Abstract
The stress that the space environment can induce on plant physiology is of both abiotic and biotic nature. The abiotic space environment is characterized by ionizing radiation and altered gravity, geomagnetic field (GMF), pressure, and light conditions. Biotic interactions include both pathogenic and beneficial interactions. Here, we provide an overall picture of the effects of abiotic and biotic space-related factors on plant physiology. The knowledge required for the success of future space missions will lead to a better understanding of fundamental aspects of plant physiological responses, thus providing useful tools for plant breeding and agricultural practices on Earth.
Similar content being viewed by others
Introduction
International space agencies are developing human space exploration capabilities required to venture beyond low-Earth orbit. The Artemis Lunar Exploration Program1 will allow testing of these systems and operations on the Moon, in preparation for future manned missions to Mars. During long-term missions to the Moon and Mars, the crew will not be able to rely on constant resupply from Earth for water, food, and breathable air. Solving this problem will require the creation of Biological Life Support Systems (BLSS)2, where resources are produced and recycled thanks to organisms with bioregenerative functions3. Plants are a fundamental component of BLSS, as they generate O2, assimilate CO2, purify water, and produce fresh food4. Exploration will move from a scenario where plants are supplements to the diet to one where in situ crop production will cover almost if not all, the nutritional requirements of the crew. To allow for sustainable and reliable plant production, plant physiologists are studying plant growth and physiology in space. It should be noted, however, that the environmental stressors and constraints that will characterize orbiting stations or bases on the surface of other celestial bodies will differ5. Indeed, while objects on orbiting stations and transit vehicles will experience microgravity conditions, partial gravity will be present on the surface of the Moon (1.62 m/s2) and Mars (3.71 m/s2). Radiation exposure will increase, and the environmental magnetic field will decrease, as exploration moves away from the protection of the geomagnetic field (GMF). Moreover, spacecraft and planetary bases differ in their constraints in terms of mass, energy, and available in situ resources. All these environmental differences not only affect plant development to different degrees but also determine the requirements and constraints.
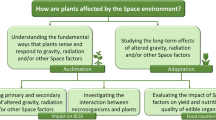
Here we describe the developmental and physiological responses of plants to the space environment by considering both abiotic and biotic (Fig. 1) interactions. Among abiotic stress conditions, altered gravity, the presence of ionizing radiation, the absence or reduction of the GMF, altered pressure and light conditions are the most important factors that can impact on plant growth and productivity. Biotic aspects include plant interactions with pathogenic or beneficial microorganisms.
Abiotic factors include elements that are features of the space environment, like altered gravity, radiations, and the lack of a magnetic field, but also characteristics of the artificial environment that can be created in space, like hypobaria, and the controlled environment for plant growth (artificial light, soil-less technologies). Biotic factors relevant for plant production in space can be both pathogenic (plant and human pathogens) and beneficial (endophytes, root-associated microbes, and paedogenic bacteria) in nature.
The abiotic environment in space
Altered gravity affects the physiology of plants at both organ and cellular level
Gravity is a major environmental factor in plant evolution, deeply affecting all aspects of plant biology. The effects of altered gravity on plant development and reproduction have been extensively studied6, but our understanding of the genetic/molecular pathways mediating this response remains incomplete.
Plants perceive gravity through specialized cells, called statocytes, located in the root columella and in the shoot endodermis. Within the statocytes, dense, starch-filled organelles (statoliths) reposition themselves according to the gravitational vector, thus providing information for the developmental response of the plant shoot7 and root8 (Fig. 1). Statolith repositioning triggers a complex biochemical cascade that is translated in a transverse auxin gradient across shoots9 and roots10. This, in turn, regulates cell expansion, causing asymmetric organ growth, and, consequently, changes in the whole plant developmental program, deeply influencing plant architecture and shape11,12. Recently, the gravity-dependent root growth mechanism has been described13. In gravity-sensing columella cells, the protein MPK3 phosphorylates the proteins LAZY3 and LAZY4, which results in the increased association of LAZY proteins with the TOC proteins on the surface of amyloplasts. Upon amyloplast sedimentation, LAZY3 and LAZY4 are released from the amyloplast and move to the plasma membrane, where they recruit the auxin efflux proteins PIN3 and PIN7, by means of interaction with RLD family proteins. The activity of PIN proteins allows the movement of auxin out of the cells and the formation of an asymmetrical gradient of the hormone, that causes the inhibition of cell elongation on the lower side of the root, thereby causing the gravitropic root growth14,15. Other plant growth regulators play a role in gravitropism, including brassinosteroids16, ethylene17, gibberellic acid18, jasmonic acid19, and Ca2+ signaling20. While the lack of a gravitropic input, whether real (spaceflight) or simulated (e.g., clinostats or Random Positioning Machines), can be compensated by other tropisms21, it is important to understand how altered gravity can affect non-vertical growth angles and, therefore, plant architecture for long-term space exploration objectives. The threshold for the onset of a gravitropic response has been estimated to be around 10−3 g, above which the magnitude of the graviresponse is only dependent on the inclination22. This suggests that altered gravity would be problematic, from a gravitropic point of view, only in orbiting stations and transit vehicles.
Gravisensitivity, i.e., the metabolic and structural adaptation to altered gravity conditions, is common to all cells, whether specialized or not specialized for gravity perception23. Reduced gravity induces changes in the content of lignin, cellulose, callose, and hemicelluloses of the plant cell wall24. Within the cell, altered gravity can cause alteration of the structural and functional organization of organelles and of the subcellular structures of mitochondria25, chloroplasts26, cortical microtubules27, and ER bodies28. While the molecular mechanism mediating these effects is still largely unknown, it has been shown that reduced gravity induces changes in the activity of peroxidases, pectinases, and cellulases and in the calcium balance in the cytoplasm and apoplast23. Other microgravity-induced alterations include a higher production of reactive oxygen species29 and of heat shock proteins30, alterations of the cell cycle and nucleolar31, and meristematic activity32. In this context, gravity alterations can significantly influence plant reproduction, as they modulate the growth of and traffic in the pollen tube33, affect the development of male and female reproductive organs, and impact seed germination34. Interestingly, studies on cell proliferation in partial gravity conducted using a modified RPM system have shown that the response is more complex than expected. Indeed, in plants grown in Moon gravity the effects on cell proliferation and cell growth were worse than in simulated microgravity. Interestingly, in the simulated Mars g-level, cell proliferation was similar to the 1 g control, suggesting, again, that some environmental conditions might not be challenging for plant growth on Mars35.
In view of the utilization of plants, as life-supporting systems in human long-term space travel, it is imperative that the effects of gravity on such crucial plant processes as development and reproduction are approached at a basic level. While we are starting to decipher the last missing details of the graviresponse13, very little is still known regarding gravisensitivity at cellular and organism levels. Moreover, phytohormones are hardly mentioned in plant-based studies in space due to technical constraints, like sample storage. In addition to the crop species, the use of plant model systems, for which numerous genetic/molecular resources and tools are available, is facilitating the study of these basic mechanisms and subsequent translational research. The knowledge gained on the control of key traits affecting plant adaptation, survival, and productivity will provide powerful tools for space-targeted precision breeding.
The impact of ionizing radiations on plants
Deep space radiation environment beyond low-earth orbits (LEO), i.e., outside Earth’s protective GMF, is characterized by a flux of ionizing radiation. Space radiation is mostly composed of protons and heavier nuclei stripped of their orbital electrons, but also includes a minority of electrons and positrons, corresponding mainly to high-energy heavy-ion (HZE), charged particles of galactic cosmic rays (GCRs) and solar energetic particles (SEPs)36. In addition, as a result of space radiation interacting with matter, e.g., the surface of planetary bodies or spacecraft structures, secondary radiation, which is constituted by neutrons of different energies, is generated37. Plants can be affected by ionizing radiations at both genetic and epigenetic levels. Genetic effects are mainly due to the occurrence of DNA double-strand breaks (DSBs), which can lead to chromosomal aberrations, structural variation, or point mutations. These are mainly the result of inaccurate repair processes such as those caused by the non-homologous end joining repair pathway, which has been shown to be prevalent in plants38. Another peculiar feature of angiosperms is that most species studied so far, including most crops, carry very active transposable elements (TEs). It is believed that TE activation has frequently caused massive amplification, which led to profound genome reorganization and extensive structural variation in intergenic regions39. Many TEs appear to be activated by different types of environmental stress, including radiation exposure40. The availability of a vast range of technologies that exploit the power of next-generation sequencing allows us today to analyze all these phenomena at the whole genome level.
The effects of ionizing radiations have been investigated only on a small proportion of plant species, and increasing inquiries on a wider number of plants will improve our understanding of the biological effects of radiation41. Plant cells show higher radiation resistance compared with their animal counterparts. Different studies, performed in space and with simulated space-like levels of radiation on the ground, have assessed the structures and mechanisms conferring radioresistance to plant cells42. Densely ionizing radiations are more efficient in inducing damage, in comparison with sparsely ionizing radiations43. For this reason, there is significant literature concerning the effects of acute high-dose-rate exposures on plant genetics, growth, and development. Much less is known regarding the effects of chronic, low-dose radiations, especially those related to the impacts of the high-energy protons and heavy ions that are encountered in the space environment44. Under chronic irradiation conditions, pollen and seed viability are reduced, growth rates are slower, and the frequency of developmental abnormalities is increased, although there is considerable variation among taxa. In addition, it has been shown that the response elicited by radiation exposure has complex interactions with other environmental stressors (e.g., temperature, drought, heavy metals)44, suggesting that the other risks associated with the space environment could play important roles in determining susceptibility to radiation-induced stress.
The reactions induced by primary radiation include the formation of various forms of reactive oxygen species (ROS) and are the cause of the observed changes in the functional activity of plants45. It is, therefore, expected that plants react to radiation by altering their redox status. X-ray exposure of Brassica rapa to doses up to 30 Gy does not induce detrimental effects on growth, while it stimulates the production of antioxidants, improving plant defence and, concurrently, nutritional value46. In Beta vulgaris, ionizing radiation (10 Gy) and specific light quality regimes interact in a complex manner to regulate photosynthesis and the accumulation of bioactive compounds in leaf edible tissues. In particular, while under a white light regime, gas exchanges of irradiated plants strongly declined compared to control, irradiation with titanium high-energy ions under red-blue light improved the water use efficiency and increased pigments, carbohydrates, and antioxidant content47. It should be noted that the exposure of seeds to these conditions negatively affected the germination rate; however, these results introduce the idea of exploiting space growth conditions to produce plant species with increased antioxidants as a supplemental functional food46,48.
Work should be done to elucidate the effects and the molecular mechanisms of response to ionizing radiation exposure, in both model and crop plants. Moreover, while considerable information is available on the effects of ionizing radiation at the genetic level, much less is known on whether it can also lead to inheritable epigenetic changes, which could determine changes in DNA methylation or histone modification and chromatin conformation changes41,49. Unfortunately, most of the current studies on plants derive from experiments conducted with different radiation types and doses50. Moreover, it is hard to reproduce the complexity of the full space radiation spectrum, which is composed of solar electromagnetic particles and high-energy protons and heavy ions from outside our solar system. A standardization of methods and protocols for irradiation experiments is necessary to validate the current knowledge of space-relevant radiation.
Magnetic field requirement for plant life in space
The GMF is a natural component of our planet. The GMF is a three-dimensional vector field, which is generated in the outer core of our planet and surrounds the circum-terrestrial environment, shielding it from energetic solar wind and harmful cosmic rays. In addition, plants, like all other living organisms, have evolved in the presence of the GMF. While phototropism, gravitropism, hydrotropism, and thigmotropism have been thoroughly studied, the plant response to magnetic field (MF) variations is not yet well understood. However, plants responses to MF have been extensively documented51,52,53,54. Concerning the current theories of magnetoperception, the mechanism involving radical pairs (i.e., magnetically sensitive chemical intermediates that are formed by photoexcitation of cryptochrome55) has been demonstrated both in animals56,57 and in plants58; moreover, the interaction between cryptochrome (Cry)59 and iron-sulphur complex assembly60, define a magnetosensing protein complex named MagR61 (see below) that is present in several living organisms62,63,64, apparently as a response to the GMF. However, the mechanisms of plant magnetoreception and adaptation to different MF remain to be elucidated65. Such information will be important to forecast the behavior of plants, and other organisms, in environments where the MF will be different, such as the Moon Space Gateway (SG), the Moon’s surface, Mars and other planets that lack a MF. On the other hand, some moons of the gas giants Jupiter and Saturn, like Europa and Enceladus, are of great astrobiological interest and are subject to the strong MFs of their planets66.
Several lines of evidence have shown that plants are able to perceive MF variations, both below and above the GMF, and respond with physiological, metabolic, and anatomical changes51,54,67. For instance, experiments performed with a triaxial Helmholtz coil system could clearly demonstrate that plants react to changes in GMF inclination68 and intensity69, with genes responding specifically to MF variations as a typical stress response. GMF variations were found to alter the plant redox status70, which in turn affects the photosynthetic activity71 and the cryptochrome biological activity58. Indeed, there is a partial association between the MF-induced changes in gene expression and an alteration in Cry activation72. A mechanism for magnetoreception has been proposed in the fruit fly (Drosophila melanogaster) and involves a magnetosensor (Drosophila CG8198, MagR) complex constituted by iron-sulphur cluster assembly (ISCA) and Cry61. In the fruit fly magnetic receptor, a linear polymerization of magnetoreceptors containing Fe–S cluster leads to the formation of a rod-like biocompass center, surrounded by photoreceptive cryptochromes61. Interestingly, four plant proteins were found to be highly similar to MagR: IscA-like 3 (At2g36260), IscA-like 1 (At2g16710), IscA-like 2 (At5g03905) and cpIscA (At1g10500)60. Arabidopsis thaliana plants grown under single or combined Fe- and S-deficient conditions and in hypomagnetic field (HMF), show alteration in Fe and S uptake and homeostasis73,74. This variation may lead to reduced Fe-S availability for ISCA formation, which in turn would reduce MF sensing in plants exposed to HMF70. In plants, the flowering time and fruit set are important for plant productivity and are regulated by the circadian clock. The amplitude of the oscillating genes that are part of the plant’s internal clock is significantly different under varying MF conditions, regardless of the lighting conditions, implying that, unlike animals, plants can respond to changes in MF in the dark72,75.
The proximity to gas giants, like Jupiter and Saturn, implies the presence of strong MFs. The magnetic field effect (MFE) depends on the strength of a MF that can be classified as weak (<1 milliTesla-mT), moderate (1 mT to 1 T), strong (1 T to 5 T), and ultra-strong (>5 T). Weak MFs, as the GMF, can be perceived by animals and plants as described above. Moderate-intensity static MFs (SMFs) influence those biological systems where function depends on the properties of excitable membranes76. Strong and ultra-strong fields affect living organisms by altering the preferred orientation of a variety of diamagnetic anisotropic organic molecules54. It has been shown that strong MFs can induce alterations of the cleavage plane during cell division77 and other cellular disorders78. In Arabidopsis, strong MFs may compromise some aspects of the transcriptional machinery, by perturbing the delicate conformational dynamics involved in gene regulation, resulting in differential gene expression and, in extreme cases, the halt of transcription79. MFs can be perceived by plants through their amyloplasts, which can be displaced by a sufficiently intense, high-gradient MF. By displacing amyloplasts with MFs, it is possible to induce curvature in roots, triggering the developmental response that is normally activated during graviresponse downstream of amyloplast sedimentation. This effect is defined as magnetotropism, although it seems that the cause of the growth response is a ponderomotive force and not the MF80.
Variations in MF intensity influence many plant biological processes. Unlike an electric field, an MF is not attenuated by living tissues and penetrates through the whole plant body. Reduction of the GMF, a condition typical of the deep space environment, alters the plant morphology and redox status by delaying flowering81 and altering the plant defence to pathogens82,83. The study of the plant response to strong MFs could also guide future research designed to explore the possibility of life on moons of planets with high MFs, such as the solar system gas giants. Plants show both light-dependent and light-independent magnetoperception and recent data have suggested that different organs may perceive MFs in a differential way, with a typical hormetic behavior69. The recent discovery that plant ISCA may play a role in magnetoreception60,71 could help understand the plant signaling cascade triggered by MF variations, which contributes to the plant responses in space. Indeed, the reduction of the GMF to HMF was shown to significantly alter the gene expression of the fruit fly homolog MagR in important crop plants such as bean71 and maize84, suggesting an important role of plant MagR homologs in plant magnetoperception. Unlike migratory birds, plants presumably have no use for a magnetic compass and may derive some other evolutionary benefit from the presence of the GMF. Optimization of growth, for example, seems more likely than the development of a new sensory modality85.
Plants respond to atmospheric variations in pressure and composition
The development of greenhouses on Mars, on the Moon, and in Earth orbit considers the use of low atmospheric pressures (hypobaria) to address systems and engineering limitations86. It is reasonable to expect that reduced-pressure atmospheres will be used to decrease the lift costs of structural components and consumables for future transit vehicles and surface missions. In fact, mass reduction increases the space mission length and launched payloads87. However, alterations in atmospheric pressure are known to have effects on the physiology and development of plants88. Clarifying the mechanisms behind the physiological adaptation of plants to hypobaria is therefore very relevant to space exploration in the effort to expand food production in orbital and extra-terrestrial controlled agriculture. Growing plants under reduced pressure affects their growth and, depending on the species, may lead to either positive or negative effects. These effects are also correlated to atmospheric O2 and CO2 concentrations89. Low atmospheric pressure also affects water movement: transpiration rates increase as atmospheric pressure is reduced, even at high relative humidity, influencing stomatal aperture independently of relative humidity90. In general, plants show adaptation not only to hypobaria but also to gradients of atmospheric pressure, which induces the activation of genes that code for metabolic processes involved in the hypoxia stress response88. Crucially, under microgravity conditions free air convection is restricted, limit heat and gas distribution, causing unfavorable conditions close to the leaf. Long-term experiments conducted onboard the ISS have shown that the precise control of environmental parameters, including air circulation, normal evapotranspiration, and net photosynthetic rates, can be achieved by the plant, even in microgravity conditions91.
The reduced partial pressure of O2 (hypoxia) is widely used in plant experiments aimed at the partial mimicking of hypobaria. Recent work aimed at uncoupling the effects of hypoxia and hypobaria has shown that the latter causes phenotypic changes in development and metabolism, and in the expression of related genes, both in roots and shoots89,92. Early experiments with peas (Pisum sativum) and bean (Phaseolus aureus) showed that plants respond to hypobaria and hypoxia with alteration in the basic metabolism, including the Krebs cycle, mitochondrial respiration, and photosynthesis93,94. Evaluation of the A. thaliana differentially expressed genes (DEGs) showed that, in shoots exposed to hypobaria and/or hypoxia, adaptation to hypobaria included metabolic pathways well beyond those activated for the adaptation to hypoxia87. It was also demonstrated that the Arabidopsis net photosynthetic rate increased in hypobaria when CO2 partial pressure was a limiting factor and did not change at normal or increased CO2 partial pressure95. This indicates that CO2 concentrations can be kept elevated in hypobaric plant growth modules without affecting photosynthesis. In lettuce (Lactuca sativa), growth under hypobaria conditions has adverse effects on plant growth, gas exchange, and resistance to hypoxic conditions96,97. In this important crop, hypobaric conditions induce a higher production, compared to sole hypoxia, of the plant growth-inhibiting phytohormone ethylene98,99,100. While uptake of NH4+ and NO3− were improved by 30 kPa hypobaria under the same O2 partial pressure101, low oxygen stress induces the production of lettuce protective phytochemicals and the free radical scavenging potential102. In another important crop, wheat (Triticum aestivum), average rates of photosynthesis and transpiration increased in hypobaria (50 kPa) and also increased when oxygen partial pressure was reduced further; however, lower oxygen partial pressure (2.5 kPa) was unsuitable for reproductive growth of wheat103. In radish (Raphanus sativus), growth can be enhanced and transpiration reduced in hypobaria by enriching the atmosphere with CO2, although at high CO2 levels leaf damage may occur104. In this crop, hypobaric conditions perturbed the shoot nitrogen-related metabolism.
Ongoing research has demonstrated that, in plants, there is a clear separation between the effects of hypoxia and water stress, and hypobaria. Root and shoot DEGs of plants exposed to hypobaric conditions display a more complex regulatory pattern than simple hypoxia, a condition that cannot be recovered by increasing the O2 partial pressure89. This important result underlines the importance of evaluating the biological consequences of hypobaric environments for the exploration of life-support habitats. Our overall understanding of how atmospheric pressure influences plants and, hence, directly plant-driven bioregenerative fluxes is still very limited, and studies of the underlying genetic/molecular mechanisms are much needed89. Other environmental factors, such as humidity and atmospheric temperature and composition (including volatile organic compounds, or VOCs, airborne contaminants, and dust), which could crosstalk with the hypobaria response, are also very important and could affect plant growth in planetary greenhouses.
Light requirements for plant growth in the space context
Light is a critical environmental factor that has an influence on plant growth, from seed germination to flowering and fruiting. Light conditions, including photoperiod, intensity, and spectral quality, are among the most relevant external factors affecting plant growth and development105. Light spectral composition and intensity influence morphology, physiology, and development by impacting processes ranging from photosynthesis to secondary metabolism106,107. Genomic studies reported that light induces extensive reprogramming of gene expression patterns, and the effect of light (mainly red light) can help in restoring meristematic competence under microgravity conditions108. Furthermore, different photoperiods can modify the expression of genes regulated by the circadian clock, affecting flowering time109. Importantly for space applications, in the presence of light, roots remain negatively phototropic in microgravity110. In Arabidopsis, both WS and Col-0 ecotypes remained negatively phototropic in microgravity, although with differences in wave and skew directions111.
BLSS involving photoautotrophic organisms is needed to sustain long-duration crewed missions beyond LEO. Sunlight for plant growth is an unreliable source that depends on local conditions, while electric lighting (or hybrid electric-solar) systems are more suitable for growing plants in space108. Experiments have been conducted to verify plant growth using fluorescent lamps in the Lada plant chamber housed in the Russian module onboard the International Space Station (ISS)112. However, currently, Light-emitting diodes (LEDs) are considered the best option for BLSS growth facilities due to the limited space present onboard future orbiting and planetary stations. Moreover, LEDs are highly energy efficient, difficult to damage with physical shocks, and more resistant to extreme temperature changes than fluorescent lamps105. LEDs can also be used to modulate spectral composition in time and, therefore, tune plant productivity and yield110,113,114. Onboard the ISS, LEDs are currently used in the VEGGIE system, which includes red (630 nm), blue (455 nm), and green (530 nm) LEDs115.
The development of the so-called “light recipes” could help increase the efficiency of plant growth and modulate nutritional qualities. Experiments on wheat and Arabidopsis, conducted in the advanced plant habitat (APH) facility onboard the ISS, have already successfully tested optimized light spectra composed of blue, green, and red LED illumination at diverse light levels (e.g., 150 μmol m−2 s−1 for Arabidopsis and 600 μmol m−2 s−1 for wheat/Arabidopsis)116. Interestingly, it has been suggested that the red-light phototropic response can compensate for the stress induced by microgravity at the cellular level117. Moreover, in the plant, the light spectrum can regulate the production of secondary metabolites, like phenolic compounds, anthocyanins, and ascorbic acid, that are often beneficial for human health118. It is therefore paramount to further understand how light affects plant physiology, in an exploration context, to design adaptive growth regimes119,120.
The biotic challenges of growing plants in space
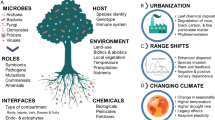
On Earth, plant growth, health and productivity are deeply influenced by a multitude of microorganisms, collectively known as the plant microbiota, that thrive on the outer surfaces as well as in internal tissues121,122. Some of these microbes are beneficial while other are commensal or pathogenic. The nature of the relationship established with the host plant depends on the partners involved, but the functional roles can also vary in response to environmental factors123,124.
Beneficial microbes, especially endophytes, significantly increase host fitness through improved nutrition and protection from biotic and abiotic stress122,125. Photosynthetic microorganisms may contribute to plant growth with their potential biostimulant effects for life in closed environments, as recently reviewed126. On the other hand, plant-microbe associations can contribute to supporting plants survival, growth, and health under harsh environmental conditions such as those of space missions. Microbes can promote plant growth through more efficient and sustainable use of nutrients—e.g., nitrogen provided by nitrogen-fixing rhizobia127 and phosphorus provided by mycorrhizal fungi128—as well as through the emission of volatile organic compounds129,130, which can even more efficiently accumulate in close environments. In addition, plant-associated microbes can provide higher tolerance to abiotic stresses (water shortage, high/low temperature, etc.) and to phytopathogens, which can be accidentally introduced in space environments, through the activation of plant immune responses131,132.
Plant pathogens: a threat also for space farming
Plant disease control in enclosed environments, especially in microgravity conditions, is regarded as a serious issue for precision farming. Prevention and control of plant pathogens in space are particularly critical because plants are known to be more susceptible to fungal pathogen infection in microgravity condition133,134. The choice in plant species, management practice, and growth conditions can dictate the presence and abundance of microbial pathogens. Moreover, spacecrafts and space habitats supporting human exploration, which harbor diverse microbial populations, can act as a reservoir of potential pathogens135. Beside good sanitation procedure, a better understanding of phytopathogen interactions is of relevance to guarantee food security and safety in space. New strategies involve the optimization of growth conditions to stir plant development towards tolerance to pathogens, e.g., inducing the production of specialized metabolites with a role in defence (including VOCs), and the use of beneficial plant-associated microbes that can lead to a priming status129.
Plant pathogens can affect the success of a mission by destroying plants serving as food sources or in as recycling systems136,137. The formation of the plant structural barriers and the activation of plant defence responses has been shown to be impaired in spaceflight conditions, while altered gravity can stimulate pathogens growth and reproduction, increasing their pathogenicity134. Interestingly, after five/seven years in space, tomato seeds did not show a relevant decrease in germination or performance, but enhanced resistance to pathogens138. However, pathogens in enclosed habitats, including seed-borne ones, can lead to extensive plant damage136. The first instance of plant pathogens in space was related to Neotyphodium chilense, a fungal plant pathogen fungus, that was found to be the underlying disease agent of wheat seedlings inside the Plant Growth Unit139. An experiment dedicated to verifying the effect of microgravity on plant-phytopathogen interaction was performed on the Shuttle STS-87 flight. Soybean roots were inoculated with oospores of the Phytophthora sojae, which is a common root-rotting pathogen in this plant species140. The disease symptoms, the extent of the infection, and the number of new oospores were significantly higher in space-flown plants compared to ground controls. The Vegetable Production System (Veggie), a facility onboard the ISS to produce fresh vegetables,115,141 uses the ISS cabin air for dehumidification and temperature control. When this air happened to be contaminated with infective propagules of the plant pathogen Fusarium oxysporum, it led to severe disease in Zinnia hybrida plants that were being grown in the Veggie module142. The development of symptoms in microgravity conditions was correlated to a reduced airflow that led to an increase in the water enveloping the leaves and stems. Generally speaking, the increase in disease severity in microgravity, reported in all the above-mentioned cases, was correlated to low-light levels, elevated relative humidity, and the environment in the spacecraft136,142,143. It has been suggested that challenges related to microbial and insect pests that occur in field, greenhouse, or vertical-farming, are also valid for future BLSS-supported missions. Specific protocols based on an Integrated Pest Management approach should therefore be developed, taking into account all aspects of plant production, to avoid the spreading of potential pathogens136.
Beneficial interactions between plants and microbes can sustain plant production for space applications
Beneficial components of the plant microbiota can significantly increase plant fitness through the modulation of regulatory networks involved in nutrient acquisition, development, and immune responses122,144,145,146. Special attention has been given to bacterial and fungal endophytes that, by colonizing plant tissues, establish a more intimate association with plants124,125,147. Well-known examples are symbiotic, nitrogen-fixing rhizobia148 and plant growth-promoting rhizobacteria (PGPR) such as Bacillus subtilis149. Beneficial fungal endophytes include the shoot grass Epichloë spp.150, the plant-growth-promoting and biocontrol agent Trichoderma spp.151, and the well-known mycorrhizal fungi, which are characterized by specialized plant-fungal interfaces for mutualistic resource exchanges128,152. Root-associated microbes activate the so-called induced systemic resistance (ISR) that can accelerate defense-related gene expression in the host, thus priming the plant immune system and improving tolerance to a broad range of pathogens and insect herbivores132.
Current knowledge about the potential of plant-associated microorganisms to support plant life in spaceflight and extra-terrestrial environments is limited153,154. There is evidence that rhizobia can improve soil fertility and plant growth in Martian regolith simulant155. However, it has been shown that microgravity can have a negative impact on the formation of nodules156. Similarly, mycorrhization and phosphate uptake were reduced in the model plant Petunia hybrida under simulated microgravity157. This effect, likely due to an inhibition of hyphal elongation and branching, could be reduced with an increase in root exudate of strigolactones, plant rhizospheric signals able to promote arbuscular mycorrhizal colonization157. This suggests the possibility of manipulating plant-fungal chemical communication for the successful establishment of mycorrhizas under challenging conditions. Careful consideration of the plant microbiota should, therefore, be included in the development of crops with improved performance and better adaptability to space conditions153,158.
Other mutualistic symbiotic systems of interest for astro-biotechnological applications are lichens, where unicellular green algae and/or cyanobacteria associate with fungi. Lichens represent ideal meta-organisms for investigating the survival and adaptability of living organisms to harsh environments as they can show tolerance to extreme space-like conditions, e.g., dehydration, extremely low temperatures, and oxygen depletion134. In addition, some lichens were shown to produce, under specific conditions, a high amount of molecular hydrogen (H2)159, which is considered one of the most promising fuels in the future.
Abiotic and biotic interactions converge in the definition of plant substrates for space farming
On Earth, plant growth is sustained by soil, a complex mixture of organic matter, minerals, gases, liquids, and organisms, that is not found on any other known planetary body. However, the transport of soil to future space settlements is not logistically feasible. While establishing an in situ paedogenic process starting from lunar and Martian regolith is a possibility in the long term, space farming will rely on the modern cultivation practices of the soilless system for the foreseeable future. Soilless growth practices require less water and nutrient application and are, overall, more sustainable to produce plants in a controlled environment. Depending on the exploration objective, plants could be grown with hydroponics (classical or nutrient film technique, NFT), aeroponics, on various solid substrates, or amended regolith and technosols. Onboard the ISS, plant growth in the Veggie is sustained by clay-based “pillows” filled with fertilizer. The pillows have been designed to help distribute water and air in an optimal way, considering fluid behavior in space160. Generally speaking, the use of soilless growth systems allows to counteract the altered behavior of fluids in microgravity and actively supplies the roots with the nutrient solution.
The properties of different growth substrates can affect water, oxygen, and nutrient availability for the plant. Localized deficiency or oversupply of nutrients and gasses can induce morphological, physiological, and biochemical adaptations161. For instance, it has been shown that plants grown in a hydroponic system differ from their solid substrate-grown counterparts in the number of leaves, stomatal density, and content of pigments, sugars, and ions162. Moreover, it has been shown that while natural fiber substrates tend to promote yield and production turnover, this is accompanied by a reduction in phytochemical content in microgreens163. It is therefore important to understand how plants adapt to different substrates, in the context of all different applications of plants in space exploration (food production, biomining, and bioremediation). Issues could arise with pH, nutrient availability, air and fluid movement, the presence of potentially toxic elements, and altered root microbiota.
Indigenous regoliths are being considered as potential substrates for plant growth164. In addition to being available in situ, regoliths could represent a solid substrate and a source of inorganic nutrients. The growth of many species of plant has been tested using Moon and Mars regolith simulants, with various degrees of success165. When present, the effects of regolith simulants ranged from stunted growth to reduced seed quality and viability165,166,167. Recently, it was shown that the use of actual lunar regolith brought back from Apollo 11, 12, and 17 missions168, slows plant development and induces severe stress-related morphologies. Plants grown in lunar soils differentially expressed genes related to ionic stresses, similar to plant reactions to salt, metal, and reactive oxygen species. These data indicate that, although in situ lunar regoliths can be useful for plant production in lunar habitats, they are not ideal substrates for plant growth168. The amendment with compost percolates and bio-weathering has already been shown to improve the fitness of plant growth regolith simulants166, so subjecting lunar and Martian regolith to a paedogenic process could make them suitable for plant space farming for future applications169,170.
The growth substrate is a critical element for space farming applications. It must fulfill specific characteristics, such as guaranteeing the respiration of roots and the absorption of nutrients. This can be achieved by the geometry, distribution and size of the pores, and the type of material used171. An emerging technology capable of providing this improvement in current space agriculture systems is 3D printing, also called “Additive Manufacturing” (AM). AM can be used to create complex and hierarchical structures by using different printing techniques and different materials, that could also be, in the future, recycled172,173. AM is a rapidly developing technology, for which new materials and technologies capable of building increasingly complex and tailored structures are constantly developed.
Figure 1 summarizes abiotic and biotic factors that characterize the space environment.
Ionizing radiations can affect plants at both the genetic and epigenetic levels. Gravity alterations can significantly influence plant reproduction and affect the development of male and female reproductive organs. Understanding plant response to magnetic fields will be important to forecast the behavior of plants in extreme environments where the magnetic fields will be different (Lunar Gateway, Moon, Mars). Understanding the plant responses to atmospheric pressure variations, like hypobaria, is very relevant to space exploration in the effort to expand food production in orbital and extra-terrestrial controlled agriculture. Light influences plant growth, from seed germination to flowering and fruiting. The effect of light quality and quantity can help restore meristematic competence under microgravity conditions, and light-emitting diodes (LEDs) are currently used in space farming to modulate spectral composition for optimal plant growth. Plant-microbe associations can contribute to support plants survival, growth and health under harsh environmental conditions such as those of space missions. Nutrient assimilation in plants can be improved by nitrogen-fixing rhizobia and mycorrhizal fungi. Pathogens can affect plant productivity and pose a risk to food safety, both on Earth and in space.
Conclusions
To achieve the current human exploration goals, future space outposts must be designed as self-sufficient, closed ecosystems that require minimum expenditure of energy and in which resources, like air, water, and food, are regenerated. Plants are a fundamental component of BLSS as they supply O2, sequester CO2, purify water, and produce fresh food. Additional research on the fundamental mechanisms underlying the interaction between plants and their adaptation to the space environment is essential to guide future applied research and guarantee the success of long-term goals (Fig. 2). Despite the wealth of knowledge produced in the past few decades, a lot of work still needs to be done to understand the synergy between different stressors and their possible impact on crop growth and productivity. Importantly, it has been shown that in the multifactorial stress combination, even if the level of each individual stressor is below the response threshold, plant growth and survival will decline dramatically with the number and complexity of stressors involved174. This is partially due to technical difficulties and the many constraints of performing biological research in space175,176. Genetic engineering (biotechnologies), guided by fundamental molecular research and applied to plant breeding, has the potential to accelerate the production of optimal crops for space applications177. Advances in these fields could have positive impacts on Earth, by creating novel, highly efficient agricultural technologies suited to meet the “From Farm to Fork” strategy objectives and innovative concepts for sustainability.
Investigations regarding plant responses and adaptation to the space environment are built on the previous work done to understand fundamental mechanisms of plant physiology, but also contribute to the understanding of universal molecular pathways and the development of tools that will benefit life on Earth. The optimization the plant adaptation to the space environment and the plant-microbe interactions will allow the design of reliable and sustainable Bioregenerative Life Support Systems (BLSS) and the obtainment of In-Situ Resource Utilization (ISRU).
The knowledge of the plant responses on Earth and the available technologies (substrates, light, etc.) for precision farming, represent the starting point to understand how plant physiology is affected by space conditions. Future trends in plant biotic interactions include optimization of space-oriented plant-microorganism interactions, whereas the abiotic conditions require a deep knowledge of the multi-omics responses and of the mechanisms adopted by plants to tolerate and adapt to the space environment.
There are different genetic engineering technologies that can result in plants that contain different types of ameliorative modifications. On Earth, current legislation concerning the growth and human consumption of biotechnological products is often country- and technology-specific. Some countries, like the US and Canada, have more relaxed rules for genetically modified organisms (GMOs) and allow growth and consumption. Being especially concerned by the environmental risks posed by GMO cultivation, the EU has only recently ruled to relax the legislation on Category-1-NGT (New Genomic Techniques) plants, i.e., plants that have been produced using targeted mutagenesis technologies, like CRISPR/Cas, and contain only genetic material that is present in the gene pool of the species used for breeding. Naturally, to ensure food security for space consumption and exclude any risk to human health, all crops would need to be severely evaluated, at least to the same standards used on Earth. Moreover, while contamination of space and celestial bodies should be avoided, as also stated in the Space Law Treaties of the United Nations Office for Outer Space Affairs, GMO products in space should not cause the same environmental concerns as on Earth, since they are equally contaminants to space than any other terrestrial biological material. As the more long-term objectives of human space exploration become closer, there is a need to start discussing the use of GMO and gene-edited organisms, beyond research purposes, for food production and human consumption.
In 2015, astronauts onboard the ISS consumed the first lettuce produced in space143. However, we are still far from achieving a reliable and sustainable plant food production system in space that can meet the dietary needs of a crew. A pragmatic approach that focuses on technological advances and applied research is needed to meet the requirements of already planned missions and stimulate the involvement of the private sector.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Angelopoulos, V. The ARTEMIS mission. Space Sci. Rev. 165, 3–25 (2011).
Binot, R. A., Tamponnet, C. & Lasseur, C. in Life Sciences and Space Research XXV. Vol. 14 Advances in Space Research (eds. MacElroy, R. D. et al.) 71–74 (Pergamon Press Ltd, 1994).
Bunchek, J. M. et al. Pick-and-eat space crop production flight testing on the International Space Station. J. Plant Interact. 19, 2292220 (2024).
Wheeler, R. M. et al. Effects of elevated and super-elevated carbon dioxide on salad crops for space. J. Plant Interact. 19, 2292219 (2024).
De Pascale, S. et al. Biology and crop production in Space environments: challenges and opportunities. Life Sci. Space Res. 29, 30–37 (2021).
Morita, M. T. & Tasaka, M. Gravity sensing and signaling. Curr. Opin. Plant Biol. 7, 712–718 (2004).
Fukaki, H. et al. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 14, 425–430 (1998).
MacCleery, S. A. & Kiss, J. Z. Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 120, 183–192 (1999).
Tasaka, M., Kato, T. & Fukaki, H. The endodermis and shoot gravitropism. Trends Plant Sci. 4, 103–107 (1999).
Ottenschlager, I. et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl Acad. Sci. USA 100, 2987–2991 (2003).
Herranz, R. & Medina, F. J. Cell proliferation and plant development under novel altered gravity environments. Plant Biol. 16 Suppl 1, 23–30 (2014).
Morris, E. C. et al. Shaping 3D root system architecture. Curr. Biol. 27, R919–R930 (2017).
Roychoudhry, S. & Kepinski, S. Things fall into place: how plants sense and respond to gravity. Nature 631, 745–747 (2024).
Chen, J. et al. Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants. Cell 186, 4788–4802.e4715 (2023).
Nishimura, T. et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 381, 1006–1010 (2023).
Bai, Q. et al. Molecular mechanism of brassinosteroids involved in root gravity response based on transcriptome analysis. BMC Plant Biol. 24, 485 (2024).
Huang, S.-J. et al. A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J. Exp. Bot. 64, 4343–4360 (2013).
Löfke, C. et al. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc. Natl Acad. Sci. USA 110, 3627–3632 (2013).
Gutjahr, C. et al. Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta 222, 575–585 (2005).
Yoder, T. L., Zheng, H. Q., Todd, P. & Staehelin, L. A. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 125, 1045–1060 (2001).
Wyatt, S. E. & Kiss, J. Z. Plant tropisms: from Darwin to the International Space Station. Am. J. Bot. 100, 1–3 (2013).
Chauvet, H., Pouliquen, O., Forterre, Y., Legué, V. & Moulia, B. Inclination not force is sensed by plants during shoot gravitropism. Sci. Rep. 6, 35431 (2016).
Kordyum, E. L. Plant cell gravisensitivity and adaptation to microgravity. Plant Biol. 16 Suppl 1, 79–90 (2014).
Nedukha, E. M. Effects of microgravity on the structure and function of plant cell walls. Int. Rev. Cytol. 170, 39–77 (1997).
Brykov, V. A., Generozova, I. P. & Shugaev, A. G. Ultrastructure and metabolic activity of pea mitochondria under clinorotation. Tsitol. Genet. 46, 20–26 (2012).
Stutte, G. W., Monje, O., Goins, G. D. & Tripathy, B. C. Microgravity effects on thylakoid, single leaf, and whole canopy photosynthesis of dwarf wheat. Planta 223, 46–56 (2005).
Kalinina, I. Microtubules spatial alterations in root cells of Brassica rapa under clinorotation. Cell. Biol. Int. 32, 581–583 (2008).
Romanchuk, S. M. Ultrastructure of statocytes and cells of distal elongation zone of Arabidopsis thaliana under clinorotation. Tsitol. Genet. 44, 3–8 (2010).
Mugnai, S. et al. Oxidative stress and NO signalling in the root apex as an early response to changes in gravity conditions. Biomed. Res. Int. 2014, 834134 (2014).
Kozeko, L. Y. & Kordyum, E. L. Heat shock proteins HSP70 and HSP90 in pea seedlings under clinorotation of different duration. J. Gravit. Physiol. 14, 115–116 (2007).
Medina, F. J. & Herranz, R. Microgravity environment uncouples cell growth and cell proliferation in root meristematic cells: the mediator role of auxin. Plant Signal. Behav. 5, 176–179 (2010).
Manzano, A. I., Herranz, R., Manzano, A., van Loon, J. J. W. A. & Medina, F. J. Early effects of altered gravity environments on plant cell growth and cell proliferation: characterization of morphofunctional nucleolar types in an Arabidopsis cell culture system. Front. Astron. Space Sci. 3, 2 (2016).
Chebli, Y. et al. Cell wall assembly and intracellular trafficking in plant cells are directly affected by changes in the magnitude of gravitational acceleration. PLoS One 8, e58246 (2013).
Karahara, I. et al. Vegetative and reproductive growth of Arabidopsis under microgravity conditions in space. J. Plant Res. 133, 571–585 (2020).
Manzano, A. et al. Novel, Moon and Mars, partial gravity simulation paradigms and their effects on the balance between cell growth and cell proliferation during early plant development. NPJ Microgravity 4, 9 (2018).
Jia, Y. & Lin, Z. W. The radiation environment on the Moon from galactic cosmic rays in a lunar habitat. Radiat. Res. 173, 238–244 (2010).
Dobynde, M. I., Shprits, Y. Y., Drozdov, A. Y., Hoffman, J. & Li, J. Beating 1 Sievert: optimal radiation shielding of astronauts on a mission to Mars. Space Weather 19, e2021SW002749 (2021).
Puchta, H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J. Exp. Bot. 56, 1–14 (2005).
Bennetzen, J. L. & Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 65, 505–530 (2014).
Alzohairy, A. M. et al. Environmental stress activation of plant long-terminal repeat retrotransposons. Funct. Plant Biol. 41, 557–567 (2014).
Caplin, N. & Willey, N. Ionizing radiation, higher plants, and radioprotection: from acute high doses to chronic low doses. Front. Plant Sci. 9, 847 (2018).
Arena, C., De Micco, V., Macaeva, E. & Quintens, R. Space radiation effects on plant and mammalian cells. Acta Astronaut. 104, 419–431 (2014).
De Micco, V., Arena, C., Pignalosa, D. & Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 50, 1–19 (2011).
Mousseau, T. A. & Moller, A. P. Plants in the light of ionizing radiation: What have we learned from Chernobyl, Fukushima, and other “hot” places? Front. Plant Sci. 11, 9 (2020).
Gudkov, S. V., Grinberg, M. A., Sukhov, V. & Vodeneev, V. Effect of ionizing radiation on physiological and molecular processes in plants. J. Environ. Radioact. 202, 8–24 (2019).
De Francesco, S. et al. Growth, anatomical, and biochemical responses of the space farming Candidate Brassica rapa L. Microgreens to low-LET ionizing radiation. Horticulturae 9, 452 (2023).
Vitale, E. et al. Light quality modulates photosynthesis and antioxidant properties of B. vulgaris L. plants from seeds irradiated with high-energy heavy ions: implications for cultivation in space. Plants 11, 18 (2022).
Mulinacci, N. et al. Effects of ionizing radiation on bio-active plant extracts useful for preventing oxidative damages. Nat. Prod. Res. 33, 1106–1114 (2019).
Horemans, N. et al. Current evidence for a role of epigenetic mechanisms in response to ionizing radiation in an ecotoxicological context. Environ. Pollut. 251, 469–483 (2019).
De Micco, V., Arena, C., Di Fino, L. & Narici, L. Radiation environment in exploration-class space missions and plants’ responses relevant for cultivation in bioregenerative life support systems. Front. Plant Sci. 13, 17 (2022).
da Silva, J. A. T. & Dobranszki, J. Magnetic fields: how is plant growth and development impacted? Protoplasma 253, 231–248 (2016).
Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 25, 1107–1119 (2019).
Sarraf, M. et al. Effect of magnetopriming on photosynthetic performance of plants. Int. J. Mol. Sci. 22, 14 (2021).
Maffei, M. E. in Bioelectromagnetism. History, Foundations and Applications (eds U. Shoogo & S. Tsukasa) Ch. 5, 191–214 (CRC Press, 2022).
Guo, J. P., Wan, H. Y., Matysik, J. & Wang, X. J. Recent advances in magnetosensing cryptochrome model systems. Acta Chim. Sin. 76, 597–604 (2018).
Hore, P. J. & Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 45, 299–344 (2016).
Golesworthy, M. J. et al. Singlet-triplet dephasing in radical pairs in avian cryptochromes leads to time-dependent magnetic field effects. J. Chem. Phys. 159, 11 (2023).
Pooam, M. et al. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 249, 319–332 (2019).
Hammad, M. et al. Cryptochrome mediated magnetic sensitivity in Arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 19, 341–352 (2020).
Parmagnani, A. S., D’Alessandro, S. & Maffei, M. E. Iron-sulfur complex assembly: potential players of magnetic induction in plants. Plant Sci. 325, 111483 (2022).
Qin, S. et al. A magnetic protein biocompass. Nat. Mater. 15, 217–226 (2016).
Yang, P. L. et al. A rationally designed building block of the putative magnetoreceptor MagR. Bioelectromagnetics 43, 317–326 (2022).
Zhou, Y. J. et al. Towards magnetism in pigeon MagR: Iron- and iron- sulfur binding work indispensably and synergistically. Zool. Res. 44, 142–152 (2023).
Guo, Z. et al. Modulation of MagR magnetic properties via iron–sulfur cluster binding. Sci. Rep. 11, 23941 (2021).
Occhipinti, A., De Santis, A. & Maffei, M. E. Magnetoreception: an unavoidable step for plant evolution? Trends Plant Sci. 19, 1–4 (2014).
Hori, K., Nilsson, A. & Tobias, S. M. Waves in planetary dynamos. Rev. Mod. Plasma Phys. 7, 5 (2023).
Teixeira da Silva, J. A. & Dobranszki, J. How do magnetic fields affect plants in vitro? Vitr. Cell Dev. Biol. Plant 51, 233–240 (2015).
Bertea, C. M., Narayana, R., Agliassa, C., Rodgers, C. T. & Maffei, M. E. Geomagnetic field (Gmf) and plant evolution: investigating the effects of Gmf reversal on Arabidopsis thaliana development and gene expression. J. Vis. Exp. 105, 53286 (2015).
Paponov, I. A., Fliegmann, J., Narayana, R. & Maffei, M. E. Differential root and shoot magnetoresponses in Arabidopsis thaliana. Sci. Rep. 11, 14 (2021).
Parmagnani, A. S., Mannino, G. & Maffei, M. E. Transcriptomics and metabolomics of reactive oxygen species modulation in near-null magnetic field-induced Arabidopsis thaliana. Biomolecules 12, 1824 (2022).
Parmagnani, A. S. et al. The Geomagnetic Field (GMF) is required for Lima bean photosynthesis and reactive oxygen species production. Int. J. Mol. Sci. 24, 2896 (2023).
Agliassa, C., Narayana, R., Christie, J. M. & Maffei, M. E. Geomagnetic field impacts on cryptochrome and phytochrome signaling. J. Photochem. Photobiol. B-Biol. 185, 32–40 (2018).
Vigani, G., Islam, M., Cavallaro, V., Nocito, F. F. & Maffei, M. E. Geomagnetic field (GMF)-dependent modulation of iron-sulfur interplay in Arabidopsis thaliana. Int. J. Mol. Sci. 22, 15 (2021).
Islam, M., Maffei, M. E. & Vigani, G. The geomagnetic field is a contributing factor for an efficient iron uptake in Arabidopsis thaliana. Front. Plant Sci. 11, 15 (2020).
Agliassa, C. & Maffei, M. E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects some Arabidopsis thaliana clock genes amplitude in a light independent manner. J. Plant Physiol. 232, 23–26 (2019).
Rosen, A. D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell. Biochem. Biophys. 39, 163–173 (2003).
Denegre, J. M., Valles, J. M. Jr., Lin, K., Jordan, W. B. & Mowry, K. L. Cleavage planes in frog eggs are altered by strong magnetic fields. Proc. Natl Acad. Sci. USA 95, 14729–14732 (1998).
Valiron, O. et al. Cellular disorders induced by high magnetic fields. J. Magn. Reson. Imaging 22, 334–340 (2005).
Paul, A. L., Wheeler, R. M., Levine, H. G. & Ferl, R. J. Fundamental plant biology enabled by the space shuttle. Am. J. Bot. 100, 226–234 (2013).
Penuelas, J., Llusia, J., Martinez, B. & Fontcuberta, J. Diamagnetic susceptibility and root growth responses to magnetic fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromag. Biol. Med. 23, 97–112 (2004).
Xu, C., Li, Y., Yu, Y., Zhang, Y. & Wei, S. Suppression of Arabidopsis flowering by near-null magnetic field is affected by light. Bioelectromagnetics 36, 476–479 (2015).
Jaworska, M., Domanski, J., Tomasik, P. & Znoj, K. Stimulation of pathogenicity and growth of entomopathogenic fungi with static magnetic field. J. Plant Dis. Prot. 123, 295–300 (2016).
Nagy, P. & Fischl, G. Effect of static magnetic field on growth and sporulation of some plant pathogenic fungi. Bioelectromagnetics 25, 316–318 (2004).
Fiorillo, A. et al. 14-3-3 proteins and the plasma membrane H+-ATPase are involved in maize (Zea mays) magnetic induction. Plants 12, 2887 (2023).
Hore, P. J., Ivanov, K. L. & Wasielewski, M. R. Spin chemistry. J. Chem. Phys. 152, 120401 (2020).
Drysdale, A. E. Life support trade studies involving plants. SAE Tech. Pap. 2001, 2362 (2001).
Paul, A. L. et al. Hypobaric biology: Arabidopsis gene expression at low atmospheric pressure. Plant Physiol. 134, 215–223 (2004).
Paul, A. L. et al. Patterns of Arabidopsis gene expression in the face of hypobaric stress. AoB Plants 9, 19 (2017).
Zhou, M. Q. et al. Dissecting low atmospheric pressure stress: Transcriptome responses to the components of hypobaria in Arabidopsis. Front. Plant Sci. 8, 528 (2017).
Bauer, H. et al. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23, 53–57 (2013).
Monje, O. & Bugbee, B. Adaptation to high CO2 concentration in an optimal environment: radiation capture, canopy quantum yield and carbon use efficiency. Plant Cell Environ. 21, 315–324 (1998).
Arce, C. C. M., Bont, Z., Machado, R. A. R., Cristaldo, P. F. & Erb, M. Adaptations and responses of the common dandelion to low atmospheric pressure in high-altitude environments. J. Ecol. 109, 3487–3501 (2021).
Musgrave, M. E., Gerth, W. A., Scheld, H. W. & Strain, B. R. Growth and mitochondrial respiration of mungbeans (Phaseolus aureus Roxb) germinated at low-pressure. Plant Physiol. 86, 19–22 (1988).
Astafurova, T. P., Vaishlya, O. B., Verkhoturova, G. S., Zaitseva, T. A. & Chirkova, T. V. Effect of hypobaric hypoxia on photosynthetic and respiratory metabolism of plants. Sov. Plant Physiol. 37, 524–529 (1990).
Richards, J. T. et al. Exposure of Arabidopsis thaliana to hypobaric environments: Implications for low-pressure bioregenerative life support systems for human exploration missions and terraforming on Mars. Astrobiology 6, 851–866 (2006).
He, C. J., Davies, F. T. & Lacey, R. E. Separating the effects of hypobaria and hypoxia on lettuce: growth and gas exchange. Physiol. Plant. 131, 226–240 (2007).
He, C. J., Davies, F. T. & Lacey, R. E. Hypobaria, hypoxia, and light affect gas exchange and the CO2 compensation and saturation points of lettuce (Lactuca sativa). Botany 87, 712–721 (2009).
He, C. J. & Davies, F. T. Ethylene reduces plant gas exchange and growth of lettuce grown from seed to harvest under hypobaric and ambient total pressure. J. Plant Physiol. 169, 369–378 (2012).
Tang, Y. K. et al. Effects of long-term low atmospheric pressure on gas exchange and growth of lettuce. Adv. Space Res. 46, 751–760 (2010).
He, C. J., Davies, F. T., Lacey, R. E., Drew, M. C. & Brown, D. L. Effect of hypobaric conditions on ethylene evolution and growth of lettuce and wheat. J. Plant Physiol. 160, 1341–1350 (2003).
Tang, Y. K., Gao, F., Yu, Q. N., Guo, S. S. & Li, F. The uptake kinetics of NH4+ and NO3− by lettuce seedlings under hypobaric and hypoxic conditions. Sci. Hortic. 197, 236–243 (2015).
Rajapakse, N. C., He, C. J., Cisneros-Zevallos, L. & Davies, F. T. Hypobaria and hypoxia affects growth and phytochemical contents of lettuce. Sci. Hortic. 122, 171–178 (2009).
Guo, S. S., Tang, Y. K., Gao, F., Ai, W. D. & Qin, L. F. Effects of low pressure and hypoxia on growth and development of wheat. Acta Astronaut. 63, 1081–1085 (2008).
Gohil, H. L., Bucklin, R. A. & Correll, M. J. The effects of CO2 on growth and transpiration of radish (Raphanus sativus) in hypobaria. Adv. Space Res. 45, 823–831 (2010).
Carillo, P. et al. Light spectral composition affects metabolic response and flowering in non-vernalized Ranunculus asiaticus L. Environ. Exp. Bot. 192, 104649 (2021).
Vandenbrink, J. P., Kiss, J. Z., Herranz, R. & Medina, F. J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 5, 563 (2014).
Darko, E., Heydarizadeh, P., Schoefs, B. & Sabzalian, M. R. Photosynthesis under artificial light: the shift in primary and secondary metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130243 (2014).
Poulet, L. et al. Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci. Space Res. 2, 43–53 (2014).
Wang, L. et al. Transcriptomic analysis of the interaction between FLOWERING LOCUS T induction and photoperiodic signaling in response to spaceflight. Front. Cell Dev. Biol. 9, 813246 (2022).
Blancaflor, E. B. et al. A Researcher’s Guide to International Space Station Plant Science. (NASA ISS Research Integration Office, 2023).
Paul, A.-L., Amalfitano, C. E. & Ferl, R. J. Plant growth strategies are remodeled by spaceflight. BMC Plant Biol. 12, 232 (2012).
Sychev, V. N., Levinskikh, M. A., Gostimsky, S. A., Bingham, G. E. & Podolsky, I. G. Spaceflight effects on consecutive generations of peas grown onboard the Russian segment of the International Space Station. Acta Astronaut. 60, 426–432 (2007).
Massa, G. D., Kim, H.-H., Wheeler, R. M. & Mitchell, C. A. Plant productivity in response to LED lighting. Hortscience 43, 1951–1956 (2008).
Lazzarin, M. et al. LEDs make it resilient: effects on plant growth and defense. Trends Plant Sci. 26, 496–508 (2021).
Massa, G. D., Wheeler, R. M., Morrow, R. C. & Levine, H. G. In Growth Chambers on the International Space Station for Large Plants. 1134 edn. 215–222 (International Society for Horticultural Science (ISHS), Leuven, Belgium, 2016).
Monje, O. et al. Hardware validation of the advanced plant habitat on ISS: Canopy photosynthesis in reduced gravity. Front. Plant Sci. 11, 673 (2020).
Millar, K. D. L. et al. A novel phototropic response to red light is revealed in microgravity. N. Phytol. 186, 648–656 (2010).
Thoma, F., Somborn-Schulz, A., Schlehuber, D., Keuter, V. & Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: a review. Front. Plant Sci. 11, 497 (2020).
Kordyum, E. & Hasenstein, K. H. Plant biology for space exploration - Building on the past, preparing for the future. Life Sci. Space Res. 29, 1–7 (2021).
Vermeulen, A. C. J., Hubers, C., de Vries, L. & Brazier, F. What horticulture and space exploration can learn from each other: the mission to mars initiative in the Netherlands. Acta Astronaut. 177, 421–424 (2020).
Fitzpatrick, C. R. et al. The plant microbiome: from ecology to reductionism and beyond. Annu. Rev. Microbiol. 74, 81–100 (2020).
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T. & Singh, B. K. Plant-microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621 (2020).
Bastìas, D. A., Balestrini, R., Pollmann, S. & Gundel, P. E. Environmental interference of plant-microbe interactions. Plant Cell Environ. 45, 3387–3398 (2022).
Porras-Alfaro, A. & Bayman, P. Hidden fungi, emergent properties: endophytes and microbiomes. Annu. Rev. Phytopathol. 49, 291–315 (2011).
Yan, L. et al. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 103, 3327–3340 (2019).
Renaud, C., Leys, N. & Wattiez, R. Photosynthetic microorganisms, an overview of their biostimulant effects on plants and perspectives for space agriculture. J. Plant Interact. 18, 2242697 (2023).
Udvardi, M. & Poole, P. S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64, 781–805 (2013).
Lanfranco, L., Fiorilli, V. & Gutjahr, C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. N. Phytol. 220, 1031–1046 (2018).
Parmagnani, A. S. et al. Bacterial volatiles (mVOC) emitted by the phytopathogen Erwinia amylovora promote Arabidopsis thaliana growth and oxidative stress. Antioxidants 12, 600 (2023).
Fincheira, P. & Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 208, 63–75 (2018).
Alagna, F., Balestrini, R., Chitarra, W., Marsico, A. D. & Nerva, L. in Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants (eds Md. A. Hossain et al.) 35–56 (Academic Press, 2020).
Pieterse, C. M. et al. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375 (2014).
Foster, J. S., Wheeler, R. M. & Pamphile, R. Host-microbe interactions in microgravity: assessment and implications. Life 4, 250–266 (2014).
Fu, Y. M. et al. Change of growth promotion and disease resistant of wheat seedling by application of biocontrol bacterium Pseudochrobactrum kiredjianiae A4 under simulated microgravity. Acta Astronaut. 139, 222–227 (2017).
Checinska Sielaff, A. et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 7, 50 (2019).
Schuerger, A. C. Integrated pest management protocols for space-based bioregenerative life support systems. Front. Astron. Space Sci. 8, 75964 (2021).
Pierson, D. L. Microbial contamination of spacecraft. Gravit. Space Biol. Bull. 14, 1–6 (2001).
Mishchenko, L., Dunich, A. & Danilova, O. Impact of a real microgravity on the productivity of tomato plants and resistance to viruses. Proceedings of the Life in Space for Life on Earth. 18–22 June 2012, at Aberdeen, UK. ESA-SP Vol. 706, Id. 48 (2013)
Bishop, D. L., Levine, H. G., Kropp, B. R. & Anderson, A. J. Seedborne fungal contamination: consequences in space-grown wheat. Phytopathology 87, 1125–1133 (1997).
Ryba-White, M. et al. Growth in microgravity increases susceptibility of soybean to a fungal pathogen. Plant Cell Physiol. 42, 657–664 (2001).
Massa, G. D. et al. VEG-01: Veggie hardware validation testing on the International Space Station. Open Agriculture 2, 33–41 (2017).
Schuerger, A. C. et al. Fusarium oxysporum as an opportunistic fungal pathogen on Zinnia hybrida plants grown on board the International Space Station. Astrobiology 21, 1029–1048 (2021).
Khodadad, C. L. M. et al. Microbiological and nutritional analysis of lettuce crops grown on the International Space Station. Front. Plant Sci. 11, 199 (2020).
Chialva, M., Lanfranco, L. & Bonfante, P. The plant microbiota: composition, functions, and engineering. Curr. Opin. Biotechnol. 73, 135–142 (2022).
Teixeira, P. et al. Specific modulation of the root immune system by a community of commensal bacteria. Proc. Natl Acad. Sci. USA 118, e2100678118 (2021).
Salas-González, I. et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 371, eabd0695 (2021).
Santoyo, G., Moreno-Hagelsieb, G., Orozco-Mosqueda Mdel, C. & Glick, B. R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99 (2016).
Roy, S. et al. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32, 15–41 (2020).
Blake, C., Christensen, M. N. & Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 34, 15–25 (2021).
Bastias, D. A., Martínez-Ghersa, M. A., Ballaré, C. L. & Gundel, P. E. Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends Plant Sci. 22, 939–948 (2017).
Lorito, M., Woo, S. L., Harman, G. E. & Monte, E. Translational research on Trichoderma: from ‘omics to the field. Annu. Rev. Phytopathol. 48, 395–417 (2010).
Genre, A., Lanfranco, L., Perotto, S. & Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660 (2020).
Koehle, A. P., Brumwell, S. L., Seto, E. P., Lynch, A. M. & Urbaniak, C. Microbial applications for sustainable space exploration beyond low Earth orbit. NPJ Microgravity 9, 47 (2023).
Hummerick, M. E. et al. Spatial characterization of microbial communities on multi-species leafy greens grown simultaneously in the vegetable production systems on the International Space Station. Life 11, 1060 (2021).
Harris, F., Dobbs, J., Atkins, D., Ippolito, J. A. & Stewart, J. E. Soil fertility interactions with Sinorhizobium-legume symbiosis in a simulated Martian regolith; effects on nitrogen content and plant health. PLoS One 16, e0257053 (2021).
Dauzart, A. J. C., Vandenbrink, J. P. & Kiss, J. Z. The effects of clinorotation on the host plant, Medicago truncatula, and its microbial symbionts. Front. Astron. Space Sci. 3, 3 (2016).
Liu, G. et al. Simulated microgravity and the antagonistic influence of strigolactone on plant nutrient uptake in low nutrient conditions. NPJ Microgravity 4, 20 (2018).
Nerva, L. et al. Breeding toward improved ecological plant-microbiome interactions. Trends Plant Sci. 27, 1134–1143 (2022).
Parasyri, A. et al. Lichen as micro-ecosystem: extremophilic behavior with astrobiotechnological applications. Astrobiology 18, 1528–1542 (2018).
Massa, G. D., Newsham, G., Hummerick, M. E., Morrow, R. C. & Wheeler, R. M. Plant pillow preparation for the veggie plant growth system on the International Space Station. Gravitat. Space Res. 5, 24–34 (2017).
Baron, D., Amaro, A. C. E., Campos, F. G., Boaro, C. S. F. & Ferreira, G. in Plant Metabolites and Regulation Under Environmental Stress (eds Ahmad, P. et al.) 415–425 (Academic Press, 2018).
Li, Q., Li, X., Tang, B. & Gu, M. Growth responses and root characteristics of lettuce grown in aeroponics, hydroponics, and substrate culture. Horticulturae 4, 35 (2018).
Kyriacou, M. C. et al. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 9, 252 (2020).
Ming, D. W. & Henninger, D. L. Use of lunar regolith as a substrate for plant growth. Adv. Space Res. 14, 435–443 (1994).
Wamelink, G. W., Frissel, J. Y., Krijnen, W. H., Verwoert, M. R. & Goedhart, P. W. Can plants grow on Mars and the moon: a growth experiment on Mars and moon soil simulants. PLoS One 9, e103138 (2014).
Yao, Z., Feng, J. & Liu, H. Bioweathering improvement of lunar soil simulant improves the cultivated wheat’s seedling length. Acta Astronaut. 193, 1–8 (2022).
Wamelink, G., Frissel, J., Krijnen, W. & Verwoert, M. in Terraforming Mars, 313–329 (Wiley, 2021).
Paul, A.-L., Elardo, S. M. & Ferl, R. Plants grown in Apollo lunar regolith present stress-associated transcriptomes that inform prospects for lunar exploration. Commun. Biol. 5, 382 (2022).
Caporale, A. G. et al. Green compost amendment improves potato plant performance on Mars regolith simulant as substrate for cultivation in space. Plant Soil 486, 217–233 (2023).
Duri, L. G. et al. The potential for lunar and martian regolith simulants to sustain plant growth: a multidisciplinary overview. Front. Astron. Space Sci. 8, 747821 (2022).
Paradiso, R. et al. Design of a module for cultivation of tuberous plants in microgravity: The ESA project “Precursor of Food Production Unit” (PFPU). Front. Plant Sci. 11, 417 (2020).
Takeuchi, Y. 3D printable hydroponics: a digital fabrication pipeline for soilless plant cultivation. IEEE Access 7, 35863–35873 (2019).
Brinkert, K., Zhuang, C. P., Escriba-Gelonch, M. & Hessel, V. The potential of catalysis for closing the loop in human space exploration. Catal. Today 423, 114242 (2023).
Zandalinas, S. I. et al. The impact of multifactorial stress combination on plant growth and survival. N. Phytologist 230, 1034–1048 (2021).
Ferranti, F., Del Bianco, M. & Pacelli, C. Advantages and limitations of current microgravity platforms for space biology research. Appl. Sci. 11, 68 (2021).
Huff, J. L. et al. Galactic cosmic ray simulation at the NASA space radiation laboratory—progress, challenges and recommendations on mixed-field effects. Life Sci. Space Res. 36, 90–104 (2023).
Land, E. S., Canaday, E., Meyers, A., Wyatt, S. & Perera, I. Y. Bridging the gap: parallel profiling of ribosome associated and total RNA species can identify transcriptional regulatory mechanisms of plants in spaceflight. J. Plant Interact. 18, 2248173 (2023).
Acknowledgements
This work was funded by the Italian Space Agency (ASI). This review paper is based upon work from the contributors to the topic “Plant Physiology” in the “Biological Systems for Life Support” of the ASI Space Life Sciences Working Groups. This work is dedicated to the memory of Prof. Laura Bruno.
Author information
Authors and Affiliations
Contributions
M.D.B. conceived the initiative and coordinated the working group. M.D.B., M.E.F., and R.B. supervised the writing of the paper. M.D.B., M.E.F., R.B., P.C., L.L., M.M., and A.B. contributed to the conceptualization and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Huiqiong Zheng, Rafael Loureiro, Tomomichi Fujita, and Sabrina Chin for their contribution to the peer review of this work. Primary handling editor: David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maffei, M.E., Balestrini, R., Costantino, P. et al. The physiology of plants in the context of space exploration. Commun Biol 7, 1311 (2024). https://doi.org/10.1038/s42003-024-06989-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06989-7