Abstract
A risk association between membranous nephropathy (MN) and lung cancer is reported, but traditional observational studies cannot provide strong evidence of its causality. This study aimed to assess genome-wide association studies data for a causal relationship between MN and lung cancer using a two-sample Mendelian randomization (MR) approach. Inverse-variance weighted, and MR Egger regression techniques were used to determine the association of genetic variants from cohorts of MN and lung cancer patients. Independent genetic variants with genome-wide significance (P < 5×10–8) were used to determine the direction of chance. Sensitivity analyses confirmed the accuracy of the results. The results suggest that MN is an exposure factor for lung cancer, validated using a second cohort of lung cancer patients (P < 0.001). There is insufficient evidence to suggest a causal relationship between lung cancer and MN; however, cigarette smoking may be a confounding factor for lung cancer due to MN. The findings provide causal evidence for the effect of MN on lung cancer risk and may be useful for patient management, especially in older patients with MN who should be systematically screened regularly.
Similar content being viewed by others
Introduction
Membranous nephropathy (MN) is a distinct glomerular lesion associated with other systemic diseases or exposures (secondary MN) in approximately 20% of patients1. With the gradual discovery of in situ antigens, the majority of previous investigations have concentrated on examining the variations in pathophysiology between primary (idiopathic) and secondary (cancer-associated) MN and exploring the differences in pathogenesis between idiopathic and secondary MN. Since Lee2 first proposed a connection between nephrotic syndrome and cancer in 1966, scientists have been identifying ways in which these diseases are related. Clinically, cancer-related nephropathy is on the rise, but the pathological types are atypical and the etiology is unclear3,4. Although several reports mention improvement in symptoms of nephrotic syndrome in patients with MN after aggressive tumor treatment5,6,7, the evidence that directly reveals this relationship is still limited, controversial, and speculative8. It has been reported that the processes of MN and malignancy often develop in parallel, with tumor antigens or tumor-reactive antibodies found within glomerular immune deposits, supporting an association between the two diseases9. However, both MN and malignancy are prone to occur in middle-aged and elderly populations, so the presence of both diseases in the same patient may also be coincidental. Thus, a causal relationship between tumor and MN has not been universally accepted, and whether malignancy-associated MN exists independently of the concept of secondary MN as a distinct entity deserves further investigation.
Several studies have reported an association between MN and cancer7,10,11, with lung cancer having the strongest correlation. Lung cancer may occur after the diagnosis of MN11, but there are few studies on whether MN leads to an increased risk of lung cancer, and on the mechanisms of association between these diseases. In observational studies, bias due to confounding and reverse causality cannot be completely excluded, so the specific causal relationship between MN and lung cancer is unclear. Therefore, we performed a two-sample bidirectional Mendelian randomization (MR) analysis to investigate the causal effect between MN and lung cancer.
Results
MR analysis results
First, we analyzed whether MN was the cause of lung cancer; the results are shown in Fig. 1. A total of 10 SNPs were identified in the lung cancer cohort. The IVW and WM models showed statistically significant estimates of the effect of MN on lung cancer (P < 0.001); the MR Egger model yielded a P = 0.115, which was not significantly different, but consistent in direction with the other models. Ten SNPs were identified in the lung cancer validation cohort, and the results of all three models were statistically significant (P < 0.001). The results of the validation cohort support the results of the exploration cohort, suggesting a causal relationship between MN and lung cancer, and that MN may lead to lung cancer.
Different methods were used to obtain OR values and 95% confidence intervals. Subtotal estimates were combined using a fixed effects meta-analysis and the results are shown in red. Subtotal indicates the summary of the analysis results for different datasets. MN membranous nephropathy, LC lung cancer, CI confidence interval, OR odds ratio, IVW inverse-variance weighted, WM weighted median.
The reverse causality between MN and lung cancer was analyzed to determine if lung cancer was the cause of MN. The calculated results for the IVW, WM, and MR Egger models are shown in Table 1. The lung cancer and lung cancer validation cohorts, gave a P > 0.05 for all three models, indicating that lung cancer was not the cause of MN. However, owing to the small number of identified SNPs, this result cannot fully represent the causal relationship between lung cancer and MN.
Sensitivity analyses results
After completing the MR analysis, sensitivity analysis was performed on the results of the MR analysis. The results of the leave-one-out sensitivity test for the causal analysis of MN and lung cancer are shown in Fig. 2. The results of the lung cancer and lung cancer validation cohorts are shown in Fig. 2a, b respectively. This shows that the results of the MR analysis are plausible.
a MN on lung cancer. b MN on lung cancer (val). The black line is the deviation of the 95% confidence interval corresponding to the estimate of the SNPs. The red line corresponds to the estimated value of the IVW test. After removing SNPs one by one, there was no difference with the final result. MN membranous nephropathy, val validation, SNPs single-nucleotide polymorphisms.
Heterogeneity testing was performed using Cochran’s Q test (Table 2), which yielded P < 0.05 for both the IVW and MR Egger models. This indicated that heterogeneity existed and that a causal relationship between MN and lung cancer was not due to sampling error. This was further confirmed by calculating the P value of the IVW analysis in the random effects model (Table 2). Thus, the occurrence of MN may lead to an increased risk of developing lung cancer.
The results of the pleiotropic tests are presented in Table 3. In both lung cancer cohorts, there was no directional pleiotropy (P > 0.05). The results of the above sensitivity analysis support the results of the MR analysis for a causal relationship between MN and lung cancer. Scatter plots, forest plots, and funnel plots of SNPs are shown in Supplementary Fig. S1–3.
The results of the sensitivity analysis of the causal relationship between lung cancer and MN are shown in Fig. 3 and Table 4. High heterogeneity was observed in the results of the MR analysis with no directional pleiotropy. The related visualization results are shown in Fig. S4–6. In the MR analysis, the number of SNPs obtained was low, and on balance, the result that there is no causal relationship between lung cancer and MN has yet to be verified.
a Lung cancer with MN. b Lung cancer (val) with MN. The black line is the deviation of the 95% confidence interval corresponding to the estimate of the SNPs. The red line corresponds to the estimated value of the IVW test. After removing SNPs one by one, there was no difference with the final result. MN membranous nephropathy, val validation, SNPs single-nucleotide polymorphisms.
Effect of confounding factors
Considering the effect of confounding factors on the outcome variables, smoking, prostate cancer, and colorectal cancer were included as exposure factors along with MN in the multivariate MR analysis. The causal relationship between each variable and lung cancer were analyzed using the IVW model. Information on the selected dataset and the results of the MR analysis are shown in Table 5. Only MN and smoking had a P < 0.05, indicating that when smoking and MN occur together, they may contribute to the development of lung cancer. Prostate cancer and colorectal cancer, on the other hand, would not be influential factors in the causal relationship between MN and lung cancer.
Discussion
The present MR study assessed the association between MN and lung cancer from a genetic perspective. The results of the exploratory cohort study supported a causal relationship between MN and lung cancer. MN may be an exposure factor that causes lung cancer, and the results of the validation cohort were identical. Many studies have identified an association between MN and lung cancer; however, the exact causality and pathogenesis of this association remain unclear.
Epidemiological evidence has shown that the incidence of cancer is significantly higher in patients with MN than in the general population. After MN is diagnosed in patients, the risk of developing cancer may persist for at least five years3, and the risk of MN-related cancers increases with patient age12,13. Meanwhile, in a meta-analysis11, the incidence of cancer-associated MN was found to be approximately 10%, with lung cancer being the most common, followed by stomach, bowel, prostate, and breast cancers. A small number of patients with lung cancer may present with clinical manifestations led by nephropathy, which resolves after treatment of lung cancer4, and in patients with cancer-associated MN, there is a strong association between reduced proteinuria and remission of clinical symptoms of cancer14. In a recent study, six patients with lung cancer and nephropathy as the first manifestation were followed up and analyzed. Most of these patients did not show clinical manifestations or specific tumor markers associated with lung cancer, but bilateral lower limb edema associated with MN was the main manifestation4. Our MR analysis revealed that MN may act as an exposure factor for lung cancer, which is consistent with the clinical presentation of symptoms. However, the malignancy has a long latency period, and although MN is the first symptom diagnosed in the clinic, the causal relationship between the two still requires further investigation. Therefore, tumor marker screening, with chest CT screening if required, should be performed as early as possible to determine the presence of cancer in elderly patients with MN and not rely solely on its clinical manifestations. In the follow-up of elderly patients with MN who are not in remission with conventional treatment, regular systemic examinations should be performed. Furthermore, cigarette smoking is the most important risk factor for lung cancer in both men and women15. In our study, the confounding factor analysis indicated that smoking may be a confounding factor when MN causes lung cancer, which is consistent with previous findings. Thus, lung cancer should be systematically investigated in patients with MN who are smokers.
The M-type phospholipase A2 receptor (PLA2R) is identified in 70–80% of idiopathic MN16. Furthermore, it has been found that the absence of glomerular PLA2R and the dominance of IgG1- and/or IgG2-restricted subclasses are common in patients with malignancy-associated MN17. Furthermore, IgG4 is the major IgG subclass in primary model glomerular immune deposits. It has been found that IgG4 deficiency in glomeruli may cause malignancy, but this has not yet been confirmed17,18,19, whereas IgG1 and IgG2 deposition were associated with malignancy19, with a predominance of Th1-type responses in cancer-associated MN. Thrombospondin type-1 ___domain-containing 7 A (THSD7A) is a MN autoantigen and a secondary autoantigen of MN20, which has been found to be associated with the development of malignant tumors, with one study showing that THSD7A positivity in malignant tumors is 15–20%21. A possible molecular link between increased THSD7A expression in tumors and the presence of positive THSD7A antibodies in MN has been reported, suggesting a similar pathogenesis between primary and malignancy-associated MN22. In a clinical study, positive THSD7A staining was found in both glomeruli and cancer cells of patients with lung cancer, and remission of MN symptoms was observed after surgical resection, confirming the association of THSD7A with lung cancer and MN23. Recently, neural epidermal growth factor-like 1 (NELL1) was identified as a new antigen in 3.8% of primary MN24. Compared to PLA2R- and THSD7A-associated cases of MN, NELL1-positive MN has a higher proportion of cases with malignancies (33%)25.
Distinguishing between idiopathic MN coexisting with malignancy and malignancy-associated MN is difficult, especially in elderly patients where the occurrence of malignancy is common. This study aimed to extend previous research by demonstrating a causal relationship between MN and lung cancer. Although the pathophysiological mechanism underlying this relationship is still unclear, there is no doubt that there are some common biological pathways between them. Evidence indicates that THSD7A is a potential tumor antigen in humans, participating in cancer progression, vascular invasion, angiogenesis, and metastasis in the tumor environment26,27,28. Additionally, THSD7A was detected in lung cancer tissue, which was also THSD7A-positive MN23. A possible explanation is that the immune system forms an anti-THSD7A antibody that also attracts antibodies against THSD7A antigens in cancer cells. Circulating immune complexes formed by the shedding of tumor antigens are trapped in the glomerular capillary wall, causing an immune response. Tumor initiation is a process in which normal cells acquire the first mutational hit, and inflammatory microenvironments can contribute to mutation rates29. In patients with MN and malignancy, renal biopsy results suggest a significantly higher number of inflammatory cells infiltrating the glomeruli14. We speculate that activated inflammatory cells can induce DNA damage and genomic instability, which are involved in tumorigenesis. Furthermore, the study found that PLA2R1 and THSD7A were expressed not only in podocytes but also in pulmonary fine bronchi22, which may also be a potential cause of lung cancer in MN. The lungs have a large surface area exposed to the outside world. Extrinsic processes, such as viral infections or potentially abnormal immune responses, may cause MN in cancer30.
To our knowledge, the present study is the first to use a two-sample MR analysis to examine the causal relationship between MN and lung cancer. Compared with observational studies, MR analysis is less prone to confounding factors, antinomial causality, and errors caused by non-differential measurements31. The results of multiple sensitivity analyses supported the MR analysis conclusion that the findings were not confounded by polymorphic factors. However, the reverse causality between MN and lung cancer could not be effectively confirmed, owing to the quality of the GWAS dataset. The total sample size of the MN dataset was small, which may have led to errors. The lung cancer dataset lacks a classification of different lung cancer types; thus, the association between lung cancer types and MN could be further explored. The GWAS dataset is European in ethnicity, and lacks data on other ethnicities, and thus has limited generalizability to other populations.
In conclusion, our study provides genetic evidence that there is a causal relationship between MN and lung cancer and that MN may be a cause of lung cancer. This suggests that awareness of the potential risk of developing lung cancer in patients with MN needs to be raised in the clinic, which could help in early disease intervention. Exploring the specific biological mechanisms that induce lung cancer in patients with MN should be the subject of further research.
Methods
Study design
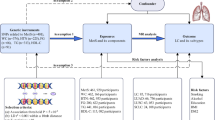
A two-sample MR analysis between MN and lung cancer, utilizing summary statistics from genome-wide association studies (GWAS), was performed to evaluate their bidirectional causal relationship and were validated using multiple datasets.
Study samples and measures
According to Mendel’s law of inheritance, during meiosis, chromosomes carry genes that are randomly assigned to offspring without confounding factors, allowing the simulation of randomized controlled experiments. Here, we obtained genetic variables between MN and lung cancer using GWAS.
The details of each cohort are presented in Table 6. The MN cohort (ebi-a-GCST010005) contained 7979 samples with 5,327,688 SNPs obtained32. All subjects were primary MN patients. And all subjects in this cohort were genotyped using high-density SNP arrays, and approximately 7 million common, high-quality markers were imputed using the latest genome-wide sequence reference panel. The lung cancer exploration cohort (ieu-a-967) contained 18,313 samples, and the lung cancer validation cohort (ieu-a-966) contained 27,209 samples33. In both lung cancer cohorts, all subjects underwent standard quality control; individuals with low call rates and very high or very low heterozygosity as well as individuals of non-European ancestry, were excluded. To ensure the quality of genotyping in all tests, duplicate samples were genotyped at each center. To exclude technical errors, cross-platform validation was performed on some samples to ensure genotyping accuracy.
Ethical approval and consent to participate were obtained from the original publication. Informed written consent was obtained from all participants in the MN and lung cancer cohorts All studies were reviewed and approved by the ethics review boards of the relevant institutions.
MR analysis
For analysis using the TwoSampleMR package, the criteria for identifying genetic variants were kb = 1000, r2 = 0.01. The specific information on MN as an exposure factor for identifying SNPs is shown in Table 7. F-statistics is a common metric for assessing bias in weak instrumental variables, F-statistics = (β/SE)2 34. The mean value of F-statistics for all SNPs was 133.6589. Fmax = 471.5235, Fmin = 30.80498. The F-statistics > 10 for each SNP indicates that there is no bias due to weak instrumental variables35. There will be no impact on the results of MR analysis. The results of lung cancer as an exposure factor for identifying SNPs are shown in Table S1–2. In the process of identifying instrument variables (IVs), no data coordination was performed. The causal relationship between MN and lung cancer was confirmed using the inverse-variance weighted (IVW) model combined with the weighted median (WM) and MR Egger models.
Sensitivity analysis
Sensitivity analysis included the leave-one-out sensitivity, heterogeneity, and pleiotropy tests. The leave-one-out sensitivity test calculates the MR results of the remaining IVs after eliminating the IVs individually. If the calculated results of the other IVs are significantly different from the final results after the elimination of a certain IV, then the MR results are sensitive to that IV. The heterogeneity test calculates the differences between individual IVs; the greater the differences between different IVs, the greater the heterogeneity of these IVs36. The pleiotropy test checks for directional pleiotropy between different IVs, expressed by the intercept of the MR Egger regression; if the intercept is very different from 0, it indicates the presence of a horizontal multi-effect37. All statistical tests were performed between two samples using the Two-Sample MR (version 0.5.6) package of the R software (Version 4.2.0).
Analysis of confounding factors
Considering complex biological factors, multivariate MR analysis was used to exclude possible confounding factors. Previous studies have found that the vast majority of tumors associated with MN are lung, prostate, and colorectal cancers11. In addition, an association between smoking38, MN, and lung cancer has been reported. In the confounding factor analysis, MN, smoking, prostate cancer, and colorectal cancer were considered exposure factors, and the causal relationship between them and lung cancer was analyzed to determine what factor had on the estimate of effect.
Statistics and reproducibility
In this study, two-sample MR analysis was used to explore the causal relationship between MN and lung cancer. Comprehensive information on SNPs, particularly effector alleles, was also included. The main MR analysis methods used were IVW, WM, and MR Egger. The risk of MN causing changes in lung cancer was expressed using odds ratios (ORs) and 95% confidence intervals (CI). P < 0.05 indicates a statistical difference.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the datasets we used are freely available from IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/). The data used for the analysis can be found in the “Supplementary Data 1”.
Code availability
The code used in this study can be found in "Supplementary Software 1".
References
Couser, W. G. Primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 12, 983–997 (2017).
Lee, J. C., Yamauchi, H. & Hopper, J. Jr The association of cancer and the nephrotic syndrome. Ann. Intern. Med. 64, 41–51 (1966).
Bjorneklett, R. et al. Long-term risk of cancer in membranous nephropathy patients. Am. J. Kidney Dis. 50, 396–403 (2007).
Xu, Q., Zou, G., Zhuo, L., Gao, H. & Li, W. Lung cancer patients with nephropathy as the first manifestation: literature review and clinical study report. Front. Oncol. 12, 1002155 (2022).
Wakabayashi, K. et al. Nivolumab-induced membranous nephropathy in a patient with stage IV lung adenocarcinoma. CEN Case Rep. 11, 171–176 (2022).
Morimoto, N. et al. Membranous nephropathy in a patient with pulmonary tuberculosis infection and lung adenocarcinoma: a case report. CEN Case Rep. 11, 126–133 (2022).
Chen, M. et al. Case report: THSD7A-positive membranous nephropathy caused by tislelizumab in a lung cancer patient. Front. Immunol. 12, 619147 (2021).
Beck, L. H. Jr Membranous nephropathy and malignancy. Semin. Nephrol. 30, 635–644 (2010).
Hoxha, E. et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 ___domain-containing 7A-specific antibodies in membranous nephropathy. J. Am. Soc. Nephrol. 28, 520–531 (2017).
Nieto-Ganan, I., Iturrieta-Zuazo, I., Rita, C. & Carrasco-Sayalero, A. Revisiting immunological and clinical aspects of membranous nephropathy. Clin. Immunol. 237, 108976 (2022).
Leeaphorn, N. et al. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am. J. Nephrol. 40, 29–35 (2014).
Zech, P. et al. The nephrotic syndrome in adults aged over 60: etiology, evolution and treatment of 76 cases. Clin. Nephrol. 17, 232–236 (1982).
O’Callaghan, C. A., Hicks, J., Doll, H., Sacks, S. H. & Cameron, J. S. Characteristics and outcome of membranous nephropathy in older patients. Int. Urol. Nephrol. 33, 157–165 (2002).
Lefaucheur, C. et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 70, 1510–1517 (2006).
Thun, M. J. et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 368, 351–364 (2013).
Beck, L. H. Jr et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Lonnbro-Widgren, J., Ebefors, K., Molne, J., Nystrom, J. & Haraldsson, B. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin. Kidney J. 8, 433–439 (2015).
Qu, Z. et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol. Dial. Transplant. 27, 1931–1937 (2012).
Ohtani, H. et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol. Dial. Transplant. 19, 574–579 (2004).
Tomas, N. M. et al. Thrombospondin type-1 ___domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 371, 2277–2287 (2014).
Ren, S. et al. An update on clinical significance of use of THSD7A in diagnosing idiopathic membranous nephropathy: a systematic review and meta-analysis of THSD7A in IMN. Ren. Fail. 40, 306–313 (2018).
von Haxthausen, F. et al. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front. Immunol. 9, 3035 (2018).
Zhang, C. et al. Features of phospholipase A2 receptor and thrombospondin type-1 ___domain-containing 7A in malignancy-associated membranous nephropathy. J. Clin. Pathol. 72, 705–711 (2019).
Sethi, S. et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 97, 163–174 (2020).
Caza, T. N. et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 99, 967–976 (2021).
Wang, C. H. et al. Thrombospondin type I ___domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J. Cell. Physiol. 222, 685–694 (2010).
Kuo, M. W., Wang, C. H., Wu, H. C., Chang, S. J. & Chuang, Y. J. Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS ONE 6, e29000 (2011).
Stahl, P. R. et al. THSD7A expression in human cancer. Genes Chromosomes Cancer 56, 314–327 (2017).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
van de Logt, A. E., Fresquet, M., Wetzels, J. F. & Brenchley, P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 96, 1292–1302 (2019).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur. J. Epidemiol. 35, 99–111 (2020).
Xie, J. et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 11, 1600 (2020).
Wang, Y. et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat. Genet. 46, 736–741 (2014).
de Klerk, J. A. et al. Altered blood gene expression in the obesity-related type 2 diabetes cluster may be causally involved in lipid metabolism: a Mendelian randomisation study. Diabetologia 66, 1057–1070 (2023).
Brion, M. J., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501 (2013).
Rees, J. M. B., Wood, A. M., Dudbridge, F. & Burgess, S. Robust methods in Mendelian randomization via penalization of heterogeneous causal estimates. PLoS ONE 14, e0222362 (2019).
Kemp, J. P., Sayers, A., Smith, G. D., Tobias, J. H. & Evans, D. M. Using Mendelian randomization to investigate a possible causal relationship between adiposity and increased bone mineral density at different skeletal sites in children. Int. J. Epidemiol. 45, 1560–1572 (2016).
Yamaguchi, M. et al. Smoking is a risk factor for the progression of idiopathic membranous nephropathy. PLoS ONE 9, e100835 (2014).
Author information
Authors and Affiliations
Contributions
K.Y.: Writing – original draft, Conceptualization. X.D.: Data curation. J.L.: Methodology. S.L.: Formal analysis. Q.L.: Validation. J.L.: Project administration. P.Z.: Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mathias Seviiri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Hélène Choquet and Joao Valente and George Inglis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, K., Ding, X., Liu, J. et al. Two-sample mendelian randomization reveals a causal association between membranous nephropathy and lung cancer. Commun Biol 6, 887 (2023). https://doi.org/10.1038/s42003-023-05111-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05111-7
This article is cited by
-
A phenome-wide Mendelian randomization analysis reveals the genetical associations of myocardial infarction, angina pectoris and Alzheimer’s disease with lung cancer
Scientific Reports (2025)
-
Exploration of the clinicopathological and prognostic significance of BRCA1 in gastric cancer
Discover Oncology (2025)