Abstract
Inflammatory diseases in poultry incurs huge economic losses and irrational use of synthetic inflammatory drugs increases the incidence of residues in poultry products. To overcome this, herbal anti-inflammatory remedies are the preferred choice as feed additives. Withania somnifera also called as Asgand Nagori (AGN), Ashwagandha and Indian Winter Cherry is used for its medicinal properties. AGN exhibits phenotypic similarities to Korean Red Ginseng Extract (KRGE) as well as comparable pharmacological effects. Nevertheless, they diverge in terms of their geographical origins, chemical makeup, and production costs. Therefore, the current study was designed to compare the anti-inflammatory activities of KRGE and AGN on chicken macrophages. Chicken peritoneal macrophages were isolated and assigned to seven treatment groups for the study: RPMI-1640 (G1), lipopolysaccharide (LPS) (G2), 0.2 µg/mL dexamethasone + LPS (G3), 100–1000 µg/mL KRGE + LPS (G4–G5), and 100–1000 µg/mL AGN + LPS (G6–G7). Anti-inflammatory properties were evaluated using adherent macrophage phagocytic activity, nitric oxide (NO) production assay, MTTassay, Real Time-PCR for pro-inflammatory mediators and cytokines, Nuclear Factor Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) pathways. Based on the results, AGN and KRGE extracts displayed potent anti-inflammatory activities as evidenced by increasing phagocytic activity, reduced NO production and decreased expressions of pro-inflammatory mediators and cytokines by downregulating the NF-κB and MAPK pathways (p < 0.001 compared with the LPS). Overall, both extracts were found to be efficacious in combating inflammation in avian macrophages.
Similar content being viewed by others
Introduction
Infectious diseases of poultry are a considerable threat to economic stability for commercial and backyard poultry farmers1. A wide range of synthetically available anti-inflammatory, parasitic, antimicrobial, and anti-coccidial drugs are available and can be administered to avian species as therapeutic and prophylactic treatment; however, their residues are present in meat and eggs and ultimately affect the end-user (i.e., humans)2. Poultry meat and eggs are considered a cheap, nutritious, and easily available source of protein throughout the world; in fact, poultry meat will be the most consumed animal meat by 20253. Pakistan ranks 176th out of 204 countries in Antimicrobial Resistance (AMR)-related mortality, with AMR being the third leading cause of death in 2019. The Global Research on Antimicrobial Resistance project reports 59,200 deaths directly caused by AMR and an additional 221,300 associated deaths4. Factors contributing to these high rates include widespread multidrug resistance, poor awareness of drug use, easy access to over-the-counter medications, weak drug regulation, lack of diagnostic facilities, unqualified suppliers, and the common use of antibiotics in livestock5.
Inflammation is the immune system’s natural response to foreign invaders, including microbes. While moderate inflammation helps eliminate pathogens and promotes tissue health, excessive inflammation can lead to tissue damage and diseases. This occurs when there is an imbalance between pro-inflammatory and anti-inflammatory mediators. In cases of heightened inflammation, macrophages release pro-inflammatory mediators, starting inflammatory pathways. For instance, lipopolysaccharides (LPS) from Gram-negative bacteria activate macrophages via Toll-like Receptor 4 (TLR4)6triggering the Nuclear Factor Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) pathways. These pathways increase the production of inflammatory cytokines such as IL-1β, IL-6, TNF-α, and IL-107. Therefore, timely regulation of NF-κB and MAPK activation is crucial for any anti-inflammatory treatments.
While there is a variety of synthetic allopathic anti-inflammatory drugs available for the treatment of inflammation, their side effects over prolonged use cannot be ignored. Therefore, to overcome the adverse effects caused by synthetic anti-inflammatory therapies, novel, affordable and effective alternatives, with minor or no side effects over prolonged use have been explored8. Korean ginseng (Panax ginseng) is a plant native to Korea, northeastern China, Japan, and few areas of Russia9. It has multiple pharmacological properties including anti-inflammatory, anti-cancer10neuroprotective11immunomodulatory12and reproductive system supportive properties13. The ethnopharmacological properties of ginseng are due to the presence of compounds called ‘Ginsenosides’14. Korean red ginseng extract (KRGE) is obtained by steaming and drying the roots of the ginseng. KRGE and its enriched fraction have been reported to have anti-inflammatory activity in a murine sepsis model15allergic rhinitis16and Helicobacter pylori-induced gastric inflammation17.
Asgand/Ashwagandha (Withania somnifera) belongs to the family Solanaceae and is commonly found in Baluchistan and the South Punjab areas of Pakistan. It is also known as Indian winter cherry/Indian ginseng due to its adaptogenic properties, which are comparable to those of Korean ginseng. The pharmacological properties of Asgand are due to the presence of withaferin A (a steroidal lactone), which is abundantly present in the roots18. There are two varieties of this plant: Asgand Nagori (AGN) and Asgand Dhakani19,20. AGN is reported to have adaptogenic21anti-inflammatory22anti-tumor23immune regulatory24,25and fertility-enhancing effects26,27. The anti-inflammatory properties of AGN are well known especially in the case of arthritis for which the effects of its root powder are comparable to those of hydrocortisone sodium succinate treatment28,29.
The rationale of this study is based on the comparison of Korean Red Ginseng Extract (KRGE) and Asgand Nagori (AGN) due to their similar biological and adaptogenic properties, with AGN being a cost-effective and indigenous (native to Sub-continent region) alternative to KRGE. Despite a lack of studies on their effects on chicken macrophages regarding inflammation, we employed cell-mediated immunity, colorimetric assays, and Real-Time PCR to evaluate their anti-inflammatory properties. This research aims to enhance understanding of the anti-inflammatory mechanisms of KRGE and AGN in relation to inflammatory diseases in chickens in Korea and Pakistan.
Materials and methods
Chemicals and reagents
Roswell Park Memorial Institute Medium (RPMI-6504) was obtained from Sigma Aldrich, MA, USA (Cat#R5504), and fetal bovine serum (FBS) was obtained from the Bio West, Nuaillé, France (Cat#S162G). Penicillin-streptomycin (Cat#PSL01) and Amphotericin-B (Cat#ABL01) were purchased from Caisson, Smithfield, Utah, USA. Sephadex G-50 was obtained from Sigma Aldrich, MA, USA (Cat#G5080). TRIzol reagent was procured from Thermofisher Scientific, MA, USA (Cat#15596-026). 6 mm cell culture plates and all necessary plastic ware and glassware were procured from Corning, NY, USA.
Sample procurement and preparation
KRGE was kindly provided by The Korean Society of Ginseng and according to its certificate of analysis, it majorly consists of single ginsenosides i.e. Rg1 + Rb1 + Rg3 not less than 5.50 mg/g of the whole extract. AGN extract was prepared by grinding 50 g of AGN roots into a powder and dissolving in 500mL of ethanol. The mixture was heated at 180 °C for 1 h and then at 130 °C for next 3 h. Nylon filtration and paper filtration method carried out to filter mixture. The ethanol was evaporated using rotary evaporator and extract was lyophilized. The product was analyzed through Gas Chromatography Mass Spectrometry (GC-MS) as described previously30 and shown in Fig. 1; Table 1 and stored at − 20 °C until further use.
Bird’s procurement
For the purpose of macrophage isolation, a total of six adult broiler chickens (Ross-300), 5 weeks old, weighing around 1500 g, disease free, healthy and properly immunized were procured from a commercial farm and provided with broiler finishing pelleted feed containing 3200 kcal/kg metabolizable energy and around 20% crude protein and water ad libitum. The standard housing facility consisted of 16/8 hr of light and dark cycle and temperature of 22 ± 1 °C. For the hyper-immune serum preparation, a total of three adult Rhode Island Red (RIR) chickens, weighing around 1200 g, aged 30 weeks, disease free, non-GMO and properly vaccinated were procured from a commercial farm and provided with laying pelleted feed containing 2900 Kcal/kg metabolizable energy and around 19% crude protein and water ad libitum. The housing conditions were same as for broilers. All the birds were acclimatized for 1 week to the housing conditions prior to the experiments. All experimental procedures were conducted following the ARRIVE guidelines, and approval was obtained from the Institutional Ethics Committee at PMAS-Arid Agriculture University Rawalpindi (Ethical approval no. PMAS-AAUR/IEC/370). All procedures were executed in compliance with the institutional applicable standards.
Preparation of sheep red blood cells (SRBCs)
Sheep blood (around 2mL) was collected through the jugular vein from the university experimental animals’ farm and centrifuged at 5000 rpm for 10 min. After the removal of plasma and buffy coat, 0.9% NaCl (Normal Saline) was added to RBC pellet. Three times washing was done to obtain a purified pellet. SRBC pellet was resuspended in 1mL saline solution and counting of SRBCs/mL of blood was done using the modified Neubauer’s chamber.
Preparation of hyper immune anti-serum
Hyper immune anti-serum was prepared according to the procedure described by31,32. Briefly, a calculated dose of 0.1% SRBCs (0.2 mL) was injected into the wings of the chickens on days 0, 3, 7, 14, and 21. Seven days after the last injection, blood was collected and allowed to clot. After the clot was dislodged (approx. 15 min), it was refrigerated for approximately 10 min. For serum separation, the sample was centrifuged for 15 min at 1500 rpm. The prepared serum was then stored at -80ºC until further use.
Isolation of peritoneal macrophages by the abdominal exudate collection (AEC) method
Macrophage isolation was done by Abdominal Exudate Cell collection method (AEC) according to previously reported literature32,33. Briefly, 3% solution of Sephadex G-50 granules was prepared in Normal Saline (0.9% NaCl) and soaked for about 1 h. Next, 6 broiler birds were carefully injected with Sephadex (10 ml/bird) intraperitoneally. After 42–48 h of Sephadex stimulation, birds were euthanized with CO2 (> 50%) in a specialized euthanizing chamber attached with CO2 gas cylinder in accordance with the American Veterinary Medical Association (AVMA) guidelines for euthanasia in animals, 2020 edition and abdominal exudate by normal saline solution flushing was carried out. Macrophages exudate was collected from each bird and pooled in the 15mL falcon tubes and centrifuged at 15,000 rpm for 15 min. After centrifugation, the supernatant was removed and macrophage pellet was washed twice with the RPMI-1640 media without supplements. Thereafter, macrophage number was counted using the modified Neubauer’s chamber. After counting, the cells were suspended in 6 cm cell culture dishes and given treatments according to the treatment regimen given in Table 2.
Preparation of opsonized SRBCs
To prepare opsonized SRBCs, the RBCs of sheep were washed for four times with PBS and then mixed with 4HA unit serum according to the procedure described by32.
Adherent AEC phagocytic activity
Macrophages were adhered to 6-mm round coverslips in the culture media, and 10 µL of opsonized SRBCs was added over the cover slips for macrophage engulfment. Adherent phagocytic activity was determined according to the previously reported literature32.
Cellular metabolism/viability (MTT) assay
A cell viability assay was performed to access the cytotoxicity in cells posed by treatment groups outlined in Table 2, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent which was added to culture medium at a final concentration of 0.1 mg/mL as per the procedure described by34.
Nitric oxide (NO) production assay
The chicken macrophages were treated with/without the samples as outlined in Table 2, and nitrite levels were measured in 48-well micro titer plates according to the procedure described by34. Briefly, Macrophages were plated in 48-well plates and exposed to LPS with varying concentrations of KRGE and AGN, followed by overnight incubation. The next day, culture supernatants were mixed with Griess reagent, and absorbance was measured at 540 nm using a spectrophotometer.
RNA extraction and real-time PCR for pro-inflammatory mediators, cytokines, and
Inflammatory pathways
Total RNA was extracted from macrophages using the Trizol reagent method and reverse transcribed with the Revert Aid First Strand cDNA synthesis kit (Thermofisher). The expression of pro-inflammatory mediators and cytokines was measured by Real-Time PCR with SYBR Green PCR Master Mix (Thermofisher)34 using primers listed in Table 3 according to the previous literature35,36,37 with GAPDH as an internal control. Gene expressions were calculated using the ΔΔCT method.
Statistical analysis
One-way Analysis of Variance (ANOVA) was used to analyze the statistical significance using GraphPad Prism software version 8.4.3. Values in the bar graphs are the means ± Standard Error of the Mean (SEM) of at least 3 independent experiments and ***p < 0.001, **p < 0.005, *p < 0.01 represent significance compared with LPS group.
Results
Effects of KRGE and AGN on the phagocytic activity of adherent abdominal exudate cells
The phagocytic activity of the macrophages was determined in the opsonized and un-opsonized groups (Fig. 2a). The macrophage engulfment was enhanced with the all the treatments in the opsonized and un-opsonized groups. Treatment with 1000 µg/mL KRGE and 1000 µg/mL AGN led to the maximum phagocytic activity as compared with the other treatment groups (Fig. 2b–c).
Adherent Macrophage phagocytic activity by KRGE and AGN. (a) Microscopic pictures of phagocytic activity performed by all treatment groups as examined by an inverted light microscope at 100× using oil immersion. (b and c) Quantification of opsonized and un-opsonized particles by KRGE and AGN. Arrows represent engulfed SRBCs by macrophages. The bar graph values present the mean ± Standard error of the mean (SEM) of at least 3 independent experiments (n = 3) and ***p < 0.001, **p < 0.005, *p < 0.01 represent significance compared with LPS group.
Reduced NO production by KRGE and AGN without cytotoxicity
In LPS-induced cells, the NO levels were significantly higher than those in the control group, and treatment of the cells with dexamethasone and both doses of KRGE and AGN reduced NO levels. Notably, both the highest doses of the extracts maximally inhibited NO production (Fig. 3a) without any cytotoxicity (Fig. 3b).
NO and MTT assay for KRGE and AGN on chicken macrophages. (a) NO production by all treatment groups. (b) MTT assay for all treatment groups. The bar graph values present the mean ± Standard error of the mean (SEM) of at least 3 independent experiments (n = 3) and ***p < 0.001, **p < 0.005, *p < 0.01 represent significance compared with LPS group.
Gene expression of pro-inflammatory mediators and cytokines via real-time PCR
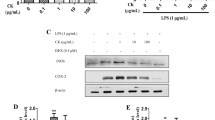
The expression levels of pro-inflammatory mediators and cytokines increase in inflammatory reactions. Therefore, we evaluated whether KRGE and AGN could reduce their levels. KRGE (100–1000 µg/mL) and AGN (100–1000 µg/mL) dose-dependently inhibited the expression of iNOS and COX-2 (Fig. 4a) (pro-inflammatory mediators) as well as Tumor Necrosis Factor (TNF)-α, (Interleukin (IL)-1β, and IL-6 (Fig. 4b) (pro-inflammatory cytokines). On the other hand, in the LPS group, significant upregulation of pro-inflammatory mediators and cytokines was observed when compared with the control group.
Expression of Pro-inflammatory mediators and cytokines by KRGE and AGN on chicken macrophages. (a) KRGE and AGN reduced the levels of pro-inflammatory mediators i.e. iNOS and COX-2. (b) Suppression in the levels of pro-inflammatory cytokines by KRGE and AGN. The bar graph values present the mean ± Standard Error of the Mean (SEM) of at least 3 independent experiments (n = 3) and ***p < 0.001, **p < 0.005, *p < 0.01 represent significance compared with LPSgroup.
Inhibition of the NF-κB and MAPK pathways by KRGE and AGN
To elucidate the pathways affected by KRGE and AGN as anti-inflammatory agents, we evaluated the effects of both extracts on the classical inflammatory pathways of NF-κB and MAPK. Both KRGE and AGN inhibited the NF-κB pathways (Fig. 5a) and ERK, JNK, P38α, P38-2β, and P38-γ factors in the MAPK pathway (Fig. 5b), reducing the inflammation.
Effects of KRGE and AGN on the gene expression of inflammatory pathways. (a) NF-κB suppression by KRGE and AGN and (b) MAPK pathway inhibition by KRGE and AGN. The bar graph values present the mean ± standard error of the mean (SEM) of at least 3 independent experiments (n = 3) and ***p < 0.001, **p < 0.005, *p < 0.01 represent significance compared with LPS group.
Discussion
In avian species, herbal extracts increase their appetite and feed intake, boost digestive enzyme production, improve immunity, and have anti-inflammatory, anti-bacterial, anthelminthic, coccidiostatic and anti-oxidant properties38. The most commonly used herbal feed additives include cinnamon, cumin, capsicum, pepper, ginger, mustard, turmeric, fenugreek, celery, parsley, oregano, mint, thyme, mustard, cloves, rosemary, and many more39.
In our study, we found that treatment with the high doses of KRGE and AGN led to maximum engulfment of opsonized and un-opsonized particles (SRBCs). In general, chicken macrophages express receptors for Fc and C3b, which makes them capable of engulfing both opsonized and un-opsonized particles40,41. Therefore, we hypothesize that KRGE and AGN enhanced the phagocytosing capability of chicken macrophages via increasing the sensitivity to Fc and C3b receptors. This result is supported by the findings of study in which glycyrrhizic acid was shown to increase the phagocytic activity of chicken macrophages from Salmonella spp. Infection42. Furthermore, polysaccharides from Platycodon grandifloras (Jacq.) and Astragalus aboriginum Richardson have also been reported to enhance the phagocytic activity of peritoneal macrophages from chickens43,44.
LPS is a vital component of Gram-negative bacterial cell walls that activates inflammation. NF-κB is a nuclear factor, and during the conversion of monocytes to macrophages, NF-κB accumulates in the cytoplasm, thereby increasing the LPS sensitivity by priming the cells and increasing the secretion of a series of pro-inflammatory mediators and cytokines such as TNF-α45. Many studies have shown that LPS is the main player in the induction of multiple inflammatory diseases. Therefore, LPS-induced inflammation in chickens is considered to be an ideal model for systemic inflammatory diseases46. Likewise, in our study, we found that both doses of KRG and AGN significantly reduced LPS induced NO production without posing cytotoxicity to chicken macrophages, indicating the alleviation of inflammation. These results are supported by a previous research which showed that garlic, onion, and purple sweet potato extracts dose-dependently decreased NO production in chicken peritoneal macrophages47. Furthermore, previous literature on Rg3-enriched Korean Red Ginseng Extract15 and an ayurvedic poly-herbal formulation containing Withania somnifera, Boswellia serrata, Zingiber officinale, and Curcuma longa48, showed potently suppressed LPS-induced NO in murine macrophages.
Activation of macrophages as a result of LPS leads to the production of pro-inflammatory mediators (iNOS & COX-2) and cytokines (IL-6, IL-1β and TNF-α) that activate the NF-κB pathway. Both KRGE and AGN decreased the levels of pro-inflammatory mediators and cytokines in a dose-dependent manner, indicating protection against LPS-induced damage. These results are also in accordance with a previously reported study in which N-Acetyl-L-cysteine reduced the levels of pro-inflammatory mediators and cytokines against cadmium- induced cytotoxicity and LPS-induced inflammation in chicken peritoneal macrophages49.
TLRs belong to membrane receptor family that recognizes specific microbial molecular patterns of foreign invaders; thus, they are also called pattern recognition receptors (PRRs). Upon recognizing foreign invaders, these PRRs activate inflammation through IKB kinase (IKK) and NF-κB, which constitute the canonical inflammatory pathway. This inflammatory pathway has critical implications in the pathogenesis of persistent inflammatory diseases including asthma, inflammatory bowel disease, rheumatoid arthritis, and chronic obstructive pulmonary disease50,51. Therefore, development of anti-inflammatory drugs targeting NF-κB pathway holds prime importance and attention. In this regard, in our study, we used LPS as an activator of TLR4 to activate NF-κB and initiate inflammation. Both doses of KRGE and AGN inhibited NF-κB expression in chicken peritoneal macrophages. This result is also in accordance with previously reported data that showed that baicalin reduced LPS-induced liver inflammation through downregulation of the TLR4-mediated NF-κB pathway52.
MAPK pathway is also the most extensively investigated intracellular signaling pathway that is activated by LPS. It consists of three major components: extracellular signal-regulated kinases 1/2 (ERK 1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK, which upon activation release proinflammatory cytokines53. In our study, both KRGE and AGN decreased the expression levels of ERK, JNK and P38 subunits, thereby acting as MAPK inhibitors and broad anti-inflammatory agents. Consistent with our results, dietary leonurine hydrochloride has also been reported to attenuate LPS-induced intestinal inflammation in broilers through the inhibition of the NF-κB/MAPK pathways54.
Interestingly, the results of GC-MS analysis for AGN revealed relatively high abundance of two active chemical compounds: 2-methyl-5-(1-methylethyl)-phenol, which is also known as carvacrol and cinnamaldehyde. Carvacrol is a phenolic monoterpene that has been reported for reducing inflammation in various mouse models through the NF-κB and MAPK pathways55. Likewise, cinnamaldehyde has also been extensively reported for its anti-inflammatory effects, especially in broilers56. Therefore, we hypothesize that the anti-inflammatory effects of AGN can be elucidated as a result of the potent activity of these isolated single compounds.
In conclusion, as shown in Fig. 6, this is the first study to elucidate the anti-inflammatory effects of KRGE and AGN on chicken peritoneal macrophages. Both extracts increased the macrophage phagocytic activity for opsonized and un-opsonized particles, reduced LPS-induced NO production as well as pro-inflammatory mediator and cytokine levels through the NF-κB and MAPK pathways.
This study has limitations, including the lack of humoral immunity assessment and in vivo inflammation trials due to budget constraints. However, the findings suggest potential applications of AGN extracts as an anti-inflammatory supplement in poultry diets to mitigate inflammation from production challenges or disease-related stress.
Data availability
The main manuscript file contains all data relevant to the study.
References
Grace, D., Knight-Jones, T. J. D., Melaku, A., Alders, R. & Jemberu, W. T. The public health importance and management of infectious poultry diseases in smallholder systems in Africa. Foods 13, 411 (2024).
Rana, M. S., Lee, S. Y., Kang, H. J. & Hur, S. J. Reducing veterinary drug residues in animal products: A review. Food Sci. Anim. Resour. 39, 687–703 (2019).
Henkuk, Y. L. Global poultry production and it’s substantial contribution to nutrition, food security and poverty alleviation in many developing countries. J Vet. Sci. Med. Diag. 7 (2018).
Qamar, M. U. Exploring one health approach to AMR in Pakistan. Microcosm Am. Soc. Microbiol. 2023, 23–26 (2023).
Chandrakar, C., Shakya, S., Patyal, A., Bhonsle, D. & Pandey, A. K. Detection of antibiotic residues in chicken meat from different agro-climatic zones of chhattisgarh, India by HPLC-PDA and human exposure assessment and risk characterization. Food Control. 148, 109667 (2023).
Fu, Y-J. et al. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pahrmacol Sin. 42, 88–96 (2021).
Yan, Z. et al. Phenethylferulate as a natural inhibitor of inflammation in LPS-stimulated RAW 264.7 macrophages: Focus on NF-B, Akt and MAPK signaling pathways. BMC Complement. Med. Ther. 23, 398 (2023).
Ayroldi, E. et al. Mechanisms of the anti-inflammatory effects of glucocorticoids: Genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 26, 4805–4820 (2012).
Zhang, H. et al. Characteristics of Panax ginseng cultivars in Korea and China. Molecules 25, 2635 (2020).
Li, X. et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 203, 112627 (2020).
Huang, X., Li, N., Pu, Y., Zhang, T. & Wang, B. Neuroprotective effects of ginseng phytochemicals: Recent perspectives. Molecules 24, 2939 (2019).
Riaz, M. et al. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci. Techonol. 83, 12–30 (2019).
Yang, W. M. et al. Effects of Panax ginseng on glial cell-derived neurotrophic factor (GDNF) expression and spermatogenesis in rats. Phytother Res. 25, 308–311 (2011).
Guo, N., Zhu, L., Song, J. & Dou, D. A new simple and fast approach to analyze chemical composition on white, red, and black ginseng. Ind. Crops Prod. 134, 185–194 (2019).
Saba, E. et al. Anti-inflammatory activity of Rg3-enriched korean red ginseng extract in murine model of sepsis. Evid. Based Complement Alternat. Med. 6874692 (2018).
Jung, J. H. et al. The effect of Korean red ginseng on symptoms and inflammation in patients with allergic rhinitis. Ear Nose Throat J. 100, 712S–719S (2021).
Bae, M. et al. Protective effect of Korean red ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils. J. Ginseng Res. 38, 8–15 (2014).
Trivedi, M. K., Mondal, S. C., Gangwar, M. & Jana, S. Effect of a novel ashwagandha-based herbomineral formulation on pro-inflammatory cytokines expression in mouse splenocyte cells: A potential Immunomodulator. Pharmacogn. Mag. 13, S90–S94 (2017).
Saiyed, A., Jahan, N., Majeedi, S. F. & Roqaiya, M. Medicinal properties, phytochemistry and pharmacology of Withania somnifera: an important drug of Unani medicine. J. Sci. Innov. Res. 5, 156–160 (2016).
Khan, A. W. et al. An updated list of neuromedicinal plants of Pakistan, their uses, and phytochemistry. Evid. Based Complement Alternat. Med. 6191505 (2019).
Chandrasekhar, K., Kapoor, J. & Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 34, 255–262 (2012).
Chandra, S., Chatterjee, P., Dey, P. & Bhattacharya, S. Evaluation of anti-inflammatory effect of ashwagandha: A preliminary study in vitro. Pharmacogn. J. 4, 47–49 (2012).
Singh, N., Verma, P., Pandey, B. & Gilca, M. Role of Withania somnifera in prevention and treatment of cancer: an overview. Int. J. Pharm. Sci. Drug Res. 3, 274–279 (2011).
Chattopadhyay, S. & Cone, R. E. Role of ashwangandha (Withania somnifera) in immune modulation: Proposed influencein immune-Regulation. SoM Articles 20, (2007). digitalcommons.lib.uconn.edu.
Tharakan, A. et al. Immunomodulatory effect of Withania somnifera (ashwagandha) extract-a randomized, double-blind, placebo controlled trial with an open label extension on healthy participants. J. Clin. Med. 10, 3644 (2021).
Nasimi Doost Azgomi, R. et al. Effects of Withania somnifera on reproductive system: A systematic review of the available evidence. Biomed. Res. Int. 4076430 (2018).
Sengupta, P. et al. Role of Withania somnifera (ashwagandha) in the management of male infertility. Reprod. Biomed. Online 36, 311–326 (2018).
Tiwari, R., Chakraborty, S., Saminathan, M., Dhama, K. & Singh, S. V. Ashwagandha (Withania somnifera): Role in safeguarding health, Immunomodulatory effects, combating infections and therapeutic applications: A review. J. Biol. Sci. 14, 77 (2014).
Yatoo, M. I. et al. Anti-Inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—a review. Recent. Pat. Inflamm. Allergy Drug Discov. 12, 39–58 (2018).
Akram, A. W., Saba, E. & Rhee, M. H. Antiplatelet and antithrombotic activities of lespedeza cuneata via pharmacological inhibition of integrin alphaiibbeta3, MAPK, and PI3K/AKT pathways and FeCl3-induced murine thrombosis. Evid. Based Complement Alternat. Med. 9927160 (2024).
Muhammad, K., Spier, R. E. & Eales, L. J. In vitro studies of phagocytic activity of chicken peritoneal macrophages. Stud. Res. Vet. Med. 2, 91–96 (1994).
Sandhu, M. A., Rahman, Z. U. & Rahman, S. U. Dynamics of macrophages in laying hens during second and third production cycles after zinc induced molting. J. Poult. Sci. 43, 286–295 (2006).
Guo, Y., Ali, R. A. & Qureshi, M. A. The influence of beta-glucan on immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 25, 461–472 (2003).
Saba, E. et al. Mediation of antiinflammatory effects of Rg3-enriched red ginseng extract from Korean red ginseng via retinoid X receptor alpha-peroxisome-proliferating receptor gamma nuclear receptors. J. Ginseng Res. 43, 442–451 (2019).
Jiang, Z-H. et al. SelW protects against H2O2-induced liver injury in chickens via inhibiting inflammation and apoptosis. RSC Adv. 7, 15158–15167 (2017).
Troung, A. D. et al. Analysis of MAPK signaling pathway genes in the intestinal mucosal layer of necrotic enteritis afflicted two inbred chicken lines. Korean J. Poult. Sci. 44, 199–209 (2017).
Xing, Z. & Schat, K. A. Expression of cytokine genes in mareck’s disease virus-infected chickens and chicken embryo fibroblast cultures. Immunology 100, 70–76 (2000).
Abd El-Hack, M. E. et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult. Sci. 101, 101696 (2022).
Suganya, T. et al. Herbal feed additives in poultry. Int. J. Sci. Environ. Techonology. 5, 1137–1145 (2016).
Duncan, R. L. Jr. & McArthur, W. P. Partial characterization and the distribution of chicken mononuclear cells bearing the Fc receptor. J. Immunol. 120, 1014–1020 (1978).
Qureshi, M. A. Avian macrophage and immune response: An overview. Poult. Sci. 82, 691–698 (2003).
Wang, B. K. et al. Glycyrrhizic acid activates chicken macrophages and enhances their salmonella-killing capacity in vitro. J.Zhejiang Univ. Sci. B 19, 785–795 (2018).
Fan, W. et al. Structure characterization of three polysaccharides and a comparative study of their Immunomodulatory activities on chicken macrophage. Carbohydr. Polym. 153, 631–640 (2016).
Zheng, P. et al. Characterization of polysaccharides extracted from Platycodon grandiflorus (Jacq.) A.DC. Affecting activation of chicken peritoneal macrophages. Int. J. Biol. Macromol. 96, 775–785 (2017).
Takashiba, S. et al. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappab. Infect. Immun. 67, 5573–5578 (1999).
Munyaka, P. M. et al. Immunomodulation in young laying hens by dietary folic acid and acute immune responses after challenge with Escherichia coli lipopolysaccharide. Poult. Sci. 91, 2454–2463 (2012).
Hanieh, H. et al. Immunomodulatory effects of alliums and Ipomoea batata extracts on lymphocytes and macrophages functions in white leghorn chickens: In vitro study. Anim. Sci. J. 83, 68–76 (2012).
Dey, D., Chaskar, S., Athavale, N. & Chitre, D. Inhibition of LPS-induced TNF-alpha and NO production in mouse macrophage and inflammatory response in rat animal models by a novel ayurvedic formulation, BV-9238. Phytother. Res. 28, 1479–1485 (2014).
Zhang, D. et al. Antagonistic effect of N-acetyl-L-cysteine against cadmium-induced cytotoxicity and abnormal immune response on chicken peritoneal macrophages. Ecotoxicol. Environ. Saf. 206, 111185 (2020).
Karin, M., Yamamoto, Y. & Wang, Q. M. The IKK NF-kappa B system: A treasure trove for drug development. Nat. Rev. Drug Discov. 3, 17–26 (2004).
Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect. Biol. 1, a001651 (2009).
Cheng, P. et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-kappaB pathway. Front. Pharmacol. 8, 547 (2017).
Zong, Y. et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One 7, e44107 (2012).
Yang, L. et al. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-kappaB/MAPK signaling pathway in broilers. J. Anim. Sci. 97, 1679–1692 (2019).
Yan, C. et al. Carvacrol protects mice against LPS-induced sepsis and attenuates inflammatory response in macrophages by modulating the ERK1/2 pathway. Sci. Rep. 13, 12809 (2023).
Kang, H. et al. Validating the use of a newly developed cinnamaldehyde product in commercial broiler production. Poult. Sci. 103, 103625 (2024).
Acknowledgements
This research was funded by The Korean Society of Ginseng, grant number GS-302-168 and the Korean government funded grant (MSIT) from National Research Foundation of Korea (NRF) (No. 2022R1A2C1012963).
Author information
Authors and Affiliations
Contributions
S.E., S.M.A.., Y.A.., and R.M.H. conceptualized, supervised and funded the study. S.E., T.T., R. A., K.A., A.A.W., N.G., A.S., M.U.D.M., K.I.A.., and H.S.M. did the experiments, investigation and formal writing. All authors have read and agreed to the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tariq, T., Yousaf, A., Sandhu, M.A. et al. A comprehensive guide on the anti-inflammatory activities of Asgand Nagori (Withania somnifera) with special reference to cinnamaldehyde and carvacrol. Sci Rep 15, 23243 (2025). https://doi.org/10.1038/s41598-025-05998-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05998-8