100% of the strains. Second, accessory genes like Softcore genes, which represent 4% include 744 genes found in 95–99% of the strains, Shell genes make up 10%, which consist of 2,203 genes present in 15–95% of the strains, and Cloud genes contribute 73% with 15,212 genes that are found in 0–15% of the strains. Figure 5b illustrates the phandango of ST 448 and the percentage of gene families out of the total pangenome, which comprises 11,416 genes. Core genes comprise 32% of strains, consisting of 3600 genes present in 99–100% of strains. Softcore genes account for 2%, which contains 234 genes found in 95–99% of strains. Shell genes, representing 15%, comprise 1709 genes present in 15–95% of strains. Lastly, cloud genes constitute 51%, with 5873 genes that can be found in 0–15% of strains. Figure 6B illustrates the distribution of gene families in ST167 within the pangenome, which contains a total of 20,010 genes. First, core genes comprise 14% of the pangenome, consisting of 2,781 genes present in 99–100% of the strains. Second, accessory genes include softcore genes, which account for 3%, comprising 692 genes found in 95–99% of the strains; shell genes, which represent 9% and consist of 1,813 genes present in 15–95% of strains; and cloud genes, contributing 74% with 14,724 genes that are present in 0–15% of the strains.
Discussion
Uropathogens, particularly E. coli, are the most common causes of illness. The misuse of antibiotics and excessive prescriptions contribute to antibiotic resistance globally8. The findings of this study are comparable to those of research from Aligarh, India where 42% of uropathogens were found to be ESBL producers, and with another study from Pakistan, which identified 66% of uropathogens as ESBL producers. Research from India and Pakistan indicated that 43% and 59% of cases found were MDR E. coli, respectively9, which has slightly increased to 66.26% in the current study. Moreover, another study from Nepal found that 54% of isolates had MDR, which is lower than the 66.26% found in the current study. This finding raises concerns regarding antimicrobial resistance, possibly due to community-acquired pathogens from superbugs10. The phenotypic analysis results are consistent with other findings indicating that the E. coli-caused UTIs are highly prevalent across diverse age groups. Earlier studies on ESBL-producing E. coli in urine samples demonstrated 46.3% prevalence in males and 53.7% in females, showing higher occurrence in females11. A similar pattern was observed in a study conducted in Meerut , India. The prevalence of UTI was significantly higher in females with 73.57%, among women aged between 26 and 28 years12.
The isolate CREC 3 possesses a serotype of O102:H6, which is similar to a study13. The highly pathogenic ST, E. coli ST405, is a leading cause of urosepsis, thus creating bacteremia, and is also implicated in antibiotic resistance due to the high prevalence of the CTX-M-15 gene responsible for resistance against third-generation cephalosporins. A study conducted among UTI strains found 19 out of 30 E. coli samples with the presence of the CTX-M-15 gene, which once again proved the high predominance of the gene in pathogenic strains14. Similar results were reported previously from Pakistan, where the most common sequence types among 118 carbapenem-resistant E. coli samples collected from clinical settings were ST405 and ST167, which accounted for 35.6% and 21.2% of the isolates, respectively. The bla CTX-M-15 gene was present in both high-risk clones, highlighting the serious problem in medication for resistance15. Additionally, a severe outbreak reported in Mozambique in 2021 underlined the impact of extraintestinal pathogenic E. coli ST405, which also carried the bla CTX-M-15 gene and extended carbapenem resistance. The outbreaks underscore the urgent need for improved antimicrobial surveillance and a globally coordinated effort to avoid the spread of resistance to drugs like clone ST40516. Taken together, these studies indicate the significant epidemiological role played by CTX-M-15-carrying ST405 in MDR infections worldwide, particularly in the healthcare setting.
E. coli ST167 is a predominant clone carrying carbapenem resistance through conjugative plasmids, potentially contributing to horizontal gene transfer14. The isolates from China, Canada, and Australia possessing O101:H9 serotypes from different sources, such as samples from cattle farms and humans, show similarities in the sequence type of ST167. Genomic analysis further shows that these isolates are similar to our isolate CREC 5 in terms of AMR genes, virulence genes, and plasmids17. According to Li et al18, carbapenemases, ESBL/AmpC enzymes, and porins deficiencies are the main causes of carbapenem resistance in CREC isolates. Similar to our study findings, NDM-5 was common among CREC isolates, with IncF being the most common outbreak incompatibility class. Our isolate CREC 5 identifies the presence of blaNDM-5-positive E. coli infections, which aligns with the current ST167 global data. This sequence type was also connected to the global spread of the NDM gene responsible for resistance worldwide. Similar strains have been reported from other nations, including Italy, Egypt, and Switzerland, with cases in kittens from Italy, which also raises the possibility of human-animal transmission19. It is more common to find NDM enzymes in E. coli, and amino acid substitution accounts for the majority of the genetic differences across NDM variants. Asia remains the region where NDM-producing bacteria are most widely distributed, with Brazil and Algeria serving as important reservoirs20.
The present study demonstrates that the three isolates showed beta-lactam class antibiotic resistance due to the presence of important and common AMR genes such as blaNDM-5. In a similar study by Kandi et al.,14 blaTEM-1, blaCTX-M-15, blaCMY-42, and blaNDM-5 AMR genes were detected, which was similar to the isolates CREC 3, CREC 4, and CREC 5 we identified. Globally distributed ST405 E. coli is typically linked to ESBLs such as CTX-M-15, which is similar to our isolate CREC 3. A recent Indian MLST report identified 16 different sequence type variants, of which 3 isolates belong to ST167 and 1 each of ST405 and ST448. A previous study21 showed a predominance of ST167, followed by ST405 and ST410. Likewise, a study from Bangladesh showed a higher prevalence of blaCTX-M (63.33%)22. The present study includes an isolate belonging to the ST448 type (CREC 4), which is reportedly the first isolate belonging to this sequence type identified using WGS in Southern India. Concerning CREC 4 (ST448), India witnessed very few isolates belonging to the ST448 type reported through pulsed-field gel electrophoresis23 and PCR-based technique24. Globally, there are a few countries such as Pakistan, Bangladesh, Australia, Netherlands, USA, Denmark, Zambia, and Qatar that have reported the ST448 type isolate based on genomic analysis25.
The unique genes of the CREC 3 isolate are mostly involved in the virulence activity of E. coli. Only fliC and shuA correspond to heme uptake. This fliC gene was found to encode the flagellin protein, which is essential for bacterial motility, allowing E. coli to move to favorable environments26. In CREC 4, only the spaS gene was found to be unique, which corresponds to its function as a type 3 secretor of inner membrane proteins that assists in the export of effector proteins into host cells. These effectors are mostly involved in promoting adherence and pathogenicity. Regarding CREC5, wcaM, iucA, iucB, and iucD are mainly involved in the iron uptake mechanism, and papF, papG, and papH come under the adherence classification, which aids in the organism’s colonization and persistence, while yigF contributes to its virulence. Plasmid IncFIA was common in all three isolates, while IncFIB(AP001918) was common in CREC 4 and CREC 5. These plasmids belong to the Incompatibility group (Inc), which may carry the drug resistance genes27.
Using BacWGSTdb, global strains of the sequence type ST 405 were analyzed. It was found that the CTX-M-15 gene is widely spread throughout many countries such as the USA, Russia, India, Lebanon, Australia, China, Nigeria, Japan, and France. However, the presence of the blaNDM-5 gene was restricted to China, the USA, and India. Although fewer countries have the presence of the blaNDM-5 gene in comparison to CTX-M-15, its presence in these countries indicates the potential hotspots for carbapenem-resistant E. coli strains. These findings suggest that the CTX-M-15 gene is more prevalent in global regions, but the blaNDM-5 gene is emerging in specific regions rather than being widely distributed. Strains from the sequenced type (ST) 448 were analyzed using BacWGSTdb. The bla-NDM-1 gene was detected in Thailand, Switzerland, Germany, the UK, India, China and Bangladesh. Moreover, the presence of the CTX-M-15 gene has been reported in India, Germany, Bangladesh, and the USA. Most importantly, instances of the presence of both the bla-NDM-1 gene and CTX-M-15 gene were found in Bangladesh and India. The region with the high incidence of the bla-NDM-1 gene was Southeast Asia comprising India, Bangladesh, and Thailand. In ST 167, the blaNDM-1 gene was found globally in regions including China, the USA, India, Lebanon, Switzerland, and South Korea with a higher incidence.
With respect to antimicrobial resistance, E. coli isolates are resistant to routinely used antimicrobial drugs such as sulfonamides, fluoroquinolones, nitrofurantoin, cephalosporins, and carbapenems in the treatment of UTIs. Specifically, carbapenem-resistant E. coli poses a major threat to people’s well-being, often characterized by limited treatment options and prolonged treatment durations, resulting in higher healthcare costs than infections caused by carbapenem susceptible E. coli28. According to the WHO’s 2022 Global Antimicrobial Resistance and Use Surveillance System (GLASS), resistance levels are particularly high in E. coli—a common cause of UTIs and bloodstream infections29. The report reveals that more than 20% of E. coli infections globally are resistant to primary treatments like ampicillin and co-trimoxazole, creating significant barriers to the effective management of these infections. Notably, over 8% of E. coli infections causing UTIs are now resistant to ciprofloxacin, a standard treatment option in many countries. In most cases, fluoroquinolones are recommended for UTIs since they have a lower risk of ciprofloxacin resistance30. However, the current study shows that this resistance has increased (55.18%) over time as a result of overuse of antibiotics. Another study from Northern India reports31 resistance rates for ampicillin (63.4%), nalidixic acid (63.4%), and cefotaxime (62.1%), which are lower than the current study data, revealing an enhanced resistance trend for ampicillin (74.16%) and several fluoroquinolone drugs, including nalidixic acid (86.04%), ofloxacin (47.21%), and norfloxacin (43.94%). This study identified that out of 90 carbapenem-resistant samples, samples were sensitive to fosfomycin (n = 75), amikacin(n = 64), nitrofurantoin (n = 58), gentamicin(n = 50), and piperacillin/tazobactam (n = 12). Our data showed that these four antibiotics can be used individually or in combination to treat infections caused by carbapenem-resistant E. coli. In the ICMR Annual Report 2023, nitrofurantoin and fosfomycin were regarded as an effective therapy option against CREs. This guideline correlates with our findings with fosfomycin exhibiting the greatest therapeutic activity. Our results are similar to recent research conducted in India where they analyzed the antibiogram data across 22 centers in 10 Indian states and 3 union territories. On examination, it was revealed that CREs were found to have an overall susceptibility of 93.6% to fosfomycin, 86.6% to nitrofurantoin, 74.3% to gentamicin, and 76.7% to piperacillin/tazobactam32. Aminoglycosides like amikacin and gentamicin are less effective when used in monotherapies, resulting in decreased clinical outcomes and prolonged use. The combination of aminoglycosides with meropenem may restore meropenem’s activity against CREC by aiding in enhancing the bacteria’s membrane permeability, resulting in a promising therapeutic approach33. The proper implementation of these antibiotics may lead to an efficient treatment plan.
One of the main causes of antimicrobial resistance in developing countries is the availability of over-the-counter drugs. People, particularly those from resource-limited backgrounds, prefer self-medicating based on assumptions rather than getting a proper consultation from clinicians, mainly due to financial constraints. This lack of knowledge coupled with the overuse and misuse of drugs has exacerbated the issue. Standard antibiotics like penicillins and cephalosporins have become increasingly ineffective against MDR and ESBL-producing E. coli, pushing clinicians to resort to more advanced combined therapies. Over the years, there have been various treatment protocols implemented. When it comes to antibiotic treatment, our findings suggest gentamicin to be more effective than other drugs, offering greater efficacy at a lower cost.
The sample analysis from a single diagnostic lab as performed in this study reveals key resistance patterns, highlighting the importance of implementing effective AMR management strategies. The findings aim to educate healthcare providers and direct the way for enforcing data surveillance across different labs and the development of rapid diagnostics using WGS, enabling them to respond to emerging antimicrobial resistance trends more efficiently. Epidemiological surveillance networks, such as EARS-Net and the Centers for Disease Control and Prevention (CDC) in the United States, have documented a significant increase in the prevalence of drug-resistant bacteria over the past decade34. Specific AMR recommendations include regular coordination and networking between clinicians and diagnostic labs on resistance patterns and treatment guidelines, which is necessary to ensure that they prescribe the most effective treatment based on the local antibiogram. Organizing public awareness campaigns to the general public emphasizing the dangers of self-medicating helps in promoting responsible medication practices. To improve patient outcomes, routine culture-based testing is recommended to identify ESBL, carbapenemase-producing bacteria, and MDR strains. The use of advanced molecular diagnostics, such as WGS, enables rapid identification of resistance profiles and tracking of resistant strains, including high-risk international clones. Antibiotic stewardship programs are essential to promote responsible antibiotic use. These programs must educate healthcare providers on appropriate empirical therapy, consider local resistance patterns, and advocate for targeted therapy based on susceptibility testing. Furthermore, restricting last-line antibiotics, such as carbapenems, and promoting narrow-spectrum alternatives when feasible will help mitigate the development of resistance.
Limitations of the study
Isolates from a limited region of Tamil Nadu, primarily in and around Chennai, were included in the study. A comprehensive investigation might provide a clearer picture and a valid comparison with the global isolates. Since only three isolates have been sequenced in the current study, there is a cause for concern at the national and international levels regarding the spread of these superbugs among humans. Further analysis and sample evaluation will suffice for the details of the studies in the future.
Conclusion
The study demonstrates a high frequency of occurrence of UTI associated with AMR among common antibiotic classes against MDR and ESBL-producing E. coli strains. Antibiotic Susceptibility Testing (VITEK 2) and genome sequencing tools could help us understand the prevalence of UTI caused by MDR E. coli. The WGS reports from India were found to be scarce and minimal MDR E. coli UTI cases have been reported since 2016–2023 due to a lack of genomic analysis, prompting a trend for urgent whole genome sequencing to identify clonality and risk factors. Global epidemiological surveillance is required to find treatment options due to the significant risk of antibiotic resistance and transmissibility of the strains and is necessary to apply these strategies for the betterment of the local community. Future in-depth analysis of plasmids and large sets of isolates may reveal several sequence types in circulation from clinical samples and their connection to animals and environmental sectors, which can be established based on one health approach. To effectively address AMR, adopting a comprehensive broad-spectrum approach is essential to thoroughly understand the various components involved in fighting infections.
Materials and methods
Sample processing and molecular characterization of MDR E. coli
Several classes of antibiotics were used, including ampicillin, amoxicillin/clavulanic acid, cefalotin, cefoxitin, cefixime, ceftazidime, ceftriaxone, ertapenem, amikacin, gentamicin, nalidixic acid, ciprofloxacin, norfloxacin, ofloxacin, fosfomycin, and nitrofurantoin for determining the minimum inhibitory concentration (MIC) using VITEK 2. MIC data of 1254 E coli isolates were obtained from a diagnostic lab of which 3 isolates (Carbapenem Resistant, MAR index > 0.89) were cultivated on MacConkey agar, followed by 24 h incubation at 37 °C, for the biochemical identification of the strain as E. coli35 (SRIHER IEC Approval Number- CSP/24/FEB/143/58). The Clinical and Laboratory Institute (CLSI) guidelines (CLSI 2022) were followed for performing antimicrobial susceptibility tests. All the analysis was performed in accordance with the relevant guidelines and regulations.
Whole genome sequencing
MALDI-TOF (Matrix Assisted Laser Desorption Ionization—Time of Flight) analysis was used as a preliminary confirmation for the presence of MDR E. coli before finalizing with whole genome sequencing. The DNeasy Ultraclean Microbial Kit was utilized to isolate the genomic DNA. The KAPA Hyper Plus Kit was used to prepare the DNA libraries. Whole-genome sequencing was performed using Illumina’s NovaSeq 6000. After the adaptor was removed, the raw data were filtered using the Phred quality score (Q score), with a Q score cutoff value of > 30. Subsequently, two steps of preprocessing were done on the raw reads (fastq files) from the sequenced genome. The Illumina adapter sequences were cut from both fastq files of paired-end data during the adapter removal process. Trim Galore was employed for adapter removal, followed by de novo assembly using Unicycler assembler v0.436.
Data analysis
Genome annotation was done using the PGAP 4.2 annotation. Multi-Locus Sequence Typing (MLST) v2.0 was used to determine the strain’s taxonomic position, and SerotypeFinder 2.0 was employed to determine its serotype. Phylogroups were identified with the web application EzClermont version 0.6.237. The Comprehensive Antibiotic Resistance Database (CARD) RGI version 6.0.3 was used to predict antibiotic resistance genes. Virulence factors were determined using PATRIC. The phylogenetic tree and plasmid replicons were identified by using the BacWGSTdb 2.0 and PlasmidFinder 2.1, respectively, which were visualized using iTOL v6. Mobile genetic elements were determined using the Proksee genome viewer. Insertion elements were determined using IS Finder. Figure 1 shows the overview of the study. The bioinformatics data analysis was performed using tools similar to those used in a recent study38. All the analysis were performed under default settings.
PanGenome analysis
Roary pipeline 3.13.0 was used to detect the core and accessory genes using annotation files generated using Prokka 1.14.6. The output files from the Roary pipeline were used for pangenome analysis using Phandango. The isolates obtained in the study belonging to the ST405, ST448, and ST167 sequence types were globally compared, along with the respective strains.
Data availability
Nucleotide accession Number: The sequence data of CREC 3, CREC 4, and CREC 5 have been deposited at GenBank, with the BioProject number: PRJNA224116, PRJNA1063618, PRJNA1063623, BioSample number: SAMN39291013, SAMN39402020, SAMN39402434 under the accession number: JAYMDK000000000, JAZGVI000000000, JAZGVJ000000000, respectively. The raw data are available at the NCBI SRA database.
References
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399, 629–655 (2022).
Bullens, M. et al. Antibiotic resistance in patients with urinary tract infections in Pakistan. Public Health Action 12, 48 (2022).
Mohapatra, S. et al. Prevalence and resistance pattern of uropathogens from community settings of different regions: an experience from India. Access Microbiol 4, 000321 (2022).
Arbab, S., Ullah, H., Wang, W. & Zhang, J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 8, 1780 (2022).
Bazaid, A. S. et al. Antimicrobial surveillance for bacterial uropathogens in Ha’il, Saudi Arabia: A five-year multicenter retrospective study. Infect. Drug Resist. 14, 1455 (2021).
Medugu, N. et al. Phenotypic and molecular characterization of beta-lactam resistant Multidrug-resistant Enterobacterales isolated from patients attending six hospitals in Northern Nigeria. Sci. Rep. https://doi.org/10.1038/s41598-023-37621-z (2023).
Zhang, W. et al. Clinical distribution characteristics of 1439 carbapenem-resistant Escherichia coli strains in china: drug resistance, geographical distribution, antibiotic MIC50/90. Infect. Drug Resist. 14, 4717 (2021).
Zhou, Y. et al. Urinary tract infections caused by uropathogenic Escherichia coli: Mechanisms of infection and treatment options. Int. J. Mol. Sci. 24(13), 10537. https://doi.org/10.3390/ijms241310537 (2023).
Islam, M. A. et al. Prevalence, etiology and antibiotic resistance patterns of community-acquired urinary tract infections in Dhaka, Bangladesh. PLOS ONE 17(9), e0274423. https://doi.org/10.1371/journal.pone.0274423 (2022).
Shakya, S. et al. High multidrug resistance in urinary tract infections in a tertiary hospital, Kathmandu, Nepal. Public Health Action 11, 24 (2021).
Anees Muhammad, N. A. et al. 18. Antibiotics resistance of extended spectrum beta lactamases uropathogenic Escherichia coli in Peshawar–Pakistan. Pure Appl. Biol. PAB 9, 1840–1848 (2020).
Prakash, D. & Saxena, R. S. Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut city, India. ISRN Microbiol 2013, 749629 (2013).
Campos, A. C. C. et al. Comprehensive molecular characterization of Escherichia coli isolates from urine samples of hospitalized patientsin Rio de Janeiro, Brazil. Front. Microbiol. https://doi.org/10.3389/fmicb.2018.00243 (2018).
Kandi, V. et al. Molecular characterization of Escherichia coli causing urinary tract infections through next-generation sequencing: A comprehensive analysis of serotypes, sequence types, and antimicrobial and virulence genes. Cureus 16, e55556 (2024).
Mujahid, F., Rasool, M. H., Shafiq, M., Aslam, B. & Khurshid, M. Emergence of carbapenem-resistant uropathogenic Escherichia coli (ST405 and ST167) strains carrying blaCTX-M-15, blaNDM-5 and diverse virulence factors in hospitalized patients. Pathogens. https://doi.org/10.3390/pathogens13110964 (2024).
Sumbana, J. J. et al. Extraintestinal pathogenic Escherichia coli ST405 isolate coharboring blaNDM-5 and blaCTXM-15: A new threat in Mozambique. Microb. Drug Resist. https://doi.org/10.1089/mdr.2020.0334 (2021).
He, W.-Y. et al. Clonal spread of Escherichia coli O101:H9-ST10 and O101:H9-ST167 strains carrying fosA3 and blaCTX-M-14 among diarrheal calves in a Chinese farm, with Australian Chroicocephalus as the possible origin of E. coli O101:H9-ST10. Zool. Res. 42, 461 (2021).
Li, F. et al. Genetic characterization of carbapenem-resistant Escherichia coli from China, 2015–2017. BMC Microbiol. 21, 1–7 (2021).
Biffignandi, G. B. et al. Genomic characterization of an O101:H9-ST167 NDM-5-producing Escherichia coli strain from a Kitten in Italy. Microbiol. Spectr. https://doi.org/10.1128/spectrum.00832-22 (2022).
Camargo, C. H. et al. Genomic Diversity of NDM-producing Klebsiella species from Brazil, 2013-2022. Antibiotics (Basel). https://doi.org/10.3390/antibiotics11101395 (2022).
Ragupathi, N. K. D. et al. First Indian report on genome-wide comparison of multidrug-resistant Escherichia coli from blood stream infections. PLoS One 15(2), e0220428. https://doi.org/10.1371/journal.pone.0220428 (2020).
Mazumder, R., Abdullah, A., Ahmed, D. & Hussain, A. High prevalence of blaCTX-M-15 gene among extended-spectrum β-lactamase-producing Escherichia coli isolates causing extraintestinal infections in Bangladesh. Antibiotics (Basel). https://doi.org/10.3390/antibiotics9110796 (2020).
Choudhury, N. A., Paul, D., Chakravarty, A., Bhattacharjee, A. & Chanda, D. D. IncX3 plasmid mediated occurrence of bla NDM-4 within Escherichia coli ST448 from India. J. Infect. Public Health 11(1), 111–114. https://doi.org/10.1016/j.jiph.2017.06.008 (2018).
Paul, D., Dhar, D., Chakravarty, A. & Bhattacharjee, A. Transcriptional analysis of IncFrepB-mediated blaOXA-48-positive plasmid characterized from Escherichia coli ST448. Microb. Drug Resist. https://doi.org/10.1089/mdr.2019.0486 (2021).
Mohsin, M. et al. Genomic characterization of high-risk Escherichia coli and Enterobacter hormaechei clones recovered from a single tertiary-care hospital in Pakistan. J. Appl. Microbiol. 132, 3907–3914 (2022).
Schwan, W. R., Flohr, N. L., Multerer, A. R. & Starkey, J. C. GadE regulates fliC gene transcription and motility in Escherichia coli. World J. Clin. Infect. Dis. 10, 14 (2020).
Pankok, F. et al. Epidemiology of plasmids in Escherichia coli and Klebsiella pneumoniae with acquired extended spectrum beta-lactamase genes isolated from chronic wounds in Ghana. Antibiotics 11, 689 (2022).
Tompkins, K. & van Duin, D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur. J. Clin. Microbiol. Infect. Dis. https://doi.org/10.1007/s10096-021-04296-1 (2021).
Ahmed, S. K. et al. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2. https://doi.org/10.1016/j.glmedi.2024.100081 (2024).
Artero, A. et al. Fluoroquinolones are useful as directed treatment for complicated UTI in a setting with a high prevalence of quinolone-resistant microorganisms. Antibiotics 12(1), 183. https://doi.org/10.3390/antibiotics12010183 (2023).
Kumar, G., Kumar, Y., Kumar, G. & Tahlan, A. K. Characterization of uropathogenic E. coli from various geographical locations in India. J. Taibah Univ. Med. Sci. 18(6), 1527–1535. https://doi.org/10.1016/j.jtumed.2023.07.003 (2023).
Rizvi, M. et al. Regional variations in antimicrobial susceptibility of community-acquired uropathogenic Escherichia coli in India: Findings of a multicentric study highlighting the importance of local antibiograms. IJID Regions 11, 100370. https://doi.org/10.1016/j.ijregi.2024.100370 (2024).
Farhan, S. M. et al. In vitro and in vivo effect of amikacin and imipenem combinations against multidrug-resistant E. coli. Trop. Med. Infect. Dis. 7(10), 281. https://doi.org/10.3390/tropicalmed7100281 (2022).
Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 7(3), 252–266. https://doi.org/10.1080/21505594.2016.1159366 (2016).
Khan, A. et al. Multidrug resistance among uropathogenic clonal group A E. Coli isolates from Pakistani women with uncomplicated urinary tract infections. BMC Microbiol. 24(1), 74. https://doi.org/10.1186/s12866-024-03221-8 (2024).
Sivarajan, V. et al. The prevalence of multidrug-resistant Escherichia coli in Chennai and whole genome sequence analysis of carbapenem-resistant Escherichia coli ST410. Indian J. Microbiol. 64(2), 467–474. https://doi.org/10.1007/s12088-023-01125-1 (2024).
Waters, N. R., Abram, F., Brennan, F., Holmes, A. & Pritchard, L. Easy phylotyping of Escherichia coli via the EzClermont web app and command-line tool. Access Microbiol. https://doi.org/10.1099/acmi.0.000143 (2020).
Mohapatra, S. et al. Genome profiling of uropathogenic E. coli from strictly defined community-acquired UTI in paediatric patients: a multicentric study. Antimicrob. Resist. Infect. Control 12 (2023).
Acknowledgements
The authors thank students (Vignesh.S, Karthic. G, Rittwika Banerjee, Janani. B, V.S Ramana Kishore) and PhD scholars (Lakshmi Srijith, Rogith. P). A special mention to V.S Ramana Kishore (SRIHER) for pangenome analysis. The authors also thank Mr. Prabhu (Apollo Diagnostics) for the support provided during the data collection. We thank Sri Ramachandra Institute of Higher Education and Research for providing all the facilities and support to carry out this research work.
Author information
Authors and Affiliations
Contributions
V.S: Writing—original draft, formal analysis, software, Figs. 2, 3; A.G: Writing—original draft, formal analysis, software, Fig. 5A,B; P.S: Writing—original draft, formal analysis, software, Fig. 1, 6A, 6B; P.G: Writing-original draft, formal analysis, software, Figs. 2, 3, 4A,B; S.P—Data collection, software; N.M—formal analysis; software; R.N. S—Review and Editing; Data analysis; M.R—Data analysis, Review and Editing; A.D—Review and Editing, Supervision; F.A—Review and Editing, Supervision; S.W—Review and Editing, Supervision; K.P: Writing—Review and Editing, Supervision.
Corresponding author
Ethics declarations
Competing of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sivarajan, V., Ganesh, A.V., Subramani, P. et al. Prevalence and genomic insights of carbapenem resistant and ESBL producing Multidrug resistant Escherichia coli in urinary tract infections. Sci Rep 15, 2541 (2025). https://doi.org/10.1038/s41598-024-84754-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84754-w