Abstract
This study aimed to investigate whether driving pressure-guided ventilation can reduce postoperative pulmonary complications in patients who have undergone heart transplantation. Patients who underwent orthotopic heart transplantation were divided into two groups according to the perioperative ventilation strategy: (1) conventional lung-protective ventilation (group C) and (2) driving pressure-guided ventilation (group D). The primary outcome was the occurrence of postoperative pulmonary complications within 30 days of surgery. Univariate and multivariate logistic regression analyses were performed to evaluate the independent risk factors associated with postoperative pulmonary complications (PPCs). Compared with group C, patients in group D exhibited lower driving pressure. Oxygenation improved significantly in the early period after surgery in patients in group D. Group C exhibited a higher number of patients with postoperative pulmonary complications, especially respiratory infections and atelectasis. Patients in group D experienced a shorter duration of postoperative mechanical ventilation and a shorter stay in the intensive care unit. The conventional ventilation strategy, the high driving pressure level and the low PaO2 value at the end of the surgery were the independent risk factors for PPCs in heart transplantation. Compared with conventional lung-protective ventilation, driving pressure-guided ventilation was associated with improved pulmonary oxygenation and lower incidences of pulmonary complications among patients after heart transplantation.
Similar content being viewed by others
Introduction
Postoperative pulmonary complications (PPCs) are a spectrum of new-onset respiratory adverse events occurring within 5 to 7 days after surgery1,2. These complications contribute to a 30-day mortality of 20–44% after cardiac surgery3,4,5,6. Driving pressure (ΔP), defined as the difference between plateau airway pressure and positive end-expiratory pressure (PEEP), was first introduced by Amato et al. in 2015 in their meta-analysis study on acute respiratory distress syndrome7. This parameter was most strongly associated with survival among various ventilation parameters. A lower ΔP has been verified to be closely relative to an ameliorative prognosis after surgery8,9. However, controversy persists regarding whether ΔP-guided ventilation can decrease the incidence of PPCs10,11,12.
In patients with end-stage heart failure, persistent positive intrathoracic pressure has the potential to reduce venous return and increase pulmonary vascular resistance, leading to hypotension. Consequently, implementing the PEEP setting, a crucial ventilation parameter in ΔP-guided ventilation, may be challenging. In addition, elevation of the right ventricular afterload due to hypopnea-induced hypercapnia poses significant challenges to anesthesiologists during perioperative hemodynamic management.
Given the need for additional evidence to confirm the relationship between ΔP and PPCs, this study aimed to assess the efficacy and safety of the ΔP-guided ventilation strategy in preventing PPCs in heart transplantation.
Methods
Consecutive patients with end-stage heart failure who underwent orthotopic heart transplantation at our center were included in this prospective observational cohort study. The exclusion criteria were: (1) being younger than 18 years; (2) sepsis within twelve weeks of surgery; (3) obstructive sleep apnoea syndrome requiring long-term ventilation assistance; (4) advanced-stage myasthenia gravis; (5) a body mass index > 30 kg·m-2; (6) reluctance to participate in the study; (7) mortality during surgery; (8) alteration of the initial ventilation strategy during surgery for reasons such as refractory hypoxemia (arterial oxygen saturation < 88% despite 100% oxygen inhalation), interference with the surgical field exposure, intolerable hypercapnia, or ventilation-related hemodynamic instability; and (9) attainment of the expected sample size. All the enrolled patients were divided into two groups at a 1:1 ratio according to the intraoperative ventilation strategy employed, with group C receiving conventional lung-protective ventilation and group D receiving driving pressure-guided ventilation. All clinical data, including baseline characteristics, intraoperative ventilation parameters, surgical information, incidence of PPCs, incidence of postoperative adverse cardiovascular events and all-cause death, length of stay in the intensive care unit (LOS-ICU), ventilation assistance time, and incidences of postoperative extrapulmonary complications were collected via the Hospital Information System and Anesthesia Information Management System. Definitions of the perioperative variables are provided in the Supplementary Data.
All patients received a standardized general anesthesia from anesthesiologists with at least 10 years of experience in cardiovascular anesthesia. A continuous pulse oximeter (BSA09001P, Berry Electronic Technology, China) measured SpO2. Catheterization was routinely performed in the peripheral, central venous, and pulmonary arteries for invasive hemodynamic monitoring (Vigilance Cell; Edwards Lifesciences Corporation, USA). The core body temperature of each patient was monitored with a nasopharyngeal detector (MR411, Mindray Biomedical Electronics Co., Ltd., Germany). Myocardial ischemia and malignant arrhythmia were routinely monitored using a 12-lead electrocardiogram. Transesophageal echocardiography (EPIQ 7 C; Philips Ultrasound, Inc., USA) was performed on all patients to evaluate their cardiac function and volume status. During the induction period, the dosages of sufentanil, etomidate, and rocuronium were 1.5 to 2.5 µg·kg-1, 0.2 to 0.6 mg·kg-1 and 0.6 to 1.2 mg·kg-1 respectively and dexmedetomidine (0.2 to 0.4 µg·kg-1·h-1), sufentanil (1.0 to 1.5 µg/kg intermittent injection), rocuronium (0.3 to 0.6 mg·kg-1·h-1) and propofol (2.0 to 4.0 mg·kg-1·h-1) were regularly administered for anaesthetic maintenance.
The application of inotropic and vasoactive agents was initiated after rewarming, with the recommended dosage regimens being as follows: dopamine 3.0 to 5.0 µg·kg-1·min-1, dobutamine 3.0 to 5.0 µg·kg-1·min-1, epinephrine 0.04 to 0.1 µg·kg-1·min-1, milrinone 0.4 to 1.0 µg·kg-1·min-1, treprostinil 0.625 to 1.25 ng·kg-1·min-1, and nitric oxide inhaled at a concentration of 15 to 30 ppm.
A similar mechanical ventilation mode and target were used in all patients until extubation (ZEUS IE; Draeger Medical Systems, Inc., USA) (Table 1). There were two different PEEP setting rules in our center, plan A was the lowest driving pressure: a 10-cycle experimental ventilation was carried out at each level of PEEP after intubation, and the ΔP of the last cycle was recorded. The PEEP value corresponding to the lowest ΔP was recognized as the optimal ventilation parameter. This parameter was subject to modification upon cessation of ventilation, ICU admission, and every morning throughout the ventilation period. Plan B was to maintain PEEP at the level facilitating optimal oxygenation during the off-pump period. The cardiovascular anesthesiologists chose the PEEP setting plans freely, and the patients receiving plan A were classified into group D, and those receiving plan B were enrolled into group C. Both of the ventilation strategies were acceptable to all of the adult patients in our center and were performed by anesthesiologists according to their specialities and preferences.
The times for the alveolar recruitment maneuvers were as follows: (1) after intubation; (2) at any time after the ventilation pause; (3) before cardiopulmonary bypass withdrawal; (4) before sternal closure; and (5) when hypoxemia occurred for > 10 s. Partial pressure of carbon dioxide (PaCO2) monitoring was employed to determine the tidal volume and respiratory rate. Inspiration/expiration pattern was adjusted based on the preoperative small airway condition. During cardiopulmonary bypass surgery, mechanical ventilation was maintained using the low-level parameters described in Table 1.
Intraoperative ventilation parameters, including ΔP (cm H2O) (plateau airway pressure minus PEEP), PEEP (cm H2O), tidal volume (mL), dynamic respiratory system compliance (CRS) (calculated as the tidal volume (mL)/ΔP (cm H2O), mL/cm H2O)13, SpO2 (%), and the end-tidal carbon dioxide (mm Hg), were measured at two time points: 15 min after intubation and the end of the operation.
Management of hypoxemia (supplementary data) was initiated immediately through the following steps: (1) carefully checking anesthesia apparatus malfunction, airway normality, and monitoring accuracy; (2) improving cardiac function, correcting fluid overload, and alleviating systemic inflammation; (3) performing alveolar recruitment maneuvers as described above; (4) increasing the tidal volume and PEEP within the upper limits, which were introduced in Table 1; (5) increasing the respiratory rate while addressing concurrent hypercapnia; (6) titrating the fraction of inspiratory oxygen until the SpO2 reaches or exceeds 90%; and (7) considering the use of extracorporeal membrane oxygenation if any following situations occurred14: (a) a PaO2/FiO2 < 50 mm Hg for more than 3 h; (b) a PaO2/FiO2 of < 80 mm Hg for more than 6 h; or (c) a critical respiratory acidosis (pH < 7.25 and PaCO2 ≥ 60 mm Hg) for more than 6 h.
The primary outcome was the occurrence of new-onset PPCs within 30 days of surgery. PPCs were defined as any postoperative respiratory system complication that occurred from admission to the ICU to 30 days post-surgery, encompassing (1) respiratory infection, (2) respiratory failure, (3) bronchospasm, (4) atelectasis, (5) pleural effusion, (6) pneumothorax, and (7) aspiration pneumonitis, as delineated in previously published literature15.
Secondary outcomes included the perioperative PaO2/FiO2, early/late death, vasoactive-inotropic score at the end of surgery, postoperative adverse cardiovascular events, LOS-ICU, ventilation assistance time, and postoperative extrapulmonary complications. GEM Premier 3500 (Wolfen Scientific, Inc., USA) was applied for arterial blood gas analyses, with the time points: T1 (before surgery), T2 (after intubation), T3 (withdrawal from cardiopulmonary bypass), T4 (end of surgery), T5 (postoperative day 1), T6 (postoperative day 3), T7 (postoperative day 5), and T8 (postoperative day 7). The average values of multiple blood gas measurements were used for statistical analysis. A systolic pressure < 80% of baseline or a mean blood pressure < 65 mmHg during ventilation was recognized as ventilation-related hypotension, excluding other risk factors for hemodynamic instability, such as low cardiac output syndrome, surgical procedures, anaphylaxis, pharmacological action, or massive hemorrhage. The vasoactive-inotropic score is the sum of the dosages of frequently used vasoactive or inotropic agents according to their weighted values16. The postoperative adverse cardiovascular events included new-onset lethal arrhythmias (supraventricular/ventricular tachycardia, ventricular fibrillation, or Adams-Stokes syndrome), acute myocardial infarction, cardiogenic shock, and thrombotic or embolic events. Early death was defined as any death occurring within 7 days of surgery, while late death was considered for deaths occurring within 30 days after the surgery was scheduled. Postoperative extrapulmonary complications included tracheotomy, rethoracotomy for exploration, wound infection, sepsis, gastrointestinal hemorrhage, and neurological complications (such as delayed recovery, delirium, cognitive dysfunction, coma, new-onset stroke, and syncope).
SPSS 25.0 (SPSS, Inc., USA), GraphPad Prism 8.0 (GraphPad Software, USA), and PASS 15.0 (NCSS, LLC, USA) were utilized in statistical analyses. Categorical variables, such as the baseline characteristics in each study group, were presented as proportions and were compared using the chi-square test or Fisher’s exact test. Continuous variables were presented as means ± standard deviations (normal distribution) or medians and interquartile ranges (abnormal distribution). They were compared using the Student’s t-test (normal distribution) or Wilcoxon test (abnormal distribution). Repeated-measures analysis of variance was applied to assess the differences in the perioperative PaO2/FiO2 and PaCO2 between patients in the two groups. Univariate and multivariate logistic regression were performed in the risk factor analysis for PPCs. Previous studies revealed that the morbidities of PPCs in cardiac surgery were reduced to 55%17with the PLV strategy, and the incidence under conventional ventilation was as high as 88%18. We calculated that the study required a minimum of 44 cases in each group (two-sided α error level of P < 0.05, β error level = 0.1, dropout rate = 0.1). Enrolment was automatically stopped when the sample size reached 44 patients in each group. Statistically significant was defined as P < 0.05.
This prospective study followed the Declaration of Helsinki and was approved by the local ethics committee at Fujian Medical University Union Hospital (2022YF022-01), and all participants provided written informed consent. All organs were procured via Organ Procurement Organizations in Fujian medical university union hospital. All methods were performed in accordance with the relevant guidelines and regulations.
Results
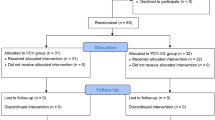
Between March 2019 and March 2023, 98 consecutive patients who underwent orthotopic heart transplantation at our center were screened for this study. The following cases were excluded: six patients were automatically excluded as enrolment reached the expected sample size for group D, two patients were younger than 18 years old, one patient experienced severe hypotension during the alveolar recruitment maneuver, and one patient underwent veno-arterial extracorporeal membrane oxygenation in the operating room due to hyperacute rejection and low cardiac output syndrome received protective ventilation according to a published reference (tidal volume: 4 mL·kg-1 predicted body weight, PEEP: 10 cm H2O, fraction of inspiratory oxygen: 0.3, respiratory rate: 4 cycles per minute)19. Finally, 88 patients were enrolled in this study and followed up for 30 days after surgery (Fig. 1). No deaths occurred during surgery. There were no statistical distinctions in any of the perioperative baseline characteristics between the patients of the two groups (Table 2).
Flow chart of the study inclusion. Between March 2019 and March 2023, 98 patients underwent orthotopic heart transplantation at our center, and 96 patients were screened for study inclusion. Eight patients who met the exclusion criteria were excluded from this study: six cases were automatically excluded as enrolment reached the expected sample size for group D, two cases were younger than 18 years, one case had severe hypotension due to alveolar recruitment maneuver, and one case had hyperacute rejection with intraoperative veno-arterial extracorporeal membrane oxygenation assistance. Finally, an observational study of 88 patients was conducted over a 30-day follow-up period.
The intraoperative mechanical ventilation parameters of all the objects are described in Table 3. Repeated-measures analysis of variance indicated a prominent difference in the perioperative PaO2/FiO2 between the two groups (F = 4.204, P < 0.001). Sidak’s multiple comparisons test found that the PaO2/FiO2 of the patients in Group D were notably higher than those in Group C at T3 (326.1 ± 35.4 vs. 291.2 ± 56.7, t = 3.869, P = 0.001), T4 (339.4 ± 42.0 vs. 301.3 ± 38.1, t = 4.223, P < 0.001) and T5 (357.8 ± 37.4 vs. 327.2 ± 45.5, t = 3.392, P = 0.006). However, no significant differences were discovered at other time points. In addition, no significant differences were observed between the two patient groups regarding the PaCO2 levels (F = 1.370, P = 0.215) (Fig. 2).
The differences in the morbidities of PPCs, postoperative adverse cardiovascular events, all-cause death, and other complications are shown in Table 4; Fig. 3. In group D, one patient died of cardiogenic shock on postoperative day 2, and no early death was observed in group C. In total, five late deaths (5/88, 5.7%) were observed, including one case of cardiogenic shock, one case of sepsis in group D, two cases of multiple organ failure, and one case of sepsis in group C (Table 4). Four patients (4/88, 4.5%) experienced adverse cardiovascular events after surgery. In group D, despite receiving veno-arterial extracorporeal membrane oxygenation, two patients died from cardiogenic shock on postoperative days 2 and 9. In addition, one patient with deep venous thrombosis in the lower limb recovered after anticoagulant therapy. In group C, one case of severe right heart failure was observed, which recovered after seven days of venoarterial extracorporeal membrane oxygenation assistance.
Following the univariate analysis, the multivariate logistic regression analysis covered four variables. Finally, the conventional ventilation strategy, the high ΔP level (after intubation, at the end of the surgery), and the low PaO2 value (at the end of the surgery) were identified as independent risk factors for PPCs in heart transplantation (Table 5).
Discussion
Orthotopic heart transplantation remains a standard surgical strategy for patients with end-stage heart failure20. No severe or persistent hypotension requiring inotropic support was observed in patients in this study, indicating that the low- to moderate-level of PEEP and alveolar recruitment maneuvers were well tolerated during heart transplantation. In our study, patients in group D exhibited higher PaO2/FiO2 in the early postoperative stages, leading to earlier intubation and a shorter LOS-ICU than patients in group C. This suggests that ΔP-guided ventilation has an advantage in promoting the recovery of postoperative pulmonary oxygenation. This could also explain why inspiratory infection and atelectasis significantly decreased in patients in group D after surgery.
ΔP represents the magnitude of cyclic parenchymal deformation applied to ventilated, preserved lung units. It is considered the most accessible and easiest way to calculate cyclic lung strain, a predictor of lung injury that outperforms tidal volume. Further, dynamic respiratory system compliance quantifies functional lung size during disease better than predicted body weight 7. In this study, although the PEEP of patients in group D was higher than in group C, the ΔP-guided ventilation strategy did not significantly elevate airway pressure. We speculated that a more individualised PEEP level plays a role in maintaining the openness of alveoli during low tidal volume ventilation. This, in turn, leads to a notable increase in dynamic respiratory system compliance. These results indicate that the ΔP might be a more suitable factor for guiding decision-making regarding the ventilation strategy employed during heart transplantation, which is in line with a previous study by Mathis and colleagues, who held the same opinion that ΔP, but not tidal volume or PEEP, was closely relative to reduced PPCs in heart surgery16.
Perioperative ventilation strategies should focus on minimising any increase in the afterload of the right ventricle21. A low tidal volume ventilation strategy significantly affects the ΔP and is one of the most critical key points in protective lung ventilation. In addition to utilizing the ΔP, international experts advocate for a low tidal volume ranging from 4 to 8 mL·kg-1 predicted body weight during surgery22. A previous prospective cohort study of 9359 patients revealed that a tidal volume of 6 mL·kg-1 improved postoperative oxygenation and in-hospital survival after cardiac surgery23. We hypothesized that the positive results were attributed to the combination of low ΔP accompanied by low tidal volume despite maintaining a relatively higher PEEP level than the conventional lung-protective ventilation strategy. However, hypoxemia and hypercapnia generated by hypoventilation cannot be neglected during heart transplantation, as they increase the risk of pulmonary arterial hypertension during surgery. Therefore, we modified the upper limit of PaCO2 to 50 mmHg (mild hypercapnia)24 during the ventilatory period. In addition, less postoperative atelectasis was observed in group D, and a similar frequency of ventilation-related hemodynamic disturbances and probability of postoperative adverse cardiovascular events was observed. The same was true for serious hypercapnia, which was evaluated using PaCO2 measurements derived from blood gas analyses and end-tidal carbon dioxide monitoring. Overall, the perioperative application of the low-tidal volume ventilation appeared safe for heart transplantation.
Currently, maintaining ventilation during extracorporeal circulation remains debatable. A meta-analysis involved 748 patients who underwent cardiac surgery (15 clinical trials) and suggested that continuous positive airway pressure ameliorated the postoperative alveolar-arterial oxygen gradient difference. However, continuous positive airway pressure or maintaining ventilation during extracorporeal circulation did not improve the prognosis in low- or moderate-risk patients undergoing selective heart surgery, and the evidence was inconclusive due to heterogeneity and small sample size25. In contrast, the PROVECS study enrolled 488 randomized patients who underwent on-pump cardiac surgery and revealed that ventilation during cardiopulmonary bypass did not reduce the incidence of PPCs compared with routine standard care. It supported the idea that patients undergoing on-pump cardiac surgery did not benefit from ventilation during the on-pump period 16. It is worth noting that intermittent inflation of the lung during cardiopulmonary bypass makes it difficult to expose the surgical field. Although low-setting ventilation during the cardiopulmonary bypass period has been habitually applied in our center, few high-quality studies have proven the relationship between on-pump period ventilation and a reduction in PPCs during cardiac surgery26,27.
This study was not without limitations. First, as a single-center prospective observational study, we could not represent the capacities of other medical institutions in the perioperative management of heart transplantation. Second, despite no significant difference in the patient baseline between the two groups, the selection bias could not be fully eliminated which originated from the anesthesiologists’ choice of intraoperative ventilation strategies. Randomized clinical trials are required to support the authors’ conclusion. Third, there were five authorized surgical teams for heart transplantation, leading to heterogeneity in perioperative management and prognosis. Forth, due to emergency operations, there were frequent instances of incomplete preoperative pulmonary function examinations, resulting in an inaccurate evaluation of perioperative status and the potential for unbalanced baseline risks.
Conclusions
Compared with conventional lung-protective ventilation, the ΔP-guided ventilation strategy enhanced oxygenation in the early period after surgery. It exhibited a strong correlation with a reduced incidence of PPCs, a shorter duration of postoperative ventilation, and a shorter LOS-ICU after heart transplantation. ΔP and SpO2 at the end of surgery are independent risk factors for PPCs. There was no evidence that this novel ventilation strategy had more obvious hemodynamic disturbances or interference with surgical procedures than conventional lung-protective ventilation during heart transplantation.
Statements & Declarations.
Data availability
The data from this study will not be shared publicly. All data included in this study are available upon request by contact with the corresponding author.
References
Jammer, I. et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 32, 88–105 (2015).
Güldner, A. et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 123, 692–713 (2015).
Canet, J. et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 113, 1338–1350 (2010).
Ibañez, J. et al. Long-term mortality after pneumonia in cardiac surgery patients: a propensity-matched analysis. J. Intensive Care Med. 31, 34–40 (2016).
Dushianthan, A., Grocott, M. P., Postle, A. D. & Cusack, R. Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 87, 612–622 (2011).
Gadre, S. & Kotloff, R. M. Noninfectious pulmonary complications of liver, heart, and kidney transplantation: an update. Clin. Chest. Med. 38, 741–749 (2017).
Amato, M. B. et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 372, 747–755 (2015).
Park, M. et al. Driving pressure during thoracic surgery: a Randomized Clinical Trial. Anesthesiology 130, 385–393 (2019).
Neto, A. S. et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 4, 272–280 (2016).
Kim, Y. J. et al. Effect of driving pressure-guided positive end-expiratory pressure on postoperative pulmonary complications in patients undergoing laparoscopic or robotic surgery: a randomised controlled trial. Br. J. Anaesth. (2023).
Park, M. et al. Driving pressure-guided ventilation and postoperative pulmonary complications in thoracic surgery: a multicentre randomised clinical trial. Br. J. Anaesth. 130, e106–e118 (2023).
Li, X. F. et al. The effect of driving pressure-guided versus conventional mechanical ventilation strategy on pulmonary complications following on-pump cardiac surgery: a randomized clinical trial. J. Clin. Anesth. 89, 111150 (2023).
Licker, M. et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit. Care. 13, R41 (2009).
Mi, M. Y., Matthay, M. A. & Morris, A. H. Extracorporeal membrane oxygenation for severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 379, 884–887 (2018).
O’Gara, B. & Talmor, D. Perioperative lung protective ventilation. BMJ (Clinical Res. ed. 362, k3030 (2018).
Mathis, M. R. et al. Intraoperative Mechanical Ventilation and postoperative pulmonary complications after cardiac surgery. Anesthesiology 131, 1046–1062 (2019).
Lagier, D. et al. Effect of open-lung vsvs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: the PROVECS randomized clinical trial. Intensive Care Med. 45, 1401–1412 (2019).
Badenes, R., Lozano, A. & Belda, F. J. Postoperative pulmonary dysfunction and mechanical ventilation in cardiac surgery. Crit. Care Res. Pract. 2015, 420513 (2015).
Schmidt, M. et al. Mechanical ventilation during extracorporeal membrane oxygenation. Crit. Care. 18, 203 (2014).
Stehlik, J. et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant Report–2011. J. Heart Lung Transpl. 30, 1078–1094 (2011).
Neethling, E. et al. Intraoperative and early postoperative management of Heart Transplantation: anesthetic implications. J. Cardiothorac. Vasc Anesth. 34, 2189–2206 (2020).
Young, C. C. et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br. J. Anaesth. 123, 898–913 (2019).
Jia, Y. et al. Low tidal volumes are Associated with slightly improved oxygenation in patients having cardiac surgery: a cohort analysis. Anesth. Analg. 130, 1396–1406 (2020).
Barnes, T., Zochios, V. & Parhar, K. Re-examining Permissive Hypercapnia in ARDS: a narrative review. Chest 154, 185–195 (2018).
Wang, Y. C., Huang, C. H. & Tu, Y. K. Effects of positive Airway pressure and mechanical ventilation of the lungs during cardiopulmonary bypass on Pulmonary adverse events after cardiac surgery: a systematic review and Meta-analysis. J. Cardiothorac. Vasc Anesth. 32, 748–759 (2018).
Chi, D. et al. Ventilation during cardiopulmonary bypass for prevention of respiratory insufficiency: a meta-analysis of randomized controlled trials. Med. (Baltim). 96, e6454 (2017).
Nguyen, L. S. et al. Low tidal volume mechanical ventilation against no Ventilation during Cardiopulmonary Bypass in Heart surgery (MECANO): a Randomized Controlled Trial. Chest 159, 1843–1853 (2021).
Funding
This work was supported by the Natural Science Foundation of Fujian Province (2021J01769 and 2019J05082) and Joint Funds for the Innovation of Science Innovation of Science and Technology, Fujian Province (2018Y9022 and 2020Y9019).
Author information
Authors and Affiliations
Contributions
Mei-fang Chen: Data curation and visualisation. Lin-fen Xie: Software. Xin-fan Lin: Validation. Ping-ping Wu: Methodology and Supervision. Jia-xin Zhang: Investigation. Liang-wan Chen: Supervision, writing, reviewing, and editing. Yong Lin: Writing-original draft preparation and conceptualisation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective study followed the Declaration of Helsinki and was approved by the local ethics committee at Fujian Medical University Union Hospital (2022YF022-01), and all participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Organs/tissues statement
No organs/tissues were procured from prisoners.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Mf., Xie, Lf., Lin, f. et al. Lung protective ventilation guided by driving pressure improves pulmonary outcomes in heart transplantation. Sci Rep 15, 856 (2025). https://doi.org/10.1038/s41598-025-85283-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85283-w