Abstract
Short and long sleep durations are associated with multiple physical, psychiatric and neurodegenerative diseases, yet their potentially shared and distinct biological mechanisms remain unclear. Here, using data from UK Biobank participants aged 38–73 years, we have characterized the in-depth genetic architecture of short (≤7 h) and long (≥7 h) sleep groups, along with their associations with behaviors, neuroimaging and blood biomarkers. The two sleep groups exhibited independent genetic architectures and distinct immunometabolic and proteomic profiles. Notably, long sleep showed more significant associations with cardiovascular-related biomarkers (for example, cholesterol), brain structures (for example, hippocampus) and plasma proteins (for example, GDF15), whereas short sleep demonstrated greater genetic overlap with psychiatric conditions, particularly depression. Mendelian randomization further supported this dissociation by showing that long sleep duration is probably a consequence of multiple brain disorders and cardiovascular diseases, whereas short sleep duration has a potential causal effect on various brain and physical illnesses. Our findings advance our understanding of the relationship between sleep and health conditions by revealing distinct biological origins and genetic mechanisms underlying short and long sleep duration.
Similar content being viewed by others
Main
Sleep is an essential function for human health, and sleep disturbances are closely related to physical, mental and neurological disorders in older adults1. Notably, sleep duration, as a critical characteristic of sleep health, has been linked to multiple health conditions in a nonlinear manner, with 7–8 h of sleep duration commonly proposed as the optimal length for adults2,3,4. Previous studies have suggested 7 h as the optimal sleep duration for middle-aged and older adults5,6,7. Deviations from this optimal sleep duration, namely short or long sleep duration, are associated with more severe mental and neurological illnesses8,9,10, as well as cardiometabolic diseases11,12. Meanwhile, sleep duration is also closely associated with lifestyle, physical and psychosocial factors13. However, a systematic investigation regarding the shared and distinct behavioral profiles and neurobiological mechanisms of short and long sleep duration is still lacking.
Sleep duration has been identified as a polygenic and heritable characteristic14,15. Furthermore, distinct genetic findings have been reported for short and long sleep duration compared to normal sleep duration. For instance, lower heritability was found for long sleep duration compared to short sleep duration16. In terms of relationships with other health conditions, long sleep duration has been genetically correlated with an increased risk of schizophrenia and type 2 diabetes17,18, and short sleep duration has been genetically linked to insomnia19. Furthermore, Mendelian randomization (MR) studies have demonstrated a causal effect of short sleep duration, but not long sleep duration, on cardiovascular disease20. These studies suggest that potentially distinct genetic mechanisms underpin short and long sleep duration. However, the underlying distinct genetic factors that influence short and long sleep duration and their potentially different causal associations with health conditions have yet to be fully elucidated.
In addition to genetic mechanisms, immunometabolic biomarkers and protein levels also play crucial roles in the associations between sleep duration and various health conditions. For instance, glucose has been identified as a crucial mediator between sleep deprivation and diabetes11,21. Inflammation, characterized by increased levels of C-reactive protein (CRP), has been associated with both short and long sleep duration. However, different interpretations of the underlying mechanisms have been proposed for these associations. For short sleep duration, inflammation might serve as an intermediate mechanism between sleep and cardiometabolic diseases and depression22,23. In contrast, for long sleep duration, the association between sleep and increased levels of CRP may be attributed to confounding factors such as obesity or a consequence of underlying diseases24. Additionally, circulating protein levels have been proposed as a potential mechanistic pathway between sleep duration and cardiometabolic health25. Furthermore, brain structure could play a crucial role in the nonlinear association between sleep duration, mental health and cognition in middle-aged to older adults5. However, the distinct and shared neurobiological implications of short and long sleep duration, especially in terms of potential causal inferences with health-related biomarkers, remain unclear.
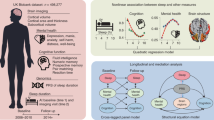
In the present study we utilized the large cohort from the UK Biobank to perform an in-depth genetic and phenotypic characterization of sleep duration as an ordinal variable in both sleep ≤7-h and sleep ≥7-h groups. Notably, in our previous studies based on the same cohort, 7 h of sleep was identified as the population-wise optimal sleep duration5,6, showing a nonlinear relationship between sleep duration and mental health. Following the same settings, we proposed using 7 h of sleep as a shared baseline (also referred to as the common reference point) for both the ≤7 h and ≥7 h groups26, where sleep duration ranges ordinally from ‘barely any sleep’ to 7 h, and from 7 h to longer durations, respectively. Specifically, we hypothesized that these two groups would exhibit distinct phenotypic (for example, lifestyle, physical and psychosocial factors), neuroimaging and blood-biomarker association profiles. Furthermore, we hypothesized that these two sleep groups would have distinct genetic architectures and neurobiological pathways, contributing to their differential roles in causal associations with health conditions in middle-aged to older adults. Finally, to explore the compatibility of our ordinal sleep definition, we conducted a sensitivity analysis using traditional stratified categories of sleep duration: normal (7 or 8 h), short (<7 h) and long (>8 h).
Results
Population characteristics
Participants aged 38–73 years with accessible data for sleep duration at baseline were utilized in the current study, including 315,628 participants in the sleep ≤7-h group (mean ± s.d. = 6.5 ± 0.72 h) and 375,028 participants in the sleep ≥7-h group (mean ± s.d. = 7.6 ± 0.79 h). A sleep duration of 7 h (n = 192,383) was utilized as the reference baseline for the group sleeping less than 7 h (n = 123,245, mean ± s.d. = 5.7 ± 0.58 h) and the group sleeping more than 7 h (n = 182,645, mean ± s.d. = 8.2 ± 0.67 h; Table 1 and Supplementary Fig. 1). Similarly, follow-up sleep duration data assessed during the imaging visit were also grouped into the sleep ≤7-h (n = 31,056) and sleep ≥7-h (n = 36,794) groups and analyzed with the neuroimaging data. A range of blood test measurements from 478,357 participants and metabolomic data of 118,000 participants acquired at baseline were also used in the present study. Extended Data Fig. 1 provides a general schema of the study. Throughout this paper, the term ‘significantly associated’ refers to statistical significance, as determined by a Bonferroni-corrected P-value threshold of P < 0.01, unless otherwise specified.
Phenome-wide associations of short and long sleep groups
The phenome-wide association study (PheWAS) encompassed 3,735 phenotypic variables across 20 categories (Supplementary Table 1). Sleep duration was treated as an ordinal variable, and specific regression models were applied based on the type of each of the 3,735 variables: linear regression for continuous variables, ordinal logistic regression for ordered categorical variables, and logistic regression for binary variables. After adjusting for covariates including age, sex, body mass index, the Townsend deprivation index measuring socioeconomic status, educational qualifications, smoking status and drinking status, 1,308 phenotypes were significantly associated with the sleep ≤7-h group, and 934 phenotypes showed significant associations with the sleep ≥7-h group (Bonferroni-corrected P < 0.01, uncorrected P < 2.68 × 10−6 (0.01/3,735); Fig. 1a). Specifically, for the sleep ≤7-h group, the most significant phenotypes included insomnia (β = −0.62, P = 1 × 10−302), trouble sleeping (β = −0.49, P = 1 × 10−302), pain (β = −0.37, P = 1.8 × 10−208), the number of treatments/medications taken (β = −0.14, P = 1.2 × 10−248) and most psychosocial factors (including frequency of tiredness/lethargy: β = −0.31, P = 1 × 10−302; frequency of depressed mood: β = −0.29, P = 1 × 10−302 and so on). The phenotypes most significantly associated with the sleep ≥7-h group included napping during the day (β = 0.34, P = 1 × 10−306), easiness of getting up in morning (β = −0.14, P = 1.9 × 10−280), in-paid employment or not (β = −0.44, P = 1 × 10−306), impedance of leg measuring the body fat percentage27 (β = 0.05, P = 5.4 × 10−283), occurrence of other disorders of brain (β = 0.28, P = 1.4 × 10−106) and usual walking pace (β = −0.20, P = 1.0 × 10−306). The impedance measure of body, as one of the measures of body fat percentage, is a relevant factor in metabolic health and cardiovascular risk, which is consistent with the previous identification of the interaction between sleep duration and body composition28. Notably, in addition to the specific association profiles for the sleep ≤7-h and sleep ≥7-h groups, both groups were also associated with some phenotypes at similar significance levels, including overall health rating (β = −0.29, P = 1 × 10−302; β = 0.18, P = 1 × 10−306), longstanding illness (β = −0.20, P = 1 × 10−302; β = 0.19, P = 1 × 10−306), occurrence of depressive episodes (β = −0.21, P = 9.7 × 10−262; β = 0.23, P = 1 × 10−306), disability allowance (β = −0.39, P = 1.0 × 10−302; β = 0.42, P = 1 × 10−306) and average total income (β = 0.17, P = 1 × 10−302; β = −0.23, P = 1 × 10−306). The full list of significant results is provided in Supplementary Data 1 and 2. Age-stratified analysis of the PheWAS showed similar results (Supplementary Fig. 2).
a, The PheWAS results of sleep ≤7 h (top) and sleep ≥7 h (bottom) with 3,735 phenotypes. The x axis represents phenotypes (20 categories) and the y axis the −log10P values (two-tailed) of associations between sleep duration and the phenotypes. These associations were calculated using linear regression for continuous variables, ordinal logistic regression for ordered categorical variables and logistic regression for binary variables. The horizontal dashed line indicates the Bonferroni-corrected significance threshold (P < 0.01). Covariates adjusted in the regression analyses include age, sex, assessment center, body mass index, Townsend deprivation index, educational qualifications, smoking status and drinking status. b, Histogram of t values of regression between short and long sleep duration with 20 categories of phenotypes. t values were transformed from β values (β/s.e.). Red color represents t values of the association between sleep ≤7 h and the phenotypes, and blue represents t values of the association between sleep ≥7 h and the phenotypes. c, Scatter plot of the t values of associations of short sleep duration versus the t values of associations of long sleep duration × −1. The point size indicates the absolute difference (Abs. diff.) between the |t| value of short sleep duration minus the |t| value of long sleep duration (||tsleep≤7| − |tsleep≥7||), with a larger point size indicating a more significant difference in associations between the two sleep groups. The color of the point indicates the |t| difference between short and long sleep duration (|tsleep≤7| − |tsleep≥7|), with red indicating phenotypes more associated with sleep ≤7 h and blue representing phenotypes more associated with sleep ≥7 h. All statistical tests were two-sided. Abs. diff. t value: ||tsleep≤7| − |tsleep≥7||; diff. t value: |tsleep≤7| − |tsleep≥7|.
To visualize and compare the association results of the two sleep groups, we transformed the β values into t values, which are directly derived from the linear model, representing the ratio of the estimated β to its standard error (s.e.). We displayed the t-value distributions for the two sleep groups across each of the 20 phenotype categories (Fig. 1b). The t values between the sleep ≤7-h group and sex-specific factors, psychosocial factors and digestive factors were higher compared with the sleep ≥7-h group. Cognitive functions showed more significant associations with the sleep ≥7-h group. Furthermore, the scatter plot of t values of the two sleep groups illustrates the specific phenotypes in each group, with insomnia, trouble sleeping, depressed mood and confide to others being more significant in the sleep ≤7-h group, and napping during the day, retired or not, and employment status being more significant in the sleep ≥7-h group (Fig. 1c).
Differentiated blood-biomarker profiles of short and long sleep
Associations between sleep duration and 229 blood biomarkers (including 31 blood count biomarkers, 30 blood biochemistry biomarkers and 168 metabolomic biomarkers) were obtained in both sleep groups after adjusting for covariates including age, sex, body mass index, the Townsend deprivation index measuring socioeconomic status, educational qualifications, smoking status and drinking status (Fig. 2a and Supplementary Tables 2 and 3). A total of 46 significant biomarkers were identified in the sleep ≤7-h group and 144 in the sleep ≥7-h group (Bonferroni-corrected P < 0.01, uncorrected P < 4.38 × 10−5 (0.01/229)), and 121 biomarkers showed significantly differentiated association strengths between the two sleep groups (permutation test, P < 0.01; Methods).
a, Associations of blood biomarkers with short sleep (≤7 h, top) and long sleep (≥7 h, bottom), with covariates adjusted. The size of the points represents the absolute correlation coefficient (|r|), with larger points indicating stronger associations. The color indicates the direction of the correlation, with red representing positive and blue representing negative associations. The y axis shows the −log10P value (two-tailed). The statistical significance of the partial correlation coefficients (r values) was determined using the Student’s t distribution in a–c. b, Partial correlations between sleep ≤7 h and sleep ≥7 h with cortical thickness and subcortical volumes, adjusted for estimated total intracranial volume, scanning site and other covariates (Bonferroni-corrected two-tailed P < 0.01). c, Partial correlations between sleep ≤7 h (top) and sleep ≥7 h (bottom) with fractional anisotropy (FA) and mean diffusivity (MD). Red color indicates positive associations and blue indicates negative associations (FDR-corrected two-tailed P < 0.05). d, Scatter plots of correlation coefficients for short sleep (rsleep≤7) and the inverse of long sleep (−rsleep≥7) for blood biomarkers (left), and gray matter (middle) and white matter (right) microstructure. The point size represents the absolute difference in |r| values between the short and long sleep groups (||rsleep≤7| − |rsleep≥7||), with larger points indicating greater differences between the two sleep groups. The color of the point represents the direction of the difference of |r| value between the short sleep group and long sleep group with phenotypes (|rsleep≤7| − |rsleep≥7|), with red indicating phenotypes more associated with sleep ≤7 h and blue representing phenotypes more associated with sleep ≥7 h. Diff. abs. r value: |rsleep≤7| − |rsleep≥7|; abs.(abs. r diff.): ||rsleep≤7| − |rsleep≥7||.
Of the 31 blood count biomarkers, 16 and 22 biomarkers were significantly associated with the sleep ≤7-h and sleep ≥7-h groups, respectively, with 13 overlapping biomarkers significantly associated with both the sleep ≤7-h and sleep ≥7-h groups simultaneously (Supplementary Table 4). Meanwhile, 16 blood count biomarkers showed significantly different associations between the two sleep groups (permutation test, P < 0.01). Fourteen of these blood counts, including neutrophil count and white blood cell count, were closely related to the immune system and inflammation29, and showed more significant associations with the long sleep group.
Furthermore, of the 30 blood biochemistry biomarkers, 16 and 23 biomarkers were significantly associated with sleep duration in the sleep ≤7-h and sleep ≥7-h groups, respectively, with 13 overlapping biochemistry biomarkers (Supplementary Table 5). Notably, 21 biochemistry biomarkers manifested significantly different correlations between the two groups (permutation test, P < 0.01). Specifically, four biomarkers, including alkaline phosphatase, vitamin D and insulin-like growth factor 1 (IGF-1), which are closely related to musculoskeletal health and body aging30, were predominantly associated in the sleep ≤7-h group (|rsleep≤7| > 0.019, Psleep≤7 < 9.4 × 10−25, and P < 2.0 × 10−4 based on permutation tests). Additionally, 12 biomarkers, of which seven biomarkers were from the immunometabolic category, including triglycerides, CRP and high-density lipoprotein (HDL) cholesterol, which are hallmarks of cardiovascular risk31,32, were more associated with the sleep ≥7-h group (|rsleep≥7| > 0.016, Psleep≥7 < 5.4 × 10−20).
In addition to the blood measures, NMR metabolomic biomarkers (n = 118,000) also showed different association patterns with sleep duration in the two sleep groups, with 14 significant metabolomic biomarkers in the sleep ≤7-h group (|rsleep≤7| ranging from 0.015 to 0.027) and 99 significant metabolomic biomarkers in the sleep ≥7-h group (|rsleep≥7| ranging from 0.014 to 0.052; Supplementary Table 6). Remarkably, 84 metabolomic biomarkers showed significantly differentiated associations between the two sleep groups (permutation test, P < 0.01). Although ten biomarkers showed predominant associations in the sleep ≤7-h group, mostly related to glycoprotein acetyls, an inflammatory biomarker that has been associated with cardiometabolic disease and multiple incident diseases33,34 (rsleep≥7) = −0.027, P = 1.5 × 10−13, permutation P < 2 × 10−4), the remaining 101 metabolomic biomarkers showed stronger associations in the sleep ≥7-h group, including cholesterol-related biomarkers such as apolipoprotein B, HDL cholesterol, total cholesterol, cholesteryl esters and so on, and glycolysis-related metabolites, such as citrate, lactate and so on (|rsleep≥7) | > 0.012, Psleep≥7 < 1.5 × 10−4, permutation P < 2 × 10−4; Fig. 2d). The results, further adjusted for technical covariates of the NMR biomarkers, including processing batch, the time difference between sample measurement and preparation dates, and spectrometer, were consistent with the current findings (Supplementary Table 7). The age-stratified association analysis between the sleep ≤7-h and sleep ≥7-h groups with blood biomarkers showed that elderly participants in the sleep ≥7-h group had the most significant associations with immunometabolic biomarkers, including triglycerides, HDL cholesterol and CRP (Supplementary Fig. 2).
Differences in associations with brain structure
Brain structure (n = 39,692) also demonstrated different association patterns with the sleep ≤7-h and sleep ≥7-h groups after adjusting for intracranial volume, sites and the aforementioned covariates. For instance, the cortical thickness of multiple brain regions showed significantly differentiated associations between the two sleep groups. There were 1 and 47 predominant brain regions identified for the sleep ≤7-h and sleep ≥7-h groups, respectively (Bonferroni-corrected P < 0.01, uncorrected P < 1.47 × 10−4 (0.01/68); Fig. 2b), and the only overlapping region, the left middle temporal gyrus, was significantly associated with both the sleep ≤7-h and sleep ≥7-h groups (rsleep≤7 = 0.025, P = 3.8 × 10−5; rsleep≥7 = −0.042, P = 5.8 × 10−14). The regions that were only significantly associated with the sleep ≥7-h group included the superior frontal gyrus, superior temporal gyrus and rostral middle frontal gyrus (r ranged from −0.021 to −0.045, Bonferroni-corrected P < 0.01, uncorrected P < 1.47 × 10−4 (0.01/68)). On the other hand, the cortical surface areas of three regions were significantly associated with the sleep ≤7-h group (rsleep≤7 = 0.023–0.0267), including the right pericalcarine cortex, the left lateral orbitofrontal cortex and the right lingual gyrus, but no significant finding was observed in the long sleep group (Supplementary Fig. 3b). The cortical volume of 10 regions (rsleep≤7 = 0.024–0.035) and 14 regions (rsleep≥7 = −0.022 to −0.031) showed significant associations with the sleep ≤7-h and sleep ≥7-h groups, respectively (Bonferroni-corrected P < 0.01, uncorrected P < 0.01/68 (1.47 × 10−4); Supplementary Fig. 3a). The cortical volume of the right isthmus cingulate cortex, the left caudal middle frontal, the right pericalcarine and the left inferior temporal gyrus was significantly associated only with the sleep ≤7-h group. Nine regions showed significant associations solely with the sleep ≥7-h group, including the left frontal pole, the superior frontal cortex and the left parahippocampal gyrus (Supplementary Table 8). The associations of subcortical volume with sleep duration also varied across the two groups (Fig. 2b and Supplementary Table 9). Significant findings were observed only for the sleep ≥7-h group, for instance, the hippocampus (left: rsleep≥7 = −0.027, P = 2.85 × 10−6; right: rsleep≥7 = −0.027, P = 2.57 × 10−6) and the thalamus (left: rsleep≥7 = −0.023, P = 6.36 × 10−5; right: rsleep≥7 = −0.023, P = 5.63 × 10−5). Age-stratified analysis showed that elderly participants in the sleep ≥7-h group showed the most significant associations with brain structures (Supplementary Fig. 2).
Brain white matter microstructure also showed different association patterns in the two sleep groups (Fig. 2c). FA of the posterior thalamic radiation was associated with sleep duration in the sleep ≥7-h group (rsleep≥7 = −0.022 to −0.032, false discovery rate (FDR)-corrected P < 0.05), with no significant findings in the sleep ≤7-h group. Similarly, MD of the posterior thalamic radiation, forceps minor, anterior thalamic radiation and uncinate fasciculus was solely associated with sleep duration in the sleep ≥7-h group (rsleep≥7 = 0.022–0.032; Fig. 2d).
Differences in genetic architecture
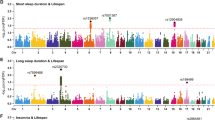
We performed genome-wide association studies (GWAS) for sleep duration in the sleep ≤7-h and sleep ≥7-h groups, respectively, and identified six independent significant loci in the sleep ≤7-h group (Fig. 3a and Supplementary Table 10) and 14 independent significant loci in the sleep ≥7-h group (Fig. 3b). The GWAS results further adjusted for batch effects and assessment centers were consistent with the original findings (Supplementary Table 11). There are no shared single nucleotide polymorphisms (SNPs) between these two sleep groups. SNP-based heritability (h2) utilizing linkage disequilibrium (LD) score regression was 6.17% (s.e. = 0.0034) for the sleep ≤7-h group and 5.31% (s.e. = 0.0031) for the sleep ≥7-h group35.
a,b, Manhattan plots for the GWAS of short sleep (≤7 h) (a) and long sleep (≥7 h) (b), respectively. The x axis represents the genomic positions across the genome and the y axis represents the −log10P value (two-tailed) of the associations derived from a linear model. A t-test was utilized to calculate the P values. The red line indicates the genome-wide significance threshold after multiple testing correction (P < 5 × 10−8). Independent significant loci are labeled. c, Genetic correlations (rg) between the short (sleep ≤7 h, n = 211,101) and long (sleep ≥7 h, n = 255,699) sleep groups, with health-related phenotypes calculated using LD-score regression (significant rg with at least one sleep group are shown; Bonferroni-corrected two-tailed P < 0.05). The x axis represents health-related phenotypes and the y axis represents the absolute value of genetic correlation: rg sleep≤7 (|rg sleep≤7| range = [0.21, 0.58], P < 4.18 × 10−5) and −1 times the absolute value of genetic correlation: rg sleep≥7 (|rg sleep≥7)| range = [0.12, 0.35], P < 4.22 × 10−5). Detailed P values for genetic correlations are available in Supplementary Data 3. Error bars indicate s.e. of the rg.
Genetic correlations (rg) were further estimated to measure the underlying genetic constructs of the two sleep groups and their genetic overlap with phenotypes of interest, respectively. Although both groups showed consistently strong genetic correlations with a previous GWAS finding for complete sleep duration (that is, without the short versus long sleep stratification, rg = 0.71 and 0.69, respectively), the genetic correlation between the sleep ≤7-h group and sleep ≥7-h group is minimal (rg = −0.058, s.e. = 0.037, P = 0.12). This result implies distinct genetic bases for the two sleep groups. Furthermore, the two groups showed distinct genetic correlation profiles with blood measures and health-related phenotypes. Specifically, the sleep ≤7-h group showed significant rg with 118 phenotypes, including 16 health-related phenotypes (12 mental health, two general health and two cognition phenotypes), five blood count biomarkers, 12 blood chemistry biomarkers and 85 metabolomic biomarkers (|rg| range = [0.21, 0.58], P < 4.18 × 10−5; Fig. 3c and Supplementary Data 3). Among them, the overall health rating, PHQ-4, longstanding illness and trauma experiences (rg range = [−0.58, −0.47]) showed the most significant genetic correlations with the sleep ≤7-h group. In contrast, the sleep ≥7-h group showed significant genetic correlations with only 18 phenotypes, including eight health-related phenotypes, three blood count biomarkers, six blood chemistry biomarkers and one metabolomic biomarker (|rg| range = [0.12, 0.35], P < 4.21 × 10−5; Fig. 3c and Supplementary Data 3). Among them, the fluid intelligence, overall health ratings, PHQ-9 and immunometabolic biomarkers, such as glycated hemoglobin (HbA1c), white blood cell and HDL cholesterol, showed the most significant genetic correlations (|rg| range = [0.14, 0.35]).
Distinct proteomic profile of short and long sleep groups
The sleep ≤7-h and sleep ≥7-h groups showed distinct associations with the proteomic profiles (n = 54,964), with the sleep ≤7-h group significantly associated with 203 of the 1,463 proteins, and the sleep ≥7-h group showing significant associations with 485 of the 1,463 proteins (Fig. 4a; Bonferroni-corrected P < 0.01, uncorrected P < 0.01/1,463 (6.84 × 10−6)). In detail, 324 proteins were solely associated with the long sleep group, and 42 proteins were only associated with the sleep ≤7-h group. Meanwhile, all four panels of protein—oncology, neurology, inflammation and cardiometabolic—showed more significant associations with the long sleep group (Fig. 4b). Notably, an aging effect was observed in the associations between the sleep ≤7-h and sleep ≥7-h groups with proteins. Elderly participants in the sleep ≥7-h group showed the most significant associations with proteins compared with other groups, with 190 unique proteins associated only with the elderly group of long sleep (Fig. 4c). Additionally, a greater difference in associations between the sleep ≤7-h and sleep ≥7-h groups with proteins was observed in the elderly group. Specifically, ITGAV and PTGDS, recognized as oncology and cardiometabolic biomarkers, respectively, exhibited significant associations solely with the long sleep group (Fig. 4d).
a, Associations between short sleep (≤7 h, left) and long sleep (≥7 h, right) with proteins using linear regression. The regression coefficients (β) and corresponding two-tailed P values were derived using a t-test. The horizontal line indicates the Bonferroni-corrected significance threshold (two-tailed P < 0.01). The x axis represents β values and the y axis represents −log10P values of the associations. b, Histogram of −log10P values (two-tailed) of linear regression between short and long sleep groups with four panels of proteins. Red represents associations between sleep ≤7 h and proteins, and blue represents associations between sleep ≥7 h and proteins. c, Overlapping significant proteins between the middle-aged and older adult groups of short and long sleep groups. Red and green represent middle-aged and older adult groups, respectively, for the long sleep group, and blue and purple represent the middle-aged and older adult groups, respectively, for the short sleep group. d, Proteins with the most significant differences in their associations with short and long sleep groups within the middle-aged (left) and older adult (right) groups, respectively. The x axis represents the difference in the absolute correlation values (|r|) between the short and long sleep groups for protein associations using linear regression (|rsleep≤7| − |rsleep≥7|). Blue indicates proteins more strongly associated with the long sleep group, and red indicates proteins more strongly associated with the short sleep group. Abs. r value diff.: |rsleep≤7| − |rsleep≥7|.
Distinct causal association pattern of short and long sleep
Bidirectional causal inference between the two sleep groups and 15 health-related phenotypes, covering mental and neurological health, cardiometabolic health and related biomarkers, was investigated using two-sample MR (TwoSample MR version 0.5.6; Fig. 5 and Supplementary Table 12) for the sleep ≤7-h and sleep ≥7-h groups separately. These traits have been previously associated with sleep disturbances in both observational and genetic studies20,36,37,38 representing major health outcomes identified to interact with sleep patterns.
Bidirectional two-sample MR identified causal associations between the short sleep group (sleep ≤7 h) and long sleep group (sleep ≥7 h) with health-related phenotypes, respectively. The causal associations were calculated using inverse-variance-weighted MR analyses. Red arrows indicate a positive causal association and blue arrows indicate a negative causal association. Arrows indicate directions of a causal association, with a double-headed arrow indicating a significant bidirectional causal association (for consistency, the P values labeled for the bidirectional causal association were from sleep ≤7 h to outcomes or from the disease to sleep ≥7 h). All statistical tests were two-sided. *FDR-corrected P < 0.05, **FDR-corrected P < 0.01, ***FDR-corrected P < 0.001.
Longer sleep duration in the sleep ≥7-h group was identified as an outcome of various health conditions, such as poor overall health (β (s.e.) = 0.11 (0.03), P = 9.7 × 10−5) and diagnoses of mental or neurodegenerative disorders, including schizophrenia (β (s.e.) = 0.025 (0.004), P = 5.0 × 10−12), bipolar disorder (β (s.e.) = 0.033 (0.007), P = 6.9 × 10−6), major depressive disorder (β (s.e.) = 0.018 (0.007), P = 7.1 × 10−3), Alzheimer’s disease (β (s.e.) = 0.008 (0.003), P = 3.5 × 10−3) and coronary artery disease (β (s.e.) = 0.014 (0.004), P = 6.8 × 10−4) (FDR-corrected P < 0.05). These results were further supported by findings from biomarkers. For instance, causal associations were found from lower cortical thickness to longer sleep duration in the sleep ≥7-h group (β (s.e.) = −0.21 (0.06), P = 5.9 × 10−4), aligning with the causal associations of brain disorders with longer sleep duration. Furthermore, a higher low-density lipoprotein (LDL) cholesterol level, a biomarker of cardiovascular risk, was causally associated with longer sleep duration (β (s.e.) = 0.017 (0.005), P = 1.6 × 10−3) in the long sleep group. Interestingly, a lower CRP level, indicating reduced inflammation, was causally related to longer sleep duration (β (s.e.) = −0.038 (0.009), P = 5.0 × 10−5). Finally, long sleep duration, but not short sleep duration, showed a causal association with a schizophrenia diagnosis (odds ratio (OR) (s.e.) = 1.49 (0.117), P = 6.8 × 10−4; Supplementary Figs. 4 and 5 and Supplementary Tables 13 and 14).
In contrast, shorter sleep duration in the sleep ≤7-h group had bidirectional causal effects with various health conditions and corresponding biomarkers. For instance, shorter sleep duration could possibly increase the risk of multiple health conditions, including coronary artery disease (OR (s.e.) = 1.55 (0.11), P = 7.5 × 10−5), major depressive disorder (OR (s.e.) = 1.36 (0.11), P = 5.3 × 10−3), class 1 obesity (OR (s.e.) = 1.69 (0.16), P = 1.4 × 10−3) and a higher BMI (β (s.e.) = 0.19 (0.06), P = 2.5 × 10−3) (FDR-corrected P < 0.05). Therefore, maintaining sleep duration close to 7 h in the ≤7-h sleep group could have a protective effect against multiple diseases and obesity (Supplementary Fig. 6 and Supplementary Table 15). In line with these findings, shorter sleep duration in the sleep ≤7-h group may lead to lower FA (β (s.e.) = −0.004 (0.002), P = 1.9 × 10−2) and decreased IGF-1 (β (s.e.) = −0.94 (0.29), P = 1.4 × 10−3), an aging biomarker with neuroprotective functions39. It could also result in increased LDL cholesterol (β (s.e.) = 0.21 (0.08), P = 4.9 × 10−3) and decreased HDL cholesterol (β (s.e.) = −0.16 (0.06), P = 6.5 × 10−3) levels. There was also evidence of a causal effect from shorter sleep duration (in the sleep ≤7-h group) on increased CRP (β (s.e.) = 0.14 (0.06), P = 0.032), indicating a higher risk of inflammation. Reversely, we identified three health-related phenotypes that show significant causal associations with shorter sleep duration in the sleep ≤7-h group, including the overall health (β (s.e.) = 0.097 (0.024), P = 4.2 × 10−5), the diagnosis of major depressive disorder (β (s.e.) = 0.019 (0.006), P = 3.42 × 10−3) and a higher LDL cholesterol level (β (s.e.) = 0.016 (0.005), P = 6.71 × 10−4) (FDR-corrected P < 0.05; Supplementary Fig. 7 and Supplementary Table 16, aligning with previous clinical observations40,41.
To validate these distinct causal associations of short and long sleep duration with health-related phenotypes, we also utilized GWAS results based on the classical stratified definition of short (<7 h) and long (>8 h) sleep versus normal sleep (7 or 8 h). The results from two-sample MR are consistent with the current findings (Supplementary Fig. 8). Long sleep duration was significantly identified as the outcome of poor overall health, schizophrenia, coronary artery disease, Alzheimer’s disease, bipolar disorder, major depressive disorder and LDL cholesterol (FDR-corrected P < 0.05). Meanwhile, short sleep duration showed a causal effect on poor overall health, coronary artery disease, class 1 obesity, higher BMI and related biomarkers, including CRP and HDL cholesterol (FDR-corrected P < 0.05; Supplementary Tables 17–20).
Sensitivity analysis
To further validate the results, we utilized the stratified definition of short (sleep <7 h, n = 123,245) and long (sleep >8 h, n = 38,346) sleep duration versus normal sleep (7 or 8 h, n = 336,682) to conduct the analysis again (Supplementary Tables 21–26, Supplementary Figs. 9–12 and Supplementary Data 4–6). We calculated the correlation coefficients between the results (r values) from the ordinal sleep duration and the results (t value) from the stratified definition of sleep groups in each analysis. The results from the stratified definition of sleep groups were highly consistent with the respective ordinal definitions of short sleep (sleep ≤7 h) and long sleep (sleep ≥7 h), with correlation coefficients for both definitions of the short sleep group ranging from −0.970 to −0.763 (with subcortical volume as a not-too-distant outlier, −0.553, where the negative sign was entirely due to reversed coding in the short sleep versus normal sleep), and both definitions of the long sleep group ranging from 0.879 to 0.995 (Supplementary Table 27). This further demonstrates the reliability of our findings. However, the classical stratified sleep definition showed lower statistical power compared to the ordinal definition, primarily due to the loss of detailed sleep duration information.
Discussion
The present study identified common and differentiated behavioral, immunometabolic, neuroimaging and protein association atlases of sleep ≤7-h and sleep ≥7-h groups. Notably, we revealed distinct genetic architectures behind these two sleep duration groups by showing that there was no evidence of a genetic correlation between them (rg = −0.058, P = 0.12). Such a claim was further supported by the MR results, which indicate that long sleep duration appears to be a consequence of poor health conditions, and short sleep duration has a potential causal effect on multiple health conditions, such as mental disorders and cardiovascular diseases, as well as their related blood and brain imaging biomarkers.
We identified specific phenotypic atlases of blood biomarkers for short and long sleep duration. In particular, long sleep duration was preferentially linked with higher levels of inflammation-related biomarkers, including CRP and elevated white blood cells. This result is consistent with a previous meta-analysis that showed significant associations between long sleep duration and increased levels of systemic inflammation biomarkers, but this was not apparent for short sleep duration42. Nonetheless, considering the existence of confounding factors, it has long been debated whether long sleep duration is indeed a risk factor for inflammation. Here, our MR results identified that there is no causal effect from long sleep duration on CRP. This finding suggests that long sleep duration and inflammation biomarkers may arise simultaneously as consequences of previously identified inflammation-related diseases, including depression and cardiovascular disease43.
Notably, even though the phenotypic associations between short sleep duration and inflammation-related biomarkers were relatively weaker compared to those of the long sleep group, we found a significant causal association of the short sleep group with CRP, depression and cardiometabolic disease. This implies that short sleep duration potentially contributes to increased CRP levels and a higher risk of inflammation-related diseases. This finding aligns with previously reported longitudinal associations between short sleep duration and increased CRP44. A potential mechanism for the causal association between short sleep duration and inflammation could be attributed to the fact that sleep disturbance may adversely affect the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system, two effector systems of the central nervous system that regulate the immune response37,43. Our results collectively support the notion that short sleep duration could be causally linked with multiple disease-related blood biomarkers, particularly immune-related biomarkers, which, in turn, may represent a plausible mechanism underpinning the link between short sleep duration and poor health conditions in adults45,46. However, as other inflammation biomarkers, such as interleukin-6 (IL-6) and IL-10, were not available in the UK Biobank, our findings should be interpreted with caution. Further validation in other populations and datasets that include a broader range of inflammatory markers is necessary.
Interestingly, we found that long sleep duration was a consequence of abnormal levels of blood biomarkers, which are closely related to cardiovascular risk and cardiovascular disease. Prior work has found no causal effect from genetically predicted long sleep duration on cardiovascular disease20. Our results support this idea and further identify the reverse causal association from cardiovascular disease to long sleep duration. Hence, our study suggests that, instead of being a risk factor for disease incidence, long sleep duration is more likely a consequence of poor health conditions. Indeed, long sleep duration has been posited to be a surrogate marker of poor physical and mental health status47. Possible mechanisms underpinning poor health’s causal effect on long sleep duration include sleep’s beneficial and restorative effects on recovery from infection and diseases48. Meanwhile, sleep plays a critical role in glymphatic function, a process in which cerebrospinal fluid enters the brain via the glymphatic system and clears metabolic waste and toxins accumulated during the awake state49. Notably, a previous study demonstrated a positive association between total sleep time and glymphatic function in older adults50. Therefore, the glymphatic system may also be intricately linked to the health conditions associated with long sleep duration, which could be crucial for brain and cardiovascular health51.
We also identified different protein profiles for short and long sleep duration, with long sleep showing more significant associations with proteins. A key protein identified here is the a cardiometabolic protein GDF15, which has been identified as a robust prognostic protein for multiple diseases, such as heart disease and dementia, in a previous study52. This distinction was identified in both the middle-aged and older adults groups. Most proteins displayed significant unique associations with the long sleep group, particularly among older adults. This suggests that disease-related protein biomarkers might serve as a potential mechanism linking aging-related conditions, such as neurodegenerative and cardiovascular diseases, to long sleep duration. These findings align with our MR results. As there is a significant aging effect in the associations of short and long sleep duration with health conditions and related biomarkers, future studies could focus on investigating the possible mechanism of aging’s influence on these associations.
This study also identified that those with short sleep were genetically distinct from those with long sleep duration. Notably, in contrast to the more significant phenotypic association between the long sleep group and most blood biomarkers, the short sleep group manifested more significant genetic correlations with health-related phenotypes, including mental health variables and biomarkers related to cardiovascular health. This implies that there may be higher genetic overlaps between the short sleep group and health-related biomarkers. This finding aligns with a previous study reporting the genetic links between insomnia, cardiovascular disease and psychiatric symptoms19,53. Meanwhile, our results suggest that the significant phenotypic associations between long sleep duration and disease-related blood biomarkers may not be derived from a shared genetic architecture, but rather from concomitant conditions. This was further identified in the MR results, where prolonged sleep duration tended to be the consequence of poor health.
Our study also has some limitations. One limitation is that we used a continuous or ordinal definition of short and long sleep duration (sleep ≤7 h and sleep ≥7 h), whereas previous work has often utilized a stratified definition of short and long sleep duration (short sleep (<7 h) versus normal sleep (7 or 8 h) and long sleep (>8 h) versus normal sleep (7 or 8 h)). Nevertheless, we demonstrated that both approaches yield highly similar results. Notably, our causal associations of ordinal short or long sleep duration with health conditions were also validated using the stratified definition. Meanwhile, the UK Biobank records self-reported sleep duration only in whole numbers. In this context, individuals reporting exactly 7 h of sleep serve as a common reference point. Their sleep duration contributes to associations with other phenotypes only when compared to individuals with shorter or longer sleep durations, that is, resembling the role of the stratified 7–8-h sleep group in the classical sleep definition. Therefore, although 7 h is used as a reference in both the ≤7-h and ≥7-h groups, this does not introduce shared information between the groups, as evidenced by the minimal genetic correlation between them.
Second, as utilized in the current study, sleep duration was derived from self-reported questionnaires. Although these are easily obtained and commonly utilized in most epidemiological studies, they can be subject to recall bias. Future studies could focus on objective sleep measures, such as those obtained from actigraphy or polysomnography, which could also reveal more about the mechanism by which sleep may underpin adverse health outcomes. Nevertheless, even the subjective estimation of sleep duration could vary in different populations across various countries due to cultural and social factors, latitude and many other factors7,54. Additionally, more detailed self-reported sleep information is not available in the UK Biobank, such as sleep variability and duration units in minutes, which should also be investigated in future studies. Third, the effect sizes of some brain findings were relatively small (for example, r = 0.02–0.03). However, the large sample size (that is, N > 27,000) of the UK Biobank brain imaging data was able to still achieve adequate statistical power (>60%) in identifying an effect size as small as r = 0.025, even under an extremely stringent Bonferroni-corrected P < 0.01. Finally, the results of our study reflect the demographic makeup of the UK Biobank and may not be representative of other populations. Therefore, caution should be exerted when extrapolating these findings to other populations.
Overall, we have dissected the recently identified nonlinear relationships between sleep duration and health conditions5 into two largely independent monotonic relationships. A population-wide minimum sleep duration of 7 h is recommended to reduce the risk of adverse health conditions, and long sleep is likely a consequence of underlying poor health states, signifying the body’s inherent attempt to initiate repair and recovery processes in response to illness. However, it is important to note that individual variability plays a crucial role, and personalized recommendations should be tailored based on an individualized optimal sleep duration. Thus, our findings make a significant and timely contribution to public health.
Methods
Participants
Data from the UK Biobank were utilized in the current study (application ID 19542), including 498,277 participants, primarily of European ancestry aged between 38 and 73 years old. The UK Biobank has research tissue bank approval from the North West Multi-centre Research Ethics Committee (https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics) and provided oversight for this study (ref. 11/NW/0382). The use of UK Biobank data in our study aligns with the terms and conditions of the UK Biobank Material Transfer Agreement. Written informed consent was obtained from all participants. The risk of participants experiencing harm from taking part is minimal, and UK Biobank has insurance in place to compensate for any harm caused by negligence. Briefly, between 2006 and 2010, information on participants’ health and lifestyle, cognitive function and a range of physical measurements were collected at the assessment center. Samples of blood were also collected and utilized for the analysis of blood biomarkers. Meanwhile, online questionnaires, including a 24-h recall questionnaire, healthy work questionnaire and mental health questionnaire, were also collected from the participants. Imaging data collection began in 2014, and neuroimaging data of 39,692 participants were available and used in the current analyses. A detailed introduction to the UK Biobank is available at http://www.ukbiobank.ac.uk.
Sleep measures
Sleep duration, as assessed in the baseline assessment (2006–2010, n = 498,277) and imaging visit (2014+, n = 48,511), was utilized in the current study. It was measured by means of touchscreen questionnaires: ‘About how many hours sleep do you get in every 24 h? (please include naps)’. Answers of less than 1 h or more than 23 h were rejected, and answers of less than 3 h or more than 12 h required confirmation by the participants. Participants who answered ‘do not know’ or ‘prefer not to answer’ were excluded from the analysis. The specific question about sleep duration was not based on any established sleep questionnaire. It was obtained from a category of self-reported sleep conditions (https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100057). Sleep duration measured at baseline was utilized in the PheWAS to determine the associations between sleep duration and phenotypes of interest measured simultaneously (n = 489,277). Sleep duration assessed at the neuroimaging visit was used to measure the associations with brain structures (n = 48,511). The sleep duration data were divided into two groups: one with sleep duration ≤7 h and the other with sleep duration ≥7 h (Supplementary Fig. 1). Considering that we identified population-wise optimal sleep duration for middle-aged and elderly participants of the UK Biobank in previous studies5,6, we utilized 7 h as the baseline to explore how changes in sleep duration—both increases and decreases—are distinctly associated with health conditions and their potential mechanisms. A sleep duration of exactly 7 h, which, as a constant, is not associated with any phenotype of interest (that is, a column of constants has no variability to correlate with any other variables), was regarded as the common reference point for both sleep <7 h and sleep >7 h. To further validate our results, we also stratified the sleep duration into three groups: short sleep (less than 7 h), normal sleep duration (7 or 8 h) and long sleep (more than 8 h), to replicate the main analysis.
Behavioral and clinical phenotypes
The phenotypes utilized in the PheWAS could be divided into 20 categories, comprising a total of 3,735 levels of variables: (1) baseline characteristics; (2) cognitive functions; (3) diet by 24-h recall; (4) digestive health; (5) early life factors; (6) family history; (7) first occurrences; (8) food preferences; (9) health and medical history; (10) health-related outcomes; (11) lifestyle and environment; (12) medical conditions; (13) medications; (14) mental health; (15) operations; (16) physical measures; (17) psychosocial factors; (18) sex-specific factors; (19) sociodemographic; (20) work environment. Details of the phenotypes are provided in Supplementary Table 1. In our study, we specifically aimed to explore the potential distinct associations of short and long sleep duration with various psychiatric disorders and neurological diseases. To this end, we intentionally did not exclude participants with neurological and psychiatric conditions from our analysis. All the phenotypes were first overlapped with the participants with sleep duration ≤7 h and ≥7 h and then utilized in the PheWAS, respectively.
Blood biomarkers
Blood samples were collected from 500,000 participants at the UK Biobank baseline assessment, which allowed for biochemical and hematologic assays. Details of the blood sample collection are available in the open-source document at https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf. Briefly, whole blood was utilized for a range of hematological parameters on a Beckman automated hematology analyzer, and 31 parameters were reported, including complete blood count data, differential data and reticulocyte data (category ID 100081). The hematological parameters were further categorized into three groups in the current study: white blood cell (basophil count and eosinophil count), red blood cell (hematocrit percentage, hemoglobin concentration and reticulocyte count) and platelet (mean platelet volume and platelet count). Meanwhile, 30 biochemistry biomarkers were analyzed from blood samples utilizing ten immunoassay analyzers and four clinical chemistry analyzers (category ID 17518). They were selected based on their established disease risk factors, diagnostic value and ease of availability at scale. One of the biochemistry biomarkers was measured in red blood cells (glycated hemoglobin) and 29 in serum. These biochemistry biomarkers were further divided into bone and joint (alkaline phosphatase, calcium and vitamin D), endocrine (testosterone, IGF-1 and sex hormone-binding globulin (SHBG)), immunometabolic (apolipoproteins A and B, cholesterol, CRP and glucose), liver function (alanine and aspartate aminotransferase, albumin and direct and total bilirubin) and renal function (creatinine, cystatin C, phosphate and total protein) as in a previous study55 (Supplementary Table 2). The blood biomarkers of the participants were analyzed in two groups with sleep duration ≤7 h and sleep duration ≥7 h.
Metabolomic biomarkers
In the baseline recruitment of the UK Biobank, ethylenediaminetetraacetic acid (EDTA) plasma samples were collected from ~118,000 participants, and processed by Nightingale Health Ltd to perform metabolic biomarker profiling. Detailed descriptions of the metabolomic biomarker profiling platform and experimentation are available in previous publications56,57. High-throughput NMR was used to measure the samples, providing 248 metabolomic biomarkers, including 168 in absolute levels and 81 ratio measure per EDTA plasma sample. Multi-metabolic pathways were covered by the biomarkers, including various measures of cholesterol, fatty acid composition and 14 lipoprotein subclasses, as well as low-molecular-weight metabolites such as amino acids, ketones and glycolysis metabolites quantified mostly in absolute concentration units. The current study utilized 168 metabolomic biomarkers in absolute levels, which were further grouped into 16 subsets, including cholesterol, triglycerides, fluid balance, phospholipids and so on (Supplementary Table 3). In the subsequent analysis, samples were divided into two groups based on sleep duration ≤7 h and sleep duration ≥7 h.
Structural magnetic resonance imaging data
After baseline assessment between 2006 and 2010, the UK Biobank invited some participants back to undergo neuroimaging collection in 2014. Imaging-derived phenotypes (IDPs) generated by an imaging-processing pipeline developed and run on behalf of UK Biobank were used in the study. Quality-controlled T1-weighted neuroimaging data and diffusion tensor imaging data, processed with FreeSurfer and FSL (FMRIB Software Library), respectively, were analyzed. Details of the imaging protocol and processing pipeline are available in the open-source document at https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/brain_mri.pdf. Neuroimaging data were collected on a standard Siemens Skyra 3T scanner with a 32-channel head coil.
T1 images were processed with FreeSurfer, and surface templates were utilized to extract IDPs referring to atlas regions’ surface area, volume and mean cortical thickness58. Subcortical regions were extracted using FreeSurfer’s aseg tool59. FreeSurfer aparc (Category 192) and ASEG (Category 190) atlas corresponding to 68 cortical regions and 40 subcortical regions were used in this study. The intracranial volume (field ID 26521) generated by aseg was utilized as a covariate in the neuroimaging analyses. The Qoala-T approach was used to check FreeSurfer outputs, supplemented by manual checking of outputs close to the threshold. Any FreeSurfer outputs that failed to pass quality control were not included in the FreeSurfer IDPs.
For diffusion-weighted imaging, briefly, the Eddy tool was first utilized to correct for eddy currents, head motion and outlier slices. Gradient distortion correction (GDC) was then applied to produce a more accurate correction60. Tractography-based analysis was then conducted on the preprocessed diffusion magnetic resonance imaging data, beginning with within-voxel modeling of multi-fiber tract orientation by the bedpostx tool, followed by probabilistic tractography by probtrackx. Twenty-seven major tracts using masks defined by AutoPtx61 were mapped in this pipeline. An IDP was generated for each tract. FA and MD of the 27 tracts were utilized in the current study (category 135).
Genotypes
The UK Biobank provided genome-wide genetic data from 488,000 participants. Two arrays, the Affymetrix UK BiLEVE Axiom and the Affymetrix UK Biobank Axiom array, were utilized to genotype the UK Biobank blood samples. Genotype data were quality-controlled and imputed into the Haplotype Reference Consortium (HRC) and UK10K haplotype resource, which increased the number of variants to ~96 million. Markers’ positions were expressed in GRCh37 coordinates. Details of genotyping, the quality-controlled pipeline, imputation and analyses are provided in a previous publication62. We also excluded SNPs with call rates <95%, minor allele frequency <0.1% and deviation from the Hardy–Weinberg equilibrium with P < 1 × 10−10, and selected subjects that were estimated to have recent British ancestry and have no more than ten putative third-degree relatives in the kinship table, consistent with the previous study63. After the quality-control procedures, we obtained 8,894,431 SNPs and 337,151 participants.
Proteomics
Blood plasma samples of 54,964 participants in the baseline assessment were utilized for proteomic analysis by the Olink Explore 3072 platform, which measured 2,941 proteins, including eight panels of cardiometabolic (II), inflammation (II), oncology (II) and neurology (II). The current study utilized 1,463 proteins (four panels) of the 2,941 proteins available for application. Details of sample selection, sample handling and quality control of the protein data are provided in previous publications64. Protein samples were first overlapped with the two sleep groups (sleep ≤7 h and sleep ≥7 h), respectively, and utilized for the subsequent analysis.
Statistical analysis
PheWAS
PheWAS associations were conducted utilizing the PHESANT package in R. Technical details and methods for the rule-based algorithm used in PHESANT are available in a previous publication65. Briefly, according to different variable types (including continuous, ordered categorical, unordered categorical and binary), specific regression was utilized to determine the associations between the trait of interest and the variables, corresponding to linear, ordinal logistic, multinomial logistic and logistic regression, respectively. In the current study, sleep duration was utilized as the trait of interest, and was divided into two groups, sleep duration ≤7 h (n = 315,630) and sleep duration ≥7 h (n = 375,030), and entered into the PHESANT as independent variables, respectively. Selected phenotypes of 20 categories were set as dependent variables. Given that sleep duration is age-dependent and closely associated with genetic factors (such as sex) and environmental influences (such as education and lifestyle), we adjusted for age, sex, assessment center, BMI, Townsend deprivation index, educational qualifications, smoking status and drinking status in the model as covariates of no interest66,67. Missing data for these covariates were imputed using the mean value for each variable. Bonferroni corrections of the 3,735 phenotypes were conducted for the model results, and standardized regression coefficients and corresponding two-sided P values were reported in the results for all regression models.
A partial correlation was utilized to analyze the associations of sleep duration with measures of interest in two groups (sleep duration ≤7 h and sleep duration ≥7 h), including blood biomarkers, metabolomic biomarkers, brain structures and protein biomarkers. The model adjusted the following variables: age, sex, BMI, scanning site of imaging, Townsend deprivation index measuring socioeconomic status, educational qualifications, smoking status and drinking status. Scanning site of imaging and intracranial volumes were further added to the linear model, examining the association between sleep duration and brain structure. Linear correlation coefficients and corresponding two-sided P values were obtained from the model to reflect the association between sleep duration and measures. Bonferroni corrections were conducted for multiple comparisons. Permutation tests were performed to compare the associations between the two sleep groups and biomarkers. In these tests, the group of participants with sleep duration of exactly 7 h served as a common reference (or shared control) and remained unchanged. The sleep durations of the remaining individuals, either less than 7 h or more than 7 h, were randomly permuted 5,000 times. Through this permutation process, the groups with permuted sleep duration—short (≤7 h) and long (≥7 h)—should theoretically show no difference in their associations with biomarkers, thus establishing the null distribution for comparing the associations of both sleep groups with biomarkers.
Aging effect between short and long sleep with health
To explore the potential aging effect in the association between short and long sleep groups with health-related biomarkers, including blood biomarkers, metabolomic biomarkers, brain structure and proteomics data, we divided the participants into two age groups, 37–57 years old (middle-aged, n = 246,209) and 58–73 years old (older adults, n = 256,283), to ensure a balanced number of subjects in each subgroup, for biomarkers measured in the baseline assessment. Similarly, for the follow-up neuroimaging assessments, the participants were grouped into 44–63 years old (n = 19,594) and 64–82 years old (n = 22,820) to maintain a balanced sample size across groups. Partial correlations between short and long sleep groups with health-related biomarkers were performed for each age group, respectively, with covariates adjusted to explore the potential interaction between short and long sleep duration with age.
GWAS
A GWAS was conducted adjusting for age, sex and the top ten ancestry principal components using PLINK 2.068 to assess the association between genotype and the phenotype of interest. Given our hypotheses, GWASs for sleep duration among 255,699 individuals who sleep ≥7 h and 211,101 individuals who sleep ≤7 h were performed separately. All other phenotypes, including blood biomarkers, metabolomic biomarkers, mental health symptoms, cognitive function and so on, were first overlapped with the two sleep duration groups, and then GWASs were performed, respectively. To assess the robustness of the GWAS results for sleep duration, we further adjusted for 106 genotype batches and 22 assessment centers in both sleep duration groups, using 105 binary covariates and 21 binary covariates (dummy variables), respectively.
Functional mapping and annotation (FUMA) was then utilized to identify the independent significant SNPs, which were selected from the significant SNPs in the GWAS analysis (P < 5 × 10−8) and were independent of other significant SNPs in the linkage equilibrium with r2 < 0.6. The lead SNPs were further selected from the independent significant SNPs and defined as having a linkage equilibrium with others of r2 < 0.1. Lead SNPs within a distance of 250 kb were further merged into the genomic locus.
SNP heritability and genetic correlation
LD-score regression was utilized to assess the SNP-based heritability of the two groups of sleep (sleep ≤7 h and sleep ≥7 h) and the genetic correlation between the two groups of sleep (GenomicSEM version 0.0.3)35. Meanwhile, the genetic correlations between the two groups’ sleep duration and blood biomarkers, metabolomic biomarkers and health-related phenotypes were calculated, respectively. Notably, as we mentioned before, the GWAS summary data of the phenotypes were obtained from participants in the two groups of sleep duration (sleep ≤7 h and sleep ≥7 h), respectively. HapMap3 SNPs were used for the reference SNP list, and LD scores of European ancestry from the 1000 Genomes Project, provided by GenomicSEM, were utilized as the background scores. Phenotypes with low heritability cannot be utilized to assess genetic correlation, and 224 phenotypes were eventually measured. Bonferroni corrections were conducted for the two-sided P value.
Two-sample MR
Bidirectional two-sample MR69 (TwoSampleMR version 0.5.6, R) was performed to measure the causal association between short and long sleep duration with 15 diseases and health-related biomarkers, including bipolar disorder70, major depressive disorder71, schizophrenia72, Alzheimer’s disease73 and coronary artery disease74, as well as the clinical blood biomarkers CRP, HDL and LDL cholesterol75 and so on (Supplementary Table 10). These domains cover conditions that are highly prevalent in the general population and have significant public health implications. Many of the selected traits, such as major depressive disorder, coronary artery disease and biomarkers such as HDL and LDL cholesterol, have direct clinical relevance and are common targets for intervention in public health76,77. The publicly available GWAS summary statistics were mainly obtained from large consortia of primarily European samples. For phenotypes without available public GWAS summary data that do not overlap with UK Biobank participants, including overall health rating, IGF-1 and so on, we performed GWAS analysis utilizing two non-overlapping samples from the UK Biobank, one for sleep duration and another for these phenotypes adjusted for age, sex and the top ten ancestry principal components using PLINK 2.0. The cortical volume, cortical thickness and FA of the brain regions significantly associated with sleep ≤7 h and sleep ≥7 h were averaged, respectively, and utilized for the GWAS analysis with age, sex, the top ten ancestry principal components, scanning site and intracranial volumes adjusted.
Inverse-variance-weighted (IVW) MR was mainly utilized to test bidirectional association with weighted median and MR-Egger as the validated methods. Instrumental variants were selected at a threshold of P < 1 × 10−5 for sleep duration and health-related phenotypes. Clumpling was performed to ensure the independence of the variants with an LD r2 threshold of 0.01 and a distance of 1,000 kb. For the highly polygenic phenotypes, including schizophrenia, bipolar disorder and CRP, the threshold for the variants was further restricted to 1 × 10−8. If the requested SNPs in the exposure were not available in the outcome GWAS, they were replaced with proxy SNPs with high LD (LD > 0.8), identified using the 1000 Genomes European sample data. The exposure and outcome data were then harmonized. Quality controls were further performed to remove the outliers with Cochran’s Q test for the IVW model and Rucker’s Q test for the MR-Egger model to prevent horizontal and directional pleiotropy effects, respectively (RadialMR version 1.0, R). Outliers with a significance level of 0.01 were removed from the instrumental variants, and the remaining variants were utilized for the MR analysis. For significant results, the MR-Egger intercept test was performed to test horizontal pleiotropy. FDR corrections were conducted separately for each group of sleep duration and each direction (sleep→health/health→sleep). Estimates were converted to odds ratios for binary outcomes, including bipolar disorder, major depression disease, bipolar disorder and so on.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This project corresponds to UK Biobank application ID 19542. Neuroimaging, genotype and behavioral data from UK Biobank dataset are available from https://biobank.ndph.ox.ac.uk/ by application. The variables utilized in this study are detailed in Supplementary Tables 1–3. Previous published GWASs of psychiatric disorders, including depression, bipolar disorder and schizophrenia, were provided by the Psychiatric Genomics Consortium, which can be downloaded from https://pgc.unc.edu/for-researchers/download-results/. GWAS summary statistics of immunometabolic phenotypes, obesity and aging disease are available in the MRC IEU OpenGWAS database (https://gwas.mrcieu.ac.uk), and the detailed PubMed identifiers (PMIDs) for the GWAS summary data are provided in Supplementary Table 10 (https://pubmed.ncbi.nlm.nih.gov/). European ancestral background LD scores from the 1000 Genomes Project were downloaded from https://alkesgroup.broadinstitute.org/LDSCORE/. The GRCh37 coordinates can be accessed via http://hgdownload.cse.ucsc.edu/goldenpath/hg19/database/.
Code availability
R version 4.2.0 was used to perform phenotype-wide association analysis. Matlab 2018b was used to perform linear association analysis. Freesurfer v6.0 was used to process the imaging data. PLINK 2.0 was used to perform GWAS analysis. R version 4.2.0 GenomicSEM version 0.0.3 was utilized to calculate heritability and genetic correlations. TwoSampleMR version 0.5.6 was utilized to measure the causal association. Scripts used to perform the analyses are available at https://github.com/yuzhulineu/UKB_short_longsleep/.
References
Mander, B. A., Winer, J. R. & Walker, M. P. Sleep and human aging. Neuron 94, 19–36 (2017).
Wang, C. et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116632 people from 21 countries. Eur. Heart J. 40, 1620–1629 (2018).
Hirshkowitz, M. et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 1, 40–43 (2015).
Mutz, J., Roscoe, C. J. & Lewis, C. M. Exploring health in the UK Biobank: associations with sociodemographic characteristics, psychosocial factors, lifestyle and environmental exposures. BMC Med. 19, 240 (2021).
Li, Y. et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat. Aging 2, 425–437 (2022).
Huang, S.-Y. et al. Sleep, physical activity, sedentary behavior, and risk of incident dementia: a prospective cohort study of 431,924 UK Biobank participants. Mol. Psychiatry 27, 4343–4354 (2022).
Coutrot, A. et al. Reported sleep duration reveals segmentation of the adult life-course into three phases. Nat. Commun. 13, 7697 (2022).
Sabia, S. et al. Association of sleep duration at age 50, 60 and 70 years with risk of multimorbidity in the UK: 25-year follow-up of the Whitehall II cohort study. PLoS Med. 19, e1004109 (2022).
Itani, O. et al. Short sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med. 32, 246–256 (2017).
Jike, M. et al. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med. Rev. 39, 25–36 (2018).
Buxton, O. M. et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra43 (2012).
Schmid, S. M., Hallschmid, M. & Schultes, B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 3, 52–62 (2015).
Reis, C. et al. Sleep duration, lifestyles and chronic diseases: a cross-sectional population-based study. Sleep Sci. 11, 217–230 (2018).
Doherty, A. et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat. Commun. 9, 5257 (2018).
Gottlieb, D. J. et al. Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol. Psychiatry 20, 1232–1239 (2015).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10, 1100 (2019).
Jones, S. E. et al. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 12, e1006125 (2016).
Byrne, E. M. et al. Genetic correlation analysis suggests association between increased self-reported sleep duration in adults and schizophrenia and type 2 diabetes. Sleep 39, 1853–1857 (2016).
Lane, J. M. et al. Biological and clinical insights from genetics of insomnia symptoms. Nat. Genet. 51, 387–393 (2019).
Ai, S. et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK Biobank. Eur. Heart J. 42, 3349–3357 (2021).
Knutson, K. L. et al. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11, 163–178 (2007).
Tobaldini, E. et al. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat. Rev. Cardiol. 16, 213–224 (2019).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Youngstedt, S. D. & Kripke, D. F. Long sleep and mortality: rationale for sleep restriction. Sleep Med. Rev. 8, 159–174 (2004).
Theorell-Haglöw, J. et al. Sleep duration is associated with protein biomarkers for cardiometabolic health: a large-scale population study. J. Sleep Res. 30, e13284 (2021).
Wojcik, G. L. et al. Opportunities and challenges for the use of common controls in sequencing studies. Nat. Rev. Genet. 23, 665–679 (2022).
Lukaski, H. C. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur. J. Clin. Nutr. 67, S2–S9 (2013).
Xiao, Q. et al. Relationship between sleep characteristics and measures of body size and composition in a nationally-representative sample. BMC Obesity 3, 48 (2016).
Zhang, Y.-R. et al. Peripheral immunity is associated with the risk of incident dementia. Mol. Psychiatry 27, 1956–1962 (2022).
Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023).
Bhargava, S. et al. Lipids and lipoproteins in cardiovascular diseases: a classification. Trends Endocrinol. Metab. 33, 409–423 (2022).
Blake, G. J. et al. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation 108, 2993–2999 (2003).
Chiesa, S. T. et al. Glycoprotein acetyls: a novel inflammatory biomarker of early cardiovascular risk in the young. J. Am. Heart Assoc. 11, e024380 (2022).
Kettunen, J. et al. Biomarker glycoprotein acetyls is associated with the risk of a wide spectrum of incident diseases and stratifies mortality risk in angiography patients. Circ. Genom. Precis. Med. 11, e002234 (2018).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Jansen, P. R. et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 51, 394–403 (2019).
Morales-Muñoz, I. et al. Role of inflammation in short sleep duration across childhood and psychosis in young adulthood. JAMA Psychiatry 81, 825–833 (2024).
Lane, J. M. et al. Genetics of circadian rhythms and sleep in human health and disease. Nat. Rev. Genet. 24, 4–20 (2023).
Bhalla, S. et al. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 142, 104896 (2022).
Plante, D. T. The evolving nexus of sleep and depression. Am. J. Psychiatry 178, 896–902 (2021).
Liew, S. C. & Aung, T. Sleep deprivation and its association with diseases—a review. Sleep Med. 77, 192–204 (2021).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep disturbance, sleep duration and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52 (2016).
Irwin, M. R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 19, 702–715 (2019).
Smagula, S. F. et al. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med. Rev. 25, 21–30 (2016).
Garbarino, S. et al. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 4, 1304 (2021).
Besedovsky, L., Lange, T. & Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 99, 1325–1380 (2019).
Stamatakis, K. A. & Punjabi, N. M. Long sleep duration: a risk to health or a marker of risk? Sleep Med. Rev. 11, 337–339 (2007).
Vyazovskiy, V. V. Sleep, recovery and metaregulation: explaining the benefits of sleep. Nat. Sci. Sleep 7, 171–184 (2015).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018).
Siow, T. Y. et al. Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology 98, e829–e838 (2022).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Walker, K. A. et al. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci. Transl. Med. 15, eadf5681 (2023).
Larsson, S. C. & Markus, H. S. Genetic liability to insomnia and cardiovascular disease risk. Circulation 140, 796–798 (2019).
Willoughby, A. R. et al. Country differences in nocturnal sleep variability: observations from a large-scale, long-term sleep wearable study. Sleep Med. 110, 155–165 (2023).
Wainberg, M. et al. Clinical laboratory tests and five-year incidence of major depressive disorder: a prospective cohort study of 433,890 participants from the UK Biobank. Transl. Psychiatry 11, 380 (2021).
Ahola-Olli, A. V. et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 62, 2298–2309 (2019).
Soininen, P. et al. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 8, 192–206 (2015).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166, 400–424 (2018).
de Groot, M. et al. Improving alignment in tract-based spatial statistics: evaluation and optimization of image registration. NeuroImage 76, 400–411 (2013).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Kang, J. et al. Increased brain volume from higher cereal and lower coffee intake: shared genetic determinants and impacts on cognition and metabolism. Cereb. Cortex 32, 5163–5174 (2022).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622, 329–338 (2023).
Millard, L. A. C. et al. Software application profile: PHESANT: a tool for performing automated phenome scans in UK Biobank. Int. J. Epidemiol. 47, 29–35 (2018).
Lane, J. M. et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat. Genet. 49, 274–281 (2017).
Kocevska, D. et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nat. Hum. Behav. 5, 113–122 (2021).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Nikpay, M. et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130 (2015).
Willer, C. J. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Yasugaki, S. et al. Bidirectional relationship between sleep and depression. Neurosci. Res. 211, 57–64 (2025).
Liu, C. et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 7, 672–680 (2022).
Acknowledgements
This study utilized the UK Biobank resource under application no. 19542. We thank all the participants and researchers from UK Biobank. This work received support from the following sources: National Key R&D Program of China (2021YFC2501400 to T.J., 2019YFA0709502 to J.F., 2018YFC1312904 to J.F., 2022CSJGG1000 to T.J., 2019YFA0709501 to T.J., 2018YFC1312900 to T.J., 2023YFE0199700 to T.J. and 2023YFC3605400 to W.C.), the National Natural Science Foundation of China (T2122005 to T.J., 82472055 to W.C., 82071997 to W.C. and 81801773 to T.J.), the 111 Project (B18015 to J.F.), the key project of Shanghai Science and Technology (16JC1420402 to J.F.), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01 to J.F.), Zhangjiang Lab (to J.F.), Shanghai Center for Brain Science and Brain-Inspired Technology (to J.F.), Shanghai Rising-Star Program (21QA1408700 to W.C.), Shanghai Pujiang Project (18PJ1400900 to T.J.), Shanghai Yangfan Project (24YF2738400 to Y.L.) and Postdoctoral Fellowship Program (Grade B) of China Postdoctoral Science Foundation (GZB20230164 to Y.L. and 2023M730697 to Y.L.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.F., W.C. and T.J. proposed the study. Y.L. and W.G. analyzed the data. W.Z. and S.H. preprocessed the data. T.J., S.N. and J.Y. contributed to interpretation of the results. Y.L. drafted the paper and T.J., S.N., B.J.S. and W.C. edited it. Y.L. and W.C. were responsible for visualization. Y.Z. and L.M. provided feedback to improve the paper. All authors contributed to discussions on data analysis and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Julian Mutz, Arezu Najafi, Masoud Tahmasian and Angeliki Tsapanou for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Guideline of the study.
Top, UK biobank data used in this study included environmental and behavioral measures, blood and imaging biomarkers, genomics, proteins, and sleep duration. Middle, A phenotype-wide association study (PheWAS) was performed to explore the associations of short and long sleep duration with these phenotypes. Biological blood biomarkers, neuroimaging biomarkers, and proteins were utilized to characterize distinct association profiles for short and long sleep duration. Bottom, Distinct genetic architectures and roles in association with health conditions were identified for short and long sleep duration. Finally, Mendelian randomization (MR) analysis showed distinct causal associations between short and long sleep duration and health-related phenotypes.
Supplementary information
Supplementary Information
Supplementary Tables 1–27 and Figs. 1–13.
Supplementary Data 1–6
Supplementary Data 1 Phenotype-wide associations study of sleep <= 7 h. Supplementary Data 2 Phenotype-wide associations study of sleep >= 7 h. Supplementary Data 3 Genetic correlations between sleep duration and health-related phenotypes. Supplementary Data 4 Phenotype-wide associations study of stratified short sleep (sleep < 7 versus sleep = 7/8 h). Supplementary Data 5 Phenotype-wide associations study of stratified long sleep (sleep > 8 versus sleep = 7/8 h). Supplementary Data 6 Genetic correlations between stratified sleep duration and health-related phenotypes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Gong, W., Sahakian, B.J. et al. Divergent biological pathways linking short and long sleep durations to mental and physical health. Nat. Mental Health 3, 429–443 (2025). https://doi.org/10.1038/s44220-025-00395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-025-00395-6