Abstract
A cognitive map is an internal model of the world’s causal structure, crucial for adaptive behaviors. The orbitofrontal cortex (OFC) is central node to decision-making and cognitive map representation. However, it remains unclear how the medial OFC (mOFC) and lateral OFC (lOFC) contribute to the formation of cognitive maps in humans. By performing a multi-step sequential task and multivariate analyses of functional magnetic resonance imaging (fMRI) data, we found that the mOFC and lOFC play complementary but dissociable roles in this process. Specifically, the mOFC represents all hidden task state components. The lOFC and dorsolateral prefrontal cortex (dlPFC) encode abstract rules governing structure knowledge across task states. Furthermore, the two orbitofrontal subregions are functionally connected to share state-hidden information for constructing a representation of the task structure. Collectively, these findings provide an account that can increase our understanding of how the brain constructs abstract cognitive maps in a task-relevant space.
Similar content being viewed by others
Introduction
Cognitive maps are internal representations of the relationships between entities in the world1, underpinning the flexibility of human and animal behavior. These maps encapsulate the relational structure of task states in the environment, by integrating directly observable stimuli with memories that are relevant to future outcomes. Extending beyond a simple repetition of previously successful actions, cognitive maps enable individuals to predict outcomes in new situations based on loosely related experiences2. This adaptability to environmental changes and the generalizability of learned strategies to similar tasks highlights the utility of cognitive maps in a variety of contexts3,4,5,6. Understanding the neural mechanisms of cognitive map formation can enhance our insight into flexible cognition and behaviors7,8.
The human orbitofrontal cortex (OFC) is known to integrate relational structures to form cognitive maps that guide decision-making behavior5,9,10,11,12,13. It is widely implicated in flexible behavior by drawing on outcomes of previous experiences14. Previous studies across species have indicated that the OFC encodes the value of outcomes and expected rewards15,16,17. In addition, the OFC has been shown to project directly to the sensory cortices18,19,20, regulating sensory processing and sensorimotor associations21,22, and integrating multisensory perceptual inputs with prior information to represent a cognitive map of task space7,9,18,23,24. It has been suggested that the OFC is involved in value calculation when such calculations are part of task state space25,26,27.
The OFC is a functionally heterogeneous brain region that can be divided into two medial OFC (mOFC) and lateral OFC (lOFC) subregions. They may have specialized functions in representing the cognitive map of task space. The cognitive map of task space is defined as the task states and their underlying relational structure, i.e., the interrelationships between these states1,28. Recent neuroimaging studies showed that the mOFC represents the current state within a task space7,9, and contains all the relevant information for the current decision. This representation becomes crucial for flexible behavior when the component information about the states cannot be directly observed from sensory inputs24,29. Although several studies suggested that the lOFC utilizes cognitive maps to mentally simulate the potential outcomes during decision-making24,30, recent findings in rodents emphasized that the lOFC is involved in both creating and modifying cognitive maps, rather than just using them31. For instance, the lOFC has been associated with encoding relationships between various entities and organizing this relational information into a cognitive map10,11,32,33. This region has also been found to integrate the current sensory inputs with prior related information, which contains the information about past events34,35. However, it remains unclear how the mOFC and lOFC might contribute differentially and interact with each other to form a cognitive map of task space in humans.
Taken together, we proposed that the mOFC and lOFC have different roles in forming a cognitive map. Specifically, the mOFC may be involved in representing individual parts that comprise the task space, i.e., the current state, particularly when these states include unobservable task-relevant information. In contrast, the lOFC, due to its capability for relational coding and integration of the current with prior information, we proposed that this region could encode relational information about how a space is structured, by representing the relationship between internal components within each task state to govern structure knowledge across task states. Since representing the current ___location necessitates encoding all components in the task state, the mOFC may provide information for the lOFC that allows it to integrate and construct its representations.
To test our hypotheses, we carried out an fMRI experiment with a multi-step sequential task adapted from a previous work by Schuck et al.9. The task required the participants to integrate both the observable and unobservable task-relevant information to make effective goal-directed decisions. Through analyzing multivoxel activities in the OFC, we showed that only the mOFC encoded all the components that define a current state within the task space, and shared this task-relevant information to the lOFC through functional connectivity. Furthermore, the lOFC represented abstract rules to encode relational information about how a space is structured. These findings collectively suggest a functional specialization and interaction between the OFC subregions in representing the cognitive map of task space.
Results
Multi-step sequential task
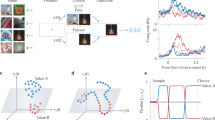
The participants completed a multi-step sequential task in which their decisions required integration of the current sensory inputs and memory of past events. On each trial, a face picture and a house picture were superimposed spatially, and the participants were requested to selectively judge the age (young or old) of either the face or the house (Fig. 1A). In the first trial of each run, a text (presented on the screen) directly instructed the category to be judged (face or house). In the following trials, the participants needed to continuously judge the age of the same category until the age of the category differed from the previous one. At this point, the category for age judging should switch to another category, e.g., from judging the category of face to house. Thus, the task rules included whether an age switch or a category switch occurred, which required the integration of prior information with current sensory inputs (Fig. 1B). These task rules involved four basic components: (1) the observable age in the current trial, (2) the category to be judged in the current trial, (3) the age in the previous trial, and (4) the category to be judged in the previous trial. Except that the current age was perceptually observable, the other three components were hidden information, which involved the memory of previous trials. The combination of these four binary-valued components formed a total of 16 task states and defined the current ___location in the task space (Fig. 1C). All the trials, based on whether the age switched and whether the category switched, were divided into three types: Enter (a category switched, regardless of whether the age switched or not), Internal (neither age nor category switched), and Exit (age switched and category not switched).
A fMRI task procedure. On each trial, the participants were presented with a compound stimulus. They had to judge the age of either a face or a house based on the information provided by the immediately previous trial. B Task design. The task started with a cue indicating a category (face or house) of stimuli to be judged (The pictures used as stimuli were courtesy of Dr. Dorothea Haemmerer and FACES datasets)54. The participants continuously judged the age of the same category until an age switch occurred. In the example, the participants were instructed to judge the age of a face. After three trials the age of the face switched from young to old. At this point, the category to be judged in the following trial switched to house. According to these task rules, we classified all trials into three types, Enter (trials where a category switch occurred), Internal (trials where no switch occurred), and Exit (trials where an age switch occurred within same category) trials. C Task space. Each node represents a particular task state that reflects the current ___location in the task, composed of observable and unobservable task-relevant information. Each black circle in the node indicates the current trial information, and white circle in the node indicates the previous trial information. Each arrow represents a possible transition between states. Transition probabilities were equal, P = 0.5. Node colors indicated the three trial types.
mOFC encodes all the task-relevant hidden state components
To test whether the mOFC represents the current ___location within the task space, we conducted multivoxel pattern analyses with leave-one-run-out cross-validation (LOOCV). Specifically, a multiclass linear support vector machine (SVM) classifier was trained on the activation pattern of the anatomically defined human mOFC and lOFC (Fig. 2A) to categorize the 16 possible task states. As shown in Fig. 2B, these task states could be decoded significantly above the chance level of 6.25% (=100%/16 states) from the activation patterns in both the mOFC and lOFC (p < 0.001 for each of the ROIs, t-test, and permutation test). The detailed decoding accuracies and t values for these ROIs are listed in Table S1 (Supplementary Information).
A Anatomical ROIs used in the data analysis. These five ROIs were the lOFC, mOFC, HP, and FFA/PPA, which were defined according to the Desikan-Killiany atlas59. The dlPFC was defined according to the Brodmann’s area (BA) template. B The decoding accuracy of the classification of these 16 states classification within the anatomically defined lOFC (in green) and mOFC (in orange). The black dashed line indicates the chance level (6.25%). Each point indicates the decoding accuracy of a participant (n = 27 participants). The red point denotes the average accuracy across the participants. The error whiskers of the box plots represent the range of the data, extending to the furthest data point within 1.5 times the interquartile range (IQR) from the upper and lower quartiles. C The decoding accuracy of the lOFC and mOFC in the four task-relevant components across all 27 participants. Only the mOFC showed a significant decoding accuracy for all of the three hidden components. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 for each state component. D The decoding accuracy of the lOFC and mOFC in the task-irrelevant information from two trials ago across all 27 participants. Neither the lOFC nor the mOFC showed significant decoding accuracy.
To determine which specific components were encoded in the mOFC and lOFC, we conducted further analyses by using a binary linear SVM. Figure 2C shows that all the hidden information, including the age and category from the previous trial as well as the current category, could be significantly decoded above the chance level of 50% from the pattern of mOFC (p < 0.05, t-test and permutation test). In contrast, from the activation of the lOFC, only information about the current and previous category (p < 0.05, t-test and permutation test; Fig. 2C), but not the previous age (p > 0.05, t-test and permutation test; Fig. 2C) was significantly decodable. We found that neither the mOFC nor lOFC represented the observable component “current age”. To test whether task-irrelevant information can be decoded by the activation pattern of the mOFC and lOFC, we developed an alternative 16-state space by incorporating task-irrelevant information about the category and age from two trials ago to replace the information from one trial ago. Neither the mOFC nor lOFC was significant in decoding these two task-irrelevant components (p > 0.05, t-test and permutation test; Fig. 2D).
In addition to the mOFC and lOFC, we performed the same analyses on three control anatomical ROIs (Fig. 2A), including the hippocampus (HP), dorsolateral PFC (dlPFC), and the combined fusiform face area and parahippocampal place area (FFA/PPA), given their previously described involvement in cognitive mapping, working memory, and the representation of faces and houses, respectively2,36,37. We found that none of these control areas represent all the hidden task-relevant components as the mOFC. Specifically, the HP significantly encoded both current and previous category information (p < 0.05, t-test and permutation test; Fig. S2A, Supplementary Information). For the dlPFC and FFA/PPA, the current age, current category, and previous category information were significantly decoded above the chance levels (p < 0.05, t-test and permutation test; Figs. S2B and S2C, Supplementary Information). Furthermore, the decoding accuracy in both the dlPFC and FFA/PPA was significant for task-irrelevant components (p < 0.05, t-test and permutation test; Figs. S2B and S2C, Supplementary Information). None of these areas showed significant encoding of all the hidden state components or exclusively represented the task-relevant information.
mOFC’s state representation predicts task performance
Accurately representing the state is a prerequisite for correct responses in the task. Therefore, we analyzed the relationship between behavioral performance and the neural representation of the task states in the mOFC. We hypothesized that if the mOFC indeed encoded task state information, then the transition between task states with highly dissimilar neural representation patterns should be more error-prone compared to transitions between states with a similar neural representation. To test this hypothesis, for each participant, we computed Pearson correlations (r) between multivoxel activation patterns associated with each pair of states by cross-correlating the patterns between runs (Fig. 3A). We focused exclusively on positive correlations in this analysis due to the interpretive difficulty associated with negative correlations. Then, we correlated these values with the participants’ error rates.
A Group-average correlations of task state representations in the mOFC across all 27 participants. Cooler green denotes higher correlations. B Pairs of states involved either a mental category switch or a category switch. Enter (in purple), Internal (in orange), Exit (in green). C Scatter plots illustrate the relationship between error rates and correlation metrics within the mOFC for states involving a mental switch. Each point in the plots represents one of the eight possible transitions, showing the average correlation coefficient (horizontal axis) and the corresponding average error rate (vertical axis) for that transition. The averages were calculated by pooling data from all 27 participants, meaning each point reflects the mean value across all the participants for that specific transition type. The horizontal and vertical error bars denote the standard error of the mean (SEM). The left plot corresponds to transitions involving a mental category switch, and the right plot corresponds to transitions involving a mental age switch.
Specifically, this analysis was performed for two separate subsets of transitions. First, for the transitions that included a mental category switch following an age switch, (e.g., from (Fy)Fo to (Fo)Ho, 8 transitions in total; Fig. 3B), the error rate was calculated based on the category switch trials (the (Fo)Ho in the example). Second, for the transitions that included a mental age switch (either between different categories, e.g., from (Fo)Fo to (Fo)Hy), or within the same category, e.g., from (Fy)Fy to (Fy)Fo, 8 transitions in total; Fig. 3B), the error rate was calculated based on the age switch trials (the (Fo)Hy and (Fy)Fo separately, in the example). The results showed that in both subsets, the higher correlation between the two states in a neural representation was significantly negatively associated with the error rates (category switches: r = −0.76, t6 = −2.87, p = 0.028, CI [−0.98, −0.17]; age switches: r = −0.88, t6 = −4.59, p = 0.003, CI [−0.98, −0.53]; Fig. 3C). No such relationships were found in activation patterns from the lOFC (p > 0.63 for both; Fig. S3, Supplementary Information), HP (p > 0.15 for both), dlPFC (p > 0.23 for both), or the FFA/PPA (p > 0.75 for both). As a control, we conducted an additional analysis in both the mOFC and lOFC that included both positive and negative values to predict task performance (see Fig. S4, Supplementary Information).
A neural circuit that includes the lOFC represents the task rules to form a relational structure of task space
We hypothesized that the brain would represent the relational structure of the task space governed by two task rules. Participants needed to leverage knowledge about whether the category or age had changed to make correct decisions. This knowledge depended on the coding and integration of the relationships between current and previous information within a state. All task states are linked by the task rules, thereby forming the relational structure of the task space (Fig. 1C). Similarity-based clustering serves as a general principle for distinguishing task states into a high-level contextual structure26. Therefore, we explored whether the neural representation of task states in the two OFC subregions form different hierarchical structures that reflect different task rules. This was achieved by hierarchically clustering the multivoxel activation pattern between states from the mOFC and lOFC separately.
The results indicated that the mOFC and lOFC exhibited distinct hierarchical structures (Fig. 4A). Specifically, in the lOFC and dlPFC (silhouette scores: 0.44 and 0.44, respectively), the activation patterns were more similar if the two states were both Exit or both non-Exit states. In contrast, in the mOFC and HP (silhouette scores: 0.39 and 0.43, respectively), the activation patterns were more similar if the two states were both Enter or both non-Enter states. Notably, all the Exit states involved the same category age switch, whereas the non-exit states did not; and all the Enter states involved a category switch, whereas the non-enter states did not.
A Results of the hierarchical clustering. Dendrograms show the similarity structure of the state representations in the 5 ROIs by hierarchical cluster analysis. The representation of states in the mOFC and HP had a similar structure, which involved whether a category switch occurred or not. The representation of states in the lOFC and dlPFC had a similar structure, which involved whether an age switch within the same category occurred or not. B Model representational dissimilarity matrixs (RDMs) for the two task rules. We had four RDMs: Enter_non-Enter, Exit_non-Exit, Control1, and Control2. In Control1, the category switch is defined as only occurring within the same age. In Control2, an age switch is defined as being able to occur across different categories. Blue color indicates greater dissimilarity.
Based on the results of the hierarchical cluster analysis and recent evidence showing that the OFC and dlPFC may represent the structure of task space or the rule of an abstract task38, we hypothesized that the OFC and dlPFC might simultaneously represent the two aspects of the task rule. If so, the state representations involving a mental category or age switch would be more similar than states that do not involve such a switch. To test this hypothesis, we generated four model representational dissimilarity matrices (RDMs) that tested whether a category or age switch had occurred (Enter_non-Enter and Exit_non-Exit, Fig. 4B), as well as two additional model RDMs to explore the potential influence of an interaction between age and category switch (Control1 and Control2; see Methods for the detailed information). We carried out an ROI-based representational similarity analysis (RSA) by using a multiple linear regression to compare the neural RDMs from pattern activity to the four model RDMs.
Figure 5A presents the group average RDMs for each of the five ROIs. Figure 5B shows that only the lOFC (Enter_non-Enter: t26 = 4.41, pBonferroni < 0.001; Exit_non-Exit: t26 = 3.22, pBonferroni = 0.003; Control1: t26 = − 0.92, pBonferroni = 0.732; Control2: t26 = 3.44, pBonferroni = 0.004) and dlPFC (Enter_non-Enter: t26 = 6.14, pBonferroni < 0.001; Exit_non-Exit: t26 = 3.41, pBonferroni = 0.002; Control1: t26 = −0.90, pBonferroni = 0.378; Control2: t26 = 3.26, pBonferroni = 0.003) had significant effects for the representation of the two rules. The significant results for Control2 imply that even though no age comparison is needed for the transition from Exit to Enter states, the participants still recognized the mental age switch. Though the mOFC could directly encode all the hidden task-relevant components, this region only represented whether a category switch had occurred or not (Enter_non-Enter: t26 = 4.50, pBonferroni < 0.001; Exit_non-Exit: t26 = 1.19, pBonferroni = 0.489; Control1: t26 =−0.16, pBonferroni < 0.877; Control2: t26 = 1.21, pBonferroni = 0.474). To further investigate these effects at the whole-brain level, we implemented whole-brain searchlight RSAs. The results were consistent with the ROI analyses, showing that the lOFC and dlPFC reached the significance level after the whole-brain family-wise error correction (FWE) (Fig. 5C and Table S2, Supplementary Information). Furthermore, searchlights in the anterior insula also reached the significance level after the FWE correction.
A Group average RDMs for each of the five ROIs. Brighter colors indicate greater dissimilarity (1 - correlation), and cooler colors greater similarity. B Distribution of the beta coefficients across the participants. The beta values were calculated from the multiple linear regression analyses by comparing the model representational dissimilarity matrices (RDMs) with the empirical RDMs estimated for each ROI. Each point represents a single participant, *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 in one-sample t-tests against zero after Bonferroni correction. C Results of the searchlight RSA. All the regions shown contain voxels significant at p < 0.001 for display. No cluster survived after FWE correction (p < 0.05) in Control1.
mOFC functional connectivity may support the formation of the state space
Our results have shown that while the lOFC simultaneously represented the task rules requiring both current and previous age and category (Fig. 5B), it only encoded information about the two hidden components relevant to category. In contrast, the mOFC represented the current ___location, encompassing all three hidden components (Fig. 2B). To determine whether the lOFC connected with the mOFC in encoding unobservable components, “previous age” in particular, we performed a connectivity-based whole-brain searchlight analysis to search for brain regions that showed state-related connectivity with the right mOFC. We found significant functional connections between the mOFC and the left lOFC (MNI: −38, 21, −11; t26 = 4.41, p < 0.001, Fig. 6 and Table S3, Supplementary Information), extending to the left insula and inferior prefrontal cortex, right putamen (MNI: 31, −7, −9; t26 = 5.20, p < 0.001), right dlPFC (MNI: 21, 25, 49; t26 = 5.88, p <0.001) and the left supramarginal gyrus (MNI: −65, −53, 33; t26 = 5.21, p < 0.001) in encoding “previous age”. This pattern of connectivity implies that despite not directly encoding all the hidden state components, these areas may utilize their functional connectivity with the mOFC to acquire task-relevant unobservable information for tracking the age switches (one of the task rules).
The functional network of encoding the unobservable component “previous age”. The blue area indicates the seed ROI used in the connectivity-based searchlight analyses. The lOFC, putamen, supramarginal gyrus, and dlPFC showed a significant connection with the mOFC in classifying the hidden component “previous age”. All regions shown contain voxels significant at p < 0.001 for display.
Discussion
There have been considerable debates about the distinct roles of the mOFC and lOFC in the formation and utilization of cognitive maps9,31,32,39,40. The goal of the current study was to investigate the functional dissociation and interaction between the mOFC and lOFC in representing the cognitive map of task space. Our results showed that the mOFC can represent hidden information to determine the current ___location within the task space (Fig. 2). The lOFC and dlPFC encode the abstract rules governing structure knowledge across task states, by integrating the immediate prior information and current sensory inputs to represent the relationships between the internal components within the task state (Fig. 5). Moreover, the acquisition of the task-relevant hidden components in the lOFC and dlPFC may be derived from the mOFC through functional connectivity (Fig. 6).
In the task, the participants integrated information from previous and current trials regarding category and age to make efficient decisions. Although the task was not spatially organized, it involved a task structure consisting of 16 states. The relational structure of these states was governed by two abstract rules, whether an age or category switch occurs, which captured the underlying relationships between these internal components.
Our results showed that the mOFC, rather than the lOFC, encodes all the hidden task-relevant state components (Fig. 2B). Neither the pattern activity in the lOFC nor the mOFC could decode the observable task state component, “current age”, which is defined as an observable visual input. In contrast, the dlPFC and FFA/PPA were found to encode this component. Previous studies have shown that the dlPFC has projections to premotor and motor regions41,42. A speculative possibility is that “current age” in the MVPA may reflect both the FFA/PPA’s visual processing response to stimuli and the dlPFC’s involvement in action-related transformation processes16,43. This may potentially interfere with the OFC’s ability to decode this component. If the component “current age” in the Multivoxel pattern analysis (MVPA) includes only observable stimuli defining the task state, it would be expected to be represented in the mOFC through visual cortex inputs. Supporting this, many studies have emphasized the OFC’s anatomical connections with regions associated with various sensory modalities, including the olfactory, gustatory, visceral, somatic, auditory, and visual areas13,44. In addition, the “current age” could be decoded by the mOFC in the subsequent trial as “previous age”. Our results support the notion that the OFC, particularly the mOFC, represents the current ___location within a task space when this ___location includes partially observable components.
We show that a neural network, including the lOFC, dlPFC, and anterior insula represents the abstract rules that govern the structure of the relationship between the task states (Fig. 4B and C). We found that the lOFC and dlPFC encode relational information about how the task states are structured, which is consistent with prior studies showing the importance of these regions in relational coding10,11,32,33 and the lOFC’s role in integrating prior with current information34,35,45. These findings dovetail with the view that prefrontal neurons encode abstract rules, a function not exclusive to the OFC but prevalent across other prefrontal areas as well38,46,47. Indeed, a previous study reported that the lOFC shows significantly greater coactivation than the mOFC with all portions of the dlPFC48. In addition, our results support the concept that the PFC is a central hub for rule-guided behavior, extending to multiple other regions including the insula38. In a rodent study using a devaluation task, Costa found that the lOFC selectively defines the specificity of associations within cognitive maps31. Our results are in line with his proposal that the lOFC might act as a cognitive “cartographer”, which is crucial not just in using maps but in creating and modifying them. Furthermore, our findings are consistent with other research indicating that the OFC represents a cognitive map reflecting task-specific relational knowledge39.
Finally, our study also extends the understanding of how the mOFC and lOFC interact in representing the task space. Representing the two task rules requires both current and previous age and category information. Although the lOFC only encodes for current and previous category information but not previous age (Fig. 2B), we found evidence for functional connectivity between the state-related mOFC and the lOFC in representing the individual hidden state component “previous age” (Fig. 6). It appears that the lOFC may utilize its functional connectivity with the mOFC to acquire age information for tracking the task rules. This finding dovetails with a recent study to some extent showing that the lOFC also plays an integral role in tracking an individual’s current “position”10. The interaction between the mOFC and lOFC may indicate that the mOFC is primarily involved in the acquisition of state component information, and the lOFC as well as dlPFC then extract this information to keep tracking the task rules that associate the task states into a structural framework, i.e., a cognitive map. Thus, besides using state component information, the mOFC may prepare this information for further mental processing. In addition to the lOFC, other regions, such as the putamen, dlPFC, and anterior insula also exhibit connectivity with the mOFC during decision-making. The interactions between these regions may suggest the OFC’s potential to influence a broad range of mental functions, including action planning and execution.
To conclude, we provide insights into how the structure of a task space can be represented in the brain. We found that the medial and lateral orbitofrontal cortex play complementary but dissociable roles in this process. Specifically, the medial orbitofrontal cortex appears to represent the task state components, especially the hidden elements. The lateral orbitofrontal cortex encodes the state’s relational structure over a wide range of abstract spaces. The two orbitofrontal subregions appear to be functionally connected to share hidden information for constructing a representation of the task structure. Collectively, these findings support a framework for understanding how the brain represents abstract cognitive maps in a non-physical, task-relevant space to guide flexible decisions.
Limitation
This study did not analyze how these regions collaborate to utilize cognitive maps for model-based inference and generalization across similar tasks, nor did it examine how inter-regional information flow regulates the sensory cortex to integrate diverse information. Methods such as informational connectivity (IC) and dynamic causal modeling (DCM) could provide deeper insights into the dynamics of information transfer between regions and the contributions of local circuits within the orbitofrontal cortex (OFC) and other areas. Future studies should consider incorporating these approaches to better understand these processes. In addition, we did not identify a role for the HP in representing either all the hidden state components state components or the relational structure of task space, consistent with previous findings9. However, previous studies have shown its involvement in forming both abstract and physical cognitive maps1,13,49. A speculative possibility is that the encoding of state components in this task may rely more on immediate representations and working memory, potentially engaging prefrontal or sensory-processing regions to a greater extent than the HP50,51,52,53. It is also possible that the task parameters in our study did not sufficiently engage the HP when encoding state information, which may occur under different experimental conditions or task demands, indicating the need for further investigation.
Materials and methods
Participants
Thirty-four right-hand healthy adults were recruited from the campus of South China Normal University (SCNU). Three participants quit the experiment, two participants were excluded due to poor behavioral performance (error rate > 3 standard deviations, SD), and another two participants were excluded from the analysis due to excessive signal dropout in the ventral regions of the prefrontal cortex that are of interest to this study. The remaining 27 participants (17 F/10 M, age = 21.6 ± 3.2 years old) were included in the subsequent analyses. All of the participants had normal or corrected-to-normal sight. None of them had a history of neurological or psychiatric disorders. The study was approved by the Institutional Review Board (IRB) of SCNU. Written informed consent was obtained from all participants prior to the experiment. All ethical regulations relevant to human research participants were followed.
Stimuli
Forty-two pictures from Haemmerer and Ebner54 were used to create the experimental stimuli, including ten young faces, twelve old faces, fourteen contemporary (i.e., young) houses, and six old houses. The pictures were accessed after registering and obtaining permission for use in this study. Each visual stimulus presented to the participants was a spatial overlap of two categories, a face and a house9,55. The face and house in each stimulus can be recognized as either young or old. Therefore, the stimuli included two classes: (i) the age of a house and a face were congruent (i.e., both of them were either young or old); (ii) the age of a house and a face were incongruent (i.e., young face overlapped with old house or vice versa).
Experimental design
The experiment consisted of two sessions, a behavior training session followed by a scanning session. In the behavior training session, each participant attended the training about 15 min to get acquainted with the experimental materials and the task rules. The first part of this session was a single-category age judgment task. The participants were shown a series of non-overlapping pictures of faces or houses on a screen and were required to judge the age of each picture. The pictures in the training session were also used in the fMRI scanning task. Each picture was displayed for 3.30 s, and if the participants made an incorrect judgment or failed to respond in time, the same picture would be shown again. The first training task ended when the participants correctly and consecutively judged the age of 17 pictures. The second part of the training session was the same task procedure as in the scanning session. This training task was completed when the participants were able to correctly judge the age of the pictures in 10 consecutive trials, which included more than 2 category switches (e.g., the age of face category changes, and participants should switch to judge the age of house). This was performed to ensure that the participants were completely acquainted with the rules of the task.
In the MRI scanning session, the participants performed the experimental task, which consisted of four runs (97 trials/run, 388 trials in 4 runs) while undergoing fMRI scanning. The experimental task requested that the participants judge the age of a certain category. At the start of each block, a cue text was displayed, indicating the initial category to be focused on (e.g., face in Fig. 1B). The participants needed to judge the age of the given category continuously until the age of the category changed (e.g., from young face to old face). When an age switch occurs, age judgment should switch to another category (e.g., from judging face to house). This structure resulted in three basic types of trials: (1) Enter trials, which indicated a category switch; (2) Internal trials, in which the same age was repeatedly present and the participants had to continue judging the same category; and (3) Exit trials, which had a different age (but within the same category) from the immediately previous trial and indicated the category switch on the next trial. No age comparison was required from the Exit trials to the Enter trials.
Task procedure
The participants responded to the stimuli using a bimanual 2-button box. They chose the age options by pressing either key “1” with the left hand (indicating the left option) or key “4” with the right hand (indicating the right option). The position of the options (young and old) were randomized in each trial to balance the left and right presses. Each trial began with the presentation of a central fixation cross and age options (young and old; Fig. 1A), which lasted between 0.70 and 4.70 s (average: 1.20 s). This was followed by an overlapped stimulus for 0.55 to 8.30 s (average: 3.30 s). The total average duration for each trial was 4.50 s, with a range from 3.25 to 8.50 s. The participants had 2.75 s to make their judgments. And the chosen option was framed by a white rectangle. The stimulus remained on the screen until the next trial started, ensuring that the response time (RT) and the stimulus duration were independent across trials. This was designed to disentangle the brain activity of decision-making and stimulus presentation. If a participant made an incorrect choice or failed to respond within 2.75 s, a repeated trial with feedback (indicating the category that needed to be judged) was displayed on the screen. If the age of the category did not change in the current trial, the age would change in the subsequent trial with a 50% probability.
To complete the task, the participants needed to keep track of four components of the task information: the ages and categories of both the current and previous trials. We used an acronym to denote each unique combination of these four components for the data analysis, which we refer to as a state. For instance, a state labeled as (Fy)Ho indicates that the participants previously judged a young face (Fy), and are currently judging an old house (Ho). Because each component of a state has two attributes (age: either o or y; category: H or F), there were a total of 24 = 16 possible states. According to the task rules, each state has an equal probability of transitioning to any of the two other states (Fig. 1C). Only one component of a state, the current age, was observable from the current visual input. The other three components were hidden and relied on the memory retrieval of the previous trial and understanding of the task rules (whether an age switch or a category switch occurred). Additionally, due to the task rules requiring the integration of prior information and current sensory inputs, they included the relationship between the current and previous category or age. This information determined which state to transition in the next trial (Fig. 1C). By capturing the underlying relationships between the internal components, the relational structure of these states was thus governed by these two abstract rules.
Scanning protocol
The MRI data were acquired using a 3-T Siemens Prisma-fit scanner equipped with a 64-channel phased-array head/neck coil at the Brain Imaging Center of SCNU. Functional images were acquired over four runs using a whole-brain single-shot simultaneous multi-slice (SMS) or multi-band (MB) gradient-echo echo-planar imaging (GE-EPI) sequence. The scanning parameters were: repetition time (TR) = 1500 ms, echo time (TE) = 31 ms, field of view (FOV) = 211 mm × 211 mm, matrix size = 88 × 88, voxel size = (2.4 mm)3, and 60 interleaved slices covering the whole brain. The slice orientation was tilted 30° backward relative to the anterior-posterior commissure axis to acquire better signals from the orbitofrontal cortex56. We also used a double-echo gradient-echo sequence to acquire the field-map of the whole brain, in order to correct the geometric distortions and MRI signal loss in the functional images caused by susceptibility effects. The field-map parameters were: TR = 620 ms, TE1 / TE2 = 4.92 ms /7.38 ms, voxel size = (2.4 mm)3, 60 slices. In addition, high-resolution T1-weighted structural images were obtained using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) pulse sequence. The parameters for this scan included: TR = 1,800 ms, TE = 2.07 ms, FOV = 256 mm × 256 mm, matrix size = 320 × 320, voxel size = (0.8 mm)3, and 208 sagittal slices covering the whole brain. Each participant underwent an MRI session consisting of 4 task-fMRI runs, a resting-state (res-fMRI) scan, a field map, and a T1-weighted brain structural scan.
Behavioral analysis
For the behavioral data, we aimed to test whether the participants showed different behavioral performances over the time course of the experiment and across different trial types. The average error rate across participants was 4.7%, and the average response time was 1137 ms. Using a linear mixed-effects model (R package lme4), which accounts for both fixed effects (time course, trial type) and random effects (participant variability), we examined whether these factors affected behavioral performance. The results indicated that performance improved over the time course (error rate: t26 = −5.50, p < 0.0001, Fig. S1A; response time: t26 = −7.54, p < 0.0001, Fig. S1B, Supplementary Information). Error rates also varied significantly by trial type (Enter: 7.1%, Internal: 3.5%, Exit: 3.7%, t26 = 12.00, p < 0.001, Fig. S1C, Supplementary Information). Similarly, response time differed by trial type (Enter: 1202 ms, Internal: 1074 ms, Exit: 1143 ms, t26 = 59.40, p < 0.001, Fig. S1D, Supplementary Information). Average error rates and response time for the 16 states are presented in FigS. S1E - S1F (Supplementary Information).
Data preprocessing
The fMRI data was preprocessed using fMRIPrep (ver 20.2.7)57, which is based on Nipype 1.6.158. The fMRI data preprocessing followed the standard procedures and included the following steps: (i) estimation and correction of head movement with 6 parameters, (ii) slice-timing correction, (iii) signal and geometric distortion correction with the field-map, (iv) spatial realignment, co-registration of the brain structural and functional images, and (v) segmentation of brain structural images into the white matter (WM) and gray matter (GM) maps and normalization into (2 mm)3 in the MNI standard space.
Regions of interest (ROI) definitions
In this study, we defined five bilateral anatomical ROIs based on the Desikan-Killiany atlas59, mOFC, lOFC, hippocampus, and PPA/FFA (bilateral fusiform face area and parahippocampal place area). These ROIs were labeled with FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). The T1-weighted structural images were preprocessed using FreeSurfer, including intensity normalization, non-brain tissue removal, transformation to Talaraich space, and segmentation of the GM, WM, and CSF. Surface-based cortical regions were converted to volumetric ROIs and transformed into individual functional native space. To align the ROIs with the functional imaging data, all of the ROIs were co-registered to each participant’s individual functional images by using the antsApplyTransformer function in ANTs, utilizing the same parameters that were used to co-register their structural images with the functional images5.
Due to the rostral middle frontal gyrus has a large medial portion, we used the WFU Pick Atlas to generate the regions of interest (ROIs) for the bilateral dorsolateral prefrontal cortex (BAs 9 and 46)60.
Multivoxel pattern analysis (MVPA)
For the MVPA, we performed a voxel-wise general linear model (GLM) to extract the trial-wise beta map by using AFNI. The first trial of each scanning run, the error trials, and the first trial following errors were excluded from all the analyses. Trial events were modeled as boxcar functions whose length reflected the response time. First-level models were conducted on the realigned but non-normalized, non-smoothed task fMRI data and included 16 regressors for the onsets of the 16 different states and 6 movement parameters for each run. 16 regressors were convolved with a canonical hemodynamic response function (HRF). By averaging the trial-wise beta maps within each run related to 16 states, we obtained the voxel-wise activation for each state. This resulted in 16 beta maps per run and 64 beta maps per participant. Before conducting the classification analysis, we Z-scored and smoothed (FWHM = 4 mm) the resulting beta maps within each ROI for each run. All the classification analyses were conducted using Scikit-learn (https://scikit-learn.org/), with a support vector machine (SVM) (kernel = “linear”, C = 0.001) and the leave-one-run-out cross-validation (LOO-CV) method. A multiclass linear SVM classifier (one vs rest) was trained and tested for the classification of the 16 possible states. Six binary linear SVM classifiers were trained and tested separately for the classification of each of the four task-relevant components (current age, previous age, current category, previous category, separately) and two task-irrelevant components (age and category from two trials ago, separately).
Representational similarity analysis (RSA)
For each ROI, we calculated the similarities between the neural state patterns by using Pearson correlations on the estimated beta maps of brain activities associated with each state, derived from the first-level GLM analysis. To avoid the temporal autocorrelations within the same run which spuriously increases the neural similarities between states61, we estimated the pattern similarity by cross-correlating the patterns between runs. Specifically, for each run, we correlated each state map with all the state maps from the other three runs. This resulted in three 16 × 16 correlation matrices per run. We then averaged these matrices across all four runs to obtain the final correlation matrices9. These obtained matrices were submitted to the hierarchical clustering algorithm by using SciPy (https://scipy.org/, version 1.10.1). The agglomerative hierarchical clustering algorithm utilized the unweighted average distance (method = “average”) between pairs of the 16 vectors62, with the default Euclidean metric used for calculating distances between data points within the linkage function.
Based on the results of the hierarchical cluster analysis, we constructed four model RDMs: Enter_non-Enter, Exit_non-Exit, Control1, and Control2. The Enter_non-Enter RDM tested whether a category switch occurred, where states involving a mental category switch were expected to be more similar to each other than those without such a switch. The Exit_non-Exit RDM examined whether an age switch occurred, where states with a mental age switch were expected to be more similar to each other than those without. Two other RDMs for Control1 and Control2 were created to analyze the potential influence of interaction between age and category switches. In Control1, the category switch was defined as occurring only within the same age, excluding the influence of age switch on category switch. In Control2, the age switch was defined as occurring within the both same (Exit) and different categories (Enter). This RDM indicated that, even though no age comparison was required in Enter, a mental age switch would happen.
In the multiple linear regression RSA, we took the Enter_non-Enter, Exit_non-Exit, Control1, and Control2 as predictors of the multiple regression analysis. The neural RDM was calculated as 1 minus the correlation matrices. The lower triangles of all the model RDMs were extracted and transformed into four vectors, along with an intercept term, which were then incorporated as predictors in a multiple linear regression analysis to estimate beta coefficients relating each model RDM to the neural RDM. This approach allowed us to parcel out the variance in representational distances due to multiple factors in the same model6.
A whole-brain RSA was conducted by passing a spherical searchlight (http://brainiak.org) with a radius of 5 mm over each voxel within the participants’ group GM mask. For each searchlight, we Z-scored the obtained beta maps within each run, and computed the correlations for 16 states by cross-correlating the patterns between runs. The four RDMs described above (Enter_non-Enter, Exit_non-Exit, Control1, and Control2) were used as predictors in the multiple regression analysis. The lower triangles of the neural RDM and the model RDMs were extracted and transformed into five vectors, along with an intercept term, to calculate the beta coefficients for each of the four predictors. These beta values were then assigned to the central node of each searchlight, thus generating a beta map for each model RDM63.
Connectivity-based searchlight
To identify which brain regions show state-related connectivity patterns with the mOFC for encoding the hidden task-relevant component “previous age”64, we carried out a connectivity-based searchlight analysis. We first selected a sphere with a radius of 8 mm at the center of the mOFC (MNI: 3, 44, −14) as the seed region (Fig. 5B) by following9, to estimate the whole-brain beta series correlation (Z-scored and smoothed) to the seed for each trial. Subsequently, we averaged the correlation maps corresponding to trials within each run for the 16 states. Next, we used these state-related connectivity estimates as input to a LOO-CV SVM (kernel = “linear”, C = 0.001) searchlight with a radius of 6 mm to classify the component “previous age”.
Statistics and reproducibility
For the ROI-based MVPA analysis, we used both a one-sample t-test (one-tailed) and a permutation test (10,000 iterations) to determine whether the average accuracy across participants was significantly higher than the chance level (6.25% and 50%, respectively). To analyze whether the state representation can predict task performance, we used a one-sample t-test (one-tailed) with bootstrapping (10,000 iterations) to evaluate whether the error rates were significantly associated with the correlation metrics. For the ROI-based RSA, we applied a one-sample t-test against 0, with Bonferroni correction for multiple comparisons (p < 0.05). In the searchlight RSA analysis, the RSA maps were analyzed at the group level using a one-sample t-test against 0. We employed a threshold-free cluster enhancement (TFCE) to identify statistically significant clusters (10,000 permutations), with multiple comparisons corrected using the family-wise error (FWE) method. Finally, for the connectivity-based searchlight analysis, statistical significance was evaluated using a one-sample t-test against the chance level of 50%, with false discovery rate (FDR) correction applied for multiple comparisons (p < 0.001).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data and statistic maps underlying Figs. 2 - 6 are supplied at https://doi.org/10.17605/OSF.IO/CD2T665.
Code availability
The behavioral data and the codes for the main analyses of the paper (i.e., MVPA and RSA) have been deposited on OSF and are publicly available as of the date of publication. The address is https://doi.org/10.17605/OSF.IO/CD2T665. Any additional information reported in this article is available from the lead contact on reasonable request.
References
Whittington, J. C. R., McCaffary, D., Bakermans, J. J. W. & Behrens, T. E. J. How to build a cognitive map. Nat. Neurosci. 25, 1257–1272 (2022).
Behrens, T. E. J. et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Bartolo, R. & Averbeck, B. B. Prefrontal cortex predicts state switches during reversal learning. Neuron 106, 1044–1054.e1044 (2020).
Boorman, E. D., Witkowski, P. P., Zhang, Y. & Park, S. A. The orbital frontal cortex, task structure, and inference. Behav. Neurosci. 135, 291–300 (2021).
Mizrak, E., Bouffard, N. R., Libby, L. A., Boorman, E. D. & Ranganath, C. The hippocampus and orbitofrontal cortex jointly represent task structure during memory-guided decision making. Cell Rep. 37, 110065 (2021).
Vaidya, A. R., Jones, H. M., Castillo, J. & Badre, D. Neural representation of abstract task structure during generalization. Elife 10, e63226 (2021).
Park, S. A., Miller, D. S., Nili, H., Ranganath, C. & Boorman, E. D. Map making: constructing, combining, and inferring on abstract cognitive maps. Neuron 107, 1226–1238.e1228 (2020).
Bellmund, J. L. S., Gardenfors, P., Moser, E. I. & Doeller, C. F. Navigating cognition: spatial codes for human thinking. Science 362, eaat6766 (2018).
Schuck, N. W., Cai, M. B., Wilson, R. C. & Niv, Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91, 1402–1412 (2016).
Witkowski, P. P., Park, S. A. & Boorman, E. D. Neural mechanisms of credit assignment for inferred relationships in a structured world. Neuron 110, 2680–2690.e2689 (2022).
Li, J. et al. Representation of the inferred relationships in a map-like space. Hum. Brain Mapp. 44, 3744–3757 (2023).
Tegelbeckers, J., Porter, D. B., Voss, J. L., Schoenbaum, G. & Kahnt, T. Lateral orbitofrontal cortex integrates predictive information across multiple cues to guide behavior. Curr. Biol. 33, 4496–4504 e4495 (2023).
Qiu, Y. et al. Forming cognitive maps for abstract spaces: the roles of the human hippocampus and orbitofrontal cortex. Commun. Biol. 7, 517 (2024).
Rushworth, M. F., Noonan, M. P., Boorman, E. D., Walton, M. E. & Behrens, T. E. Frontal cortex and reward-guided learning and decision-making. Neuron 70, 1054–1069 (2011).
Padoa-Schioppa, C. & Assad, J. A. Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226 (2006).
Padoa-Schioppa, C. & Conen, K. E. Orbitofrontal cortex: a neural circuit for economic decisions. Neuron 96, 736–754 (2017).
Kahnt, T., Heinzle, J., Park, S. Q. & Haynes, J. D. The neural code of reward anticipation in human orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 107, 6010–6015 (2010).
Cavada, C., Company, T., Tejedor, J. & Cruz-Rizzolo, R. J. & Reinoso-Suarez, F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 10, 220–242 (2000).
Ongur, D. & Price, J. L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219 (2000).
Rolls, E. T., Cheng, W. & Feng, J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2, fcaa196 (2020).
Banerjee, A. et al. Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature 585, 245–250 (2020).
Liu, D. et al. Orbitofrontal control of visual cortex gain promotes visual associative learning. Nat. Commun. 11, 2784 (2020).
Wikenheiser, A. M., Marrero-Garcia, Y. & Schoenbaum, G. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron 95, 1197–1207 e1193 (2017).
Wilson, R. C., Takahashi, Y. K., Schoenbaum, G. & Niv, Y. Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–279 (2014).
Stalnaker, T. A., Cooch, N. K. & Schoenbaum, G. What the orbitofrontal cortex does not do. Nat. Neurosci. 18, 620–627 (2015).
Niv, Y. Learning task-state representations. Nat. Neurosci. 22, 1544–1553 (2019).
Stalnaker, T. A., Raheja, N. & Schoenbaum, G. Orbitofrontal State Representations are related to choice adaptations and reward predictions. J. Neurosci. 41, 1941–1951 (2021).
VM, K. N. & Stuber, G. D. The learning of prospective and retrospective cognitive maps within neural circuits. Neuron 109, 3552–3575 (2021).
Bradfield, L. A., Dezfouli, A., van Holstein, M., Chieng, B. & Balleine, B. W. Medial orbitofrontal cortex mediates outcome retrieval in partially observable task situations. Neuron 88, 1268–1280 (2015).
Rustichini, A. & Padoa-Schioppa, C. A neuro-computational model of economic decisions. J. Neurophysiol. 114, 1382–1398 (2015).
Costa, K. M. et al. The role of the lateral orbitofrontal cortex in creating cognitive maps. Nat. Neurosci. 26, 107–115 (2023).
Boorman, E. D., Sweigart, S. C. & Park, S. A. Cognitive maps and novel inferences: a flexibility hierarchy. Curr. Opin. Behav. Sci. 38, 141–149 (2021).
Blumenfeld, R. S., Parks, C. M., Yonelinas, A. P. & Ranganath, C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J. Cogn. Neurosci. 23, 257–265 (2011).
Nogueira, R. et al. Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat. Commun. 8, 14823 (2017).
Hocker, D. L., Brody, C. D., Savin, C. & Constantinople, C. M. Subpopulations of neurons in lOFC encode previous and current rewards at time of choice. Elife 10, e70129 (2021).
Curtis, C. E. & D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7, 415–423 (2003).
Wang, L., Baumgartner, F., Kaule, F. R., Hanke, M. & Pollmann, S. Individual face- and house-related eye movement patterns distinctively activate FFA and PPA. Nat. Commun. 10, 5532 (2019).
Mansouri, F. A., Freedman, D. J. & Buckley, M. J. Emergence of abstract rules in the primate brain. Nat. Rev. Neurosci. 21, 595–610 (2020).
Wang, F., Schoenbaum, G. & Kahnt, T. Interactions between human orbitofrontal cortex and hippocampus support model-based inference. PLoS Biol. 18, e3000578 (2020).
Bradfield, L. A. & Hart, G. Rodent medial and lateral orbitofrontal cortices represent unique components of cognitive maps of task space. Neurosci. Biobehav Rev. 108, 287–294 (2020).
Cieslik, E. C. et al. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. cortex 23, 2677–2689 (2013).
Grafton, S. T. & Volz, L. J. From ideas to action: The prefrontal–premotor connections that shape motor behavior. Handb. Clin. Neurol. 163, 237–255 (2019).
Nee, D. E. & D’Esposito, M. The hierarchical organization of the lateral prefrontal cortex. Elife 5, e12112 (2016).
Way, B. M., Lacan, G., Fairbanks, L. A. & Melega, W. P. Architectonic distribution of the serotonin transporter within the orbitofrontal cortex of the vervet monkey. Neuroscience 148, 937–948 (2007).
Cazares, C., Schreiner, D. C., Valencia, M. L. & Gremel, C. M. Orbitofrontal cortex populations are differentially recruited to support actions. Curr. Biol. 32, 4675–4687 e4675 (2022).
Knudsen, E. B. & Wallis, J. D. Taking stock of value in the orbitofrontal cortex. Nat. Rev. Neurosci. 23, 428–438 (2022).
Wallis, J. D., Anderson, K. C. & Miller, E. K. Single neurons in prefrontal cortex encode abstract rules. Nature 411, 953–956 (2001).
Zald, D. H. et al. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb. Cortex 24, 232–248 (2014).
Lin, H. & Zhou, J. Hippocampal and orbitofrontal neurons contribute to complementary aspects of associative structure. Nat. Commun. 15, 5283 (2024).
Zarahn, E., Rakitin, B., Abela, D., Flynn, J. & Stern, Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb. Cortex 15, 303–316 (2005).
Funahashi, S. Working memory in the prefrontal cortex. Brain Sci. 7, 49 (2017).
Jeneson, A., Mauldin, K. N. & Squire, L. R. Intact working memory for relational information after medial temporal lobe damage. J. Neurosci. 30, 13624–13629 (2010).
Sawangjit, A. et al. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature 564, 109–113 (2018).
Ebner, N. C., Riediger, M. & Lindenberger, U. FACES–a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav. Res Methods 42, 351–362 (2010).
Schuck, N. W. & Niv, Y. Sequential replay of nonspatial task states in the human hippocampus. Science 364, eaaw5181 (2019).
Deichmann, R., Gottfried, J. A., Hutton, C. & Turner, R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19, 430–441 (2003).
Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019).
Gorgolewski, K. et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 5, 13 (2011).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Cohen, J. D. et al. Computational approaches to fMRI analysis. Nat. Neurosci. 20, 304–313 (2017).
Farovik, A. et al. Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. J. Neurosci. 35, 8333–8344 (2015).
Tucciarelli, R., Wurm, M., Baccolo, E. & Lingnau, A. The representational space of observed actions. Elife 8, e47686 (2019).
Howard, J. D., Gottfried, J. A., Tobler, P. N. & Kahnt, T. Identity-specific coding of future rewards in the human orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 112, 5195–5200 (2015).
Tan, L. The medial and lateral orbitofrontal cortex jointly represent the cognitive map of task space. https://doi.org/10.17605/OSF.IO/CD2T6 (2025).
Acknowledgements
This work was supported by funding from the Key Technologies R&D Program of Guangdong Province (Grant number: 2023B0303020002), National Natural Science Foundation of China (Grant numbers: 32371101 and 82171914), Guangdong Natural Science Foundation (Grant number: 2022A1515011022), striving for the first-class, improving weak links and highlighting features (SIH) key discipline for psychology in South China Normal University, and National Key Research and Development Program of China (Grant number: 2018YFC1705006). The authors thank Professor Nicolas Schuck for providing the code and the materials of the experimental paradigm. The authors appreciate Rhoda E., PhD, and Edmund F. Perozzi, PhD, for editing the manuscript.
Author information
Authors and Affiliations
Contributions
Liwei Tan: Conceptualization, Investigation, Methodology, Formal analysis, Writing-original draft, Writing-review and editing, Project administration. Yidan Qiu: Validation, Writing-review and editing. Lixin Qiu: Investigation, Writing-review and editing. Shuting Lin: Investigation, Writing-review and editing. Jinhui Li:Writing-review and editing. Jiajun Liao: Writing-review and editing. Yuting Zhang: Writing-review and editing. Wei Zou: Writing-review and editing. Ruiwang Huang: Conceptualization, Resources, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jacqueline Gottlieb and Jasmine Pan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, L., Qiu, Y., Qiu, L. et al. The medial and lateral orbitofrontal cortex jointly represent the cognitive map of task space. Commun Biol 8, 163 (2025). https://doi.org/10.1038/s42003-025-07588-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07588-w