Abstract
Foodborne viruses are significant public health threats, capable of causing life-threatening infections and posing major risks for future pandemics. However, the development of vaccines and treatments remains limited due to gaps in understanding their biophysical properties. Among these viruses, noroviruses are currently the leading cause of viral gastroenteritis globally and are responsible for numerous foodborne outbreaks. In this review, we explore the use of biophysical methods, with a focus on atomic force microscopy (AFM), to study foodborne viruses. We demonstrate how AFM can provide crucial insights into virus-host interactions, transmission dynamics, and environmental stability. We also show that the integration of various biophysical approaches offers new opportunities for advancing our understanding of foodborne viruses, ultimately guiding the development of effective prevention strategies and antiviral therapies.
Similar content being viewed by others
Introduction
Access to safe and nutritious food is essential for global health. According to the World Health Organization (WHO), foodborne hazards caused 600 million illnesses and 420,000 deaths globally in 20101. Foodborne illnesses come from the consumption of contaminated food or beverages, which contain known toxins, chemicals or microbial pathogens2,3. Among these pathogens, foodborne viruses are behind a significant number of infections. Foodborne viruses include numerous virus families that generally replicate in the gastrointestinal (GI) tract and that can be transmitted through the fecal-oral route. The viruses most commonly found as food contaminants are noroviruses (NoV), which are also the largest contributors to cases of gastroenteritis. Viral gastroenteritis remains a common cause for disease and mortality worldwide, with more dramatic impact in developing countries4,5. The increasing use of molecular diagnosis worldwide allows for an estimation of the norovirus role in foodborne disease. However, its prevalence is most likely underestimated, due to lack of user-friendly and widely accessible diagnostic tools6. Albeit less prevalent, hepatitis A virus (HAV) is considered another serious foodborne virus, as it is a causative agent of hepatitis, a hepatic disorder. Even though there are effective vaccines for HAV, no specific treatment is available for hepatitis A and outbreaks still occur7,8,9.
Viruses are obligate intracellular parasites, as they can only reproduce inside host organisms10. The spread via foods is enhanced for foodborne viruses due to their highly infectious nature, environmental stability, and resistance to physical agents5,11. In order to implement mitigation strategies and to develop prevention measures such as vaccines or treatments, it is important to understand the biophysical properties of these viruses, as well as understand how their structure is related to function12,13. It is known that virus attachment, in general, is crucial for transmission dynamics. In particular, the selective binding to tissues in vivo, as well as the non-selective adhesion to inanimate surfaces ex vivo, are major determinants in foodborne viral infections14,15,16. By gaining insight into the physical interaction between viruses and food surfaces or packaging materials, it is possible to improve surface treatments, coatings, and sanitization protocols that could prevent viral spread. Also, understanding the interaction mechanisms of viruses with host cells, including the identification of viral proteins or cell receptors essential for replication, could provide valuable information for the development of effective antiviral treatments. Lastly, quantifying virus binding kinetics and affinity can help identify specific viral antigens that elicit an immune response, thus allowing the enhancement of vaccine efficacy.
Various biophysical methods can be used to study the physical properties of foodborne viruses. Techniques such as surface plasmon resonance (SPR), electron microscopy (EM), and atomic force microscopy (AFM), have been used not only to for virus detection, but also to characterize virus-host interactions17,18,19. Among these, AFM stands out as a particularly powerful technique due to its ability to image the sample at high resolution under physiological conditions and measure forces with piconewton sensitivity. This technology, which emerged from scanning probe microscopy (SPM), was developed in 1986 by Binnig, Gerber, and Quate and has gone through many technological advancements, resulting in a variety of different operation modes. AFM is a highly sensitive, label-free technique that measures forces with exceptional spatial and temporal resolution, using a nanometer-scale tip to scan surfaces. This single-molecule technique has become a prominent tool in physical virology and enables imaging, force spectroscopy, and nanomechanical analysis of biological samples, making it a powerful tool in virology research for applications such as virus imaging, nanoindentation, and mechanical property characterization. Additionally, AFM’s ability to monitor dynamic processes, as viral assembly or genome uncoating, provides critical insights into the life cycle of viruses and their interaction mechanisms with host cells20,21,22.
In this review, we will emphasize on the use of AFM as a central tool for investigating the biophysical properties of foodborne viruses. We will provide an overview of foodborne viruses, with a particular look on noroviruses, since they appear to be among the most studied foodborne viruses using AFM and due to their major contribution to foodborne viral infections. General features of transmission dynamics and approaches for the prevention and control of these infections will also be presented. Furthermore, we will explore how other biophysical methods contribute to understanding foodborne viral infections and how some of these can be combined with AFM. By focusing on AFM, we aim to highlight its unique contributions to the field of physical virology and its role in advancing strategies to prevent and combat foodborne viral infections. Lastly, current challenges faced by these strategies are discussed, along with future perspectives for the field.
Common Foodborne Viruses and their Characteristics
Foodborne viruses are typically classified as such for their ability to cause disease after ingestion and possibility of foodborne transmission. The well-recognized foodborne viruses are transmitted via fecal-oral routes and share certain generally accepted features. First, there should be a clear link between relevant illnesses and the consumption of virus-contaminated foods. Secondly, there should be some evidence of foodborne viruses’ presence in foods when monitoring studies are conducted. Lastly, these viruses usually present high stability and resistance to environmental factors. The most reported viral foodborne illnesses are gastroenteritis or hepatitis, caused by NoV and HAV, respectively23,24. Additional common foodborne viruses that infect humans comprise human rotavirus (HRV), human adenovirus (HAdV), Hepatitis E virus (HEV), human astrovirus (HAstV), and sapovirus (SaV). Worldwide, NoV is the leading cause of viral gastroenteritis5,25,26,27. Gastroenteritis in young children is also associated with infection by rotaviruses, astroviruses, HAdV, and SaV28,29,30,31. Hepatitis A virus (HAV) and Hepatitis E virus (HEV) are also both enterically transmitted, though their main replication site is in the liver28,32,33,34. A list of these viruses and some of their characteristics are summarized in Table 1. Other viruses, that might not clearly meet all the criteria listed above, can also be considered foodborne viruses, because of their potential to be transmitted via food. These include enterovirus and poliovirus, which primarily infect neurological or respiratory systems35. Here, we will only take into account enterotropic and hepatotropic viruses. The development of prevention and mitigation strategies focused on the foodborne transmission of these viruses is highly relevant, especially since widely available vaccines or treatments are rare for foodborne viruses.
From a structural point of view, a viral particle is composed of nucleic acid, either single stranded (ss) or double stranded (ds) DNA or RNA, surrounded by a protein shell, called capsid. The viral capsid is required not only for virus cell attachment and infection, but also for protection of the viral genome against degradation. Additionally, viruses can have an outer lipid envelope, derived from the host cell membrane23. Foodborne viruses are typically nonenveloped particles, providing them with very good environmental stability. The highly stable viral capsid allows these viruses to be resistant to various physico-chemical conditions, such as refrigerated and frozen storage, high temperatures and low pH23. HAV and HEV share a particular feature of circulating in a quasi-enveloped state in the blood36,37,38,39. In a different case, rotavirus possesses a special feature of comprising a triple-layered capsid, which provides additional protection against GI tract conditions and enables it to persist for longer on surfaces and food40.
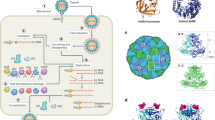
The general viral life cycle (Fig. 1) consists of host cell infection, where viral particles release their genome and replicate, and the emergence of new particles that infect other cells in the primary host or that can be transmitted to a new host41. The pathogenicity of foodborne viruses depends on the stability of the virus in the environment and on virus-host interactions42. Most foodborne viruses target glycoproteins as receptors in the GI tract, since the oral-fecal route is their primary mode of transmission43. Human NoVs use host cell glycans as cellular receptors, namely HBGAs that are expressed in cells of the intestinal epithelium (enterocytes). A good overview of attachment factors and attachment receptors of human NoV is summarized by Zhan et al.43. In short, NoV tropism and replication cycle are still poorly understood, as NoV research has been significantly hindered by the lack of robust replication models, as cell culture systems, and small animal models43. The recent development of nontransformed tissue stem cell-derived human intestinal enteroids (HIEs) provided a new model for cultivation of NoVs44. NoV models, as well as their role in antiviral treatment and vaccine development, are well summarized in Prasad et al.45.
NoV is mainly transmitted through the fecal-oral route, often by contaminated food or water. In the host, NoV replicates in the GI tract, by infecting host cells such as enterocytes. In the first step of the viral cycle, human NoV is known to bind to cell-surface attachment factors, including HBGAs. Then, the viral particle is internalized by the cell, which replicates the viral components needed for the assembly of new viral particles. Lastly, new viruses are released by the cell, allowing infection of surrounding cells and hosts. Figure created in BioRender.com.
Since the presence of virus receptors at the cell surface determines virus susceptibility, these receptor molecules are potential targets for treatment of infectious diseases. Therefore, understanding virus-receptor interactions provides crucial insight into the development of vaccines or antiviral treatments46.
Transmission Dynamics and Stability in the Food Matrix
Viruses do not multiply or produce toxins in food, acting as vehicle for their passive transfer. Foodborne viruses are normally transmitted through the fecal–oral route, and when the virus is shed in the feces of an infected individual, it can be transmitted to others by coming into contact with contaminated surfaces, food, or water. The most common food categories linked to outbreaks include shellfish, which can bio-accumulate viral particles from a large volume of water, and fresh produce, especially berry fruits and leafy green vegetables23. These products can be contaminated during the primary production, and are generally consumed uncooked or without effective decontamination treatment. After ingestion, foodborne viruses survive the defense barriers of the body like the acidic environment of the stomach and digestive enzymes. Infected individuals shed the virus through feces, vomit, or aerosolized particles. Transmission to new hosts can occur directly person-to-person or indirectly through contaminated surfaces, water, or food, often leading to outbreaks29. A comprehensive view on transmission of foodborne viruses is given by Todd and Greig47. Even though transmission by a food handler or the specific food item behind outbreaks are difficult to know with certainty, it is very clear that they present a significant risk for virus transmission through direct contact with food and are important in the prevention of norovirus infections48. Foodborne transmission of viruses is facilitated by the fact that they generally need a low infectious dose, increasing the likelihood of infection when consuming contaminated food or water12 and that virus shedding may continue long after illness, increasing the likelihood of secondary spread49.
While viruses need a host to replicate, they are highly capable of surviving prolonged time periods outside a host, being especially stable in the environment. Viruses can persist on food-related surfaces for a significant amount of time, such as days to weeks on hard surfaces, including stainless steel and plastic50. Viral contamination of water is of particular concern. Depending on the water source, NoV is reported to remain infectious for 60 to 728 days50. In mineral water, HAV and poliovirus type 1 survive for up to 1 year at temperatures of 4 °C and 23 ± 3 °C51. Also, HEV can be stable in water for several weeks52,53. The consumption of contaminated food from infected animals also poses a significant infection risk for humans. HEV is known to reside in the liver and tissues of these animals, and inadequate cooking or handling can result in the virus persisting in the food11. Additionally, most foodborne viruses are stable during freezing54. NoV is reported to be stable and infectious despite 14 freeze/thaw cycles (-80 °C/ + 22 °C) or after -80 °C storage for up to 120 days55. NoV persistence in water has been studied using the recently-developed HIE model, which shows comparable decay rates to studies using other surrogate models such as murine NoV56. An elegant overview of models and detection methods of NoV in food and water matrixes is provided by Chandran and Gibson57, including studies with the most recent HIE model57.
It is notable that noroviruses showed great survivability on food surfaces28. For example, NoV remains infectious in lettuce and spinach for up to 2 weeks58. Contributing to the accumulation and anchoring of NoV, HBGA-like polysaccharides are found in foods like oysters, mussels, clams, lettuce, and several microorganisms43.
Finally, there is a major gap in understanding the stability of viruses in the food matrix and environment. This is of particular concern for future pandemic preparedness for inactivation and mitigation strategies. Understanding of the stability of foodborne viruses is essential to find suitable methods of inactivation. However, different viruses have varying levels of resilience creating the need for reliable methods reusable in different strains.
Inactivation & Mitigation Strategies
In order to prevent large foodborne virus outbreaks and to increase food safety, inactivation and mitigation strategies have been developed, to which a detailed understanding of the biophysical properties of the viruses may contribute.
Heat treatment is widely used to enhance food safety. While light cooking or steaming is not sufficient to inactivate NoV in bivalve molluscs59, heating at 70–72 °C for 2 min reduces infectious titers of NoV, HAV and HEV60. HEV infection primarily occurs through the consumption of infected pork, camel, or game products that were not or not sufficiently heat inactivated. Several studies have been conducted with different time/temperature combinations for HEV inactivation, which were summarized by Cook and Poel61. Even though more studies are needed to determine the exact effect of heat on HEV, it appears that a treatment of 71 oC for 20 min is sufficient to completely inactivate the virus in contaminated liver61. Another study has shown that pig sausages still contained infectious HEV after a food processing-relevant heat treatment of 55 oC for 120 min62. However, it has also become clear that the effect of temperature is influenced by the matrix where the virus is embedded63. Therefore, the effectiveness of treatments can vary from cell culture to in vivo conditions, highlighting the need for more robust cell culture models.
For fresh products, non-thermal processing methods such as high-pressure processing, cold plasma, ultraviolet light, and irradiation may be implemented by the manufacturer, which were summarized by Pexara and Govaris12,64,65. Plasma activated water was shown to inactivate MNV-1 on blueberries and still had an inactivation effect after 45 days of storage at 4 °C66. Another inactivation technique commonly used is ultraviolet-C light (200–280 nm), which damages viral nucleic acids12. Finally, a study has shown that irradiation (gamma rays, X-rays, electron beams) can lead to a reduction of viral titers, but was not effective in completely inactivating NoV67. Further research highlights the need for deeper understanding of viral inactivation mechanisms.
Overall, in order to achieve optimal water and food quality and safety, synergistic effects of different treatment strategies need to be further explored in the future68. It is also of note that vaccines are critical preventive measures and are available for HAV and rotavirus, but not so far for other enteric viruses. However, ongoing research is needed to answer the change of virus susceptibility to vaccines, due to their continuous evolution and genetic shifts69, as well as for the development of new vaccines.
Atomic Force Microscopy in Foodborne Virus Research
Advances in biophysical methods, emergence of novel techniques, and discovery of new viruses, have aided immensely in expanding our understanding of foodborne viral infections. The high demand for rapid and specific identification of viruses and how to inactivate them emphasizes the need for research in particular including physical virology methods. Biophysical methods, such as AFM, have been especially valuable in investigating the interaction of viruses with transmission sources and are a core element in fighting foodborne virus outbreaks. AFM allows extraction of biophysical properties of samples and detection of single molecular interactions under physiologically-relevant conditions70,71. Therefore, the following sections of this review will focus on the AFM and its potential applications as a biophysical method in studying foodborne viruses.
Principles and Applications of AFM
The AFM is a particularly interesting multiparametric tool in quantifying biophysical properties and molecular interactions, due to its ability to detect forces in the range of a few (< 10) piconewtons, image the topography of samples with subnanometer resolution in air or liquid, and obtain kinetic and thermodynamic parameters of such interactions (Fig. 2A-F). Moreover, AFM does not require labelling, coating, or chemical fixation of the samples, in contrast to other techniques, such as immunocytochemistry approaches, scanning electron microscopy (SEM), and transmission electron microscopy (TEM)72. The AFM is a mechanical microscope and its imaging is based on the physical rendering of the surface topography by raster-scanning of a probe across the sample. This probe is composed by a flexible cantilever with a sharp tip that interacts with the sample surface, acting as a force transducer20,73,74. The operating principle of AFM is based on precisely sensing the interaction between tip and sample by a laser beam that is focused onto the cantilever and is reflected from the cantilever surface into a position-sensitive photodiode. During topographic imaging surface variations induce bending, or deflection of the cantilever, leading to a change in the position of the reflected laser spot on the photodiode. The deflection (d) can be translated into the force (F) acting between the tip and the sample, through the relationship F = -k × d, where k is the spring constant of the cantilever. The x-y-z position of the sample in relation to the tip is adjusted by a piezoelectric scanner, which is controlled by a feedback system75,76. AFM-based force spectroscopy (AFM-FS) relies on using the cantilever as a force sensor to measure interaction forces between tip and sample. In this mode, the AFM tip is cyclically approached and retracted from the surface while recording the variation of force over time or with respect to the tip-sample distance (Fig. 2A). Force-distance (FD) and force-time (FT) curves can then be collected pixel-by-pixel across the surface of the sample, which allows the extraction of biophysical properties, such as elasticity, stiffness, and adhesion (Fig. 2D-F)77,78.
A Basic principle of AFM. An AFM setup includes a flexible cantilever with a tip at the end. A laser is focused on the cantilever and reflected into a photodiode, allowing detection of the movement of the tip, which is transmitted to the computer and can be translated into interacting forces between tip and sample. An area of the sample can be scanned by approaching and retracting the tip at every point corresponding to a pixel of the AFM image. The force applied or experienced by the AFM tip can be recorded as a function of tip-sample distance, providing force-distance (FD) curves. B Tip functionalization strategies and variety of samples that can be used in AFM. The AFM tip can be converted into a biosensor by its functionalization with various biological partners, such as an antibody, a virus, or a cellular receptor. Their interaction with a wide range of samples can be probed, including the surface of living cells, material surfaces such as glass or plastic components, and other biomolecules grafted on a substrate. (C–F) Example of applying AFM to studying biophysical parameters of virus-host interactions, adapted from the study: Mohammed, D., et al.171. C Schematic representation of the experimental setup, comprising living cells being probed by an AFM tip functionalized with a reovirus particle. Height (D), Elasticity (E), and Adhesion (F) maps collected form the AFM experiment171. Schematics of panels A-C were created in BioRender.com.
A further powerful feature of AFM is the possibility of converting the AFM tip into a biosensor by functionalizing it with proteins, ligands, chemical groups, lipids, or viruses. Single molecule force spectroscopy (SMFS) is based on this ability of tip functionalization in combination with force measurements of the interaction between molecules on the tip and the sample (Fig. 2B, C). Therefore, SMFS allows the detection and localization of specific interactions, including adhesion and bond dissociation, in a wide range of biological systems. When performing FD curve-based AFM, the cantilever is moved vertically, so that the tip contacts the sample in each pixel. At first, tip and sample are distant enough not to interact, resulting in recorded zero force. Then, the tip is approached until it contacts the sample, which induces an upward deflection of the cantilever. Upon reaching a predefined force setpoint, the tip is retracted away from the sample, losing contact with the sample surface and returning to the baseline of measured forces. If a bond is formed between the molecules on the tip and the sample surface, a deflection downwards is recorded during retraction of the tip, due to the adhesive forces of the interaction. This binding event can be identified by the presence of an adhesion peak in collected FD and FT retraction curves, from which adhesion forces can be measured. When the bond is ruptured, the cantilever returns to its zero-force position75,79,80. Tip functionalization can be achieved through different strategies, though the most prominent approach to attaching molecules to the AFM tip is the use of flexible PEG linkers between the tip and the sensor molecule. The use of these linkers is a highly attractive option due to their ability to undergo stretching, inertness, stability in liquid environment, versatility, and the fact that they provide a fingerprint for identifying specific adhesion peaks72. In addition, the use of photocleavable linkers can be advantageous, as they provide controlled release of biomolecules from AFM tips, which can be used, for example, for specificity controls81. Regarding the substrate, a large variety of samples can be probed with AFM, including material surfaces82, biomolecule-grafted model surfaces83, and bacterial84 or mammalian cells85. Model surfaces typically comprise flat supports, such as mica or gold, that allow grafting of, for example, cellular receptors86. Currently, a common experimental setup for studying viruses includes probing either model surfaces grafted with cellular receptors, or directly probing the surface of living cells, with a virus-functionalized AFM tip. The option to select specific molecules of interest, combined with the possibility to work under physiologically-relevant conditions, has widened the scope of the AFM to be used in an increasing number of studies that shed light on the viral infection process. Within the infection cycle, viral adhesion is crucial for successful infection and for transmission dynamics. As proof-of-concept, an early study used an AFM tip functionalized with a single virus particle to probe the interaction between rhinovirus and cellular receptors, allowing the measurement of forces governing virus attachment to the cell87. Since then, the above-mentioned methods have propelled AFM-based force spectroscopy to emerge as a powerful tool in investigating virus-receptor interactions. In particular, this technique detects and provides thermodynamical characterization of virus attachment to specific cellular receptors at the single-virus level, expanding our knowledge on the viral life cycle and opening doors for targeted antiviral treatments or preventive measures.
Practical Applications of AFM in Studying Foodborne Viruses
Soon after its invention, AFM was already used to image viruses88 and it has, since then, allowed for remarkable discoveries about virus structure and mechanics89. A deeper understanding of foodborne viruses at the fundamental level, in particular with regard to their biophysical properties, is clearly needed for developing more specialized detection, prevention, and treatment strategies. Within studies at the fundamental level, AFM has been shown to be a powerful tool for extracting physical properties of viruses. Particularly, this technique holds great potential in studying foodborne viruses, by providing information on morphology and mechanical properties of viruses, and biophysical parameters of virus-host interactions.
In the context of foodborne viruses, AFM has been used to image the capsid of noroVLPs and to reveal that their mechanical stability is dependent on pH90. Recently, AFM-based imaging and nanoindentation methods were used to characterize the mechanical properties of noroVLPs, revealing differences in size and physical stability of strains belonging to the same genogroup91 (Fig. 3A). A study by Yang, J., et al.92 elegantly portrays the use of FD curve-based AFM for quantitative and dynamic characterization of rotavirus-receptor interactions, wherein the properties of rotavirus binding to sialic acid and integrins are reported (Fig. 3B)92. Mechanical properties of multi-layered rotavirus particles have also been extracted using AFM, which provided relevant insight into the role of their protein shells in the virus replication cycle93. In other studies, by using AFM to induce mechanical fatigue, knowledge was gained on the stepwise disassembly dynamics of adenovirus particles94 (Fig. 3C). These studies help portray how biophysical methods can be used to investigate the physical properties and dynamics of viruses, while bridging the gap between structure and function.
A AFM topography images of noroVLPs of NoV variants (GI.1, GII.17, and GII.10) for comparison of their sizes. Images reproduced from Feng, Y., et al.91 B Probing of rotavirus single virion binding to an SA-coated model surface (left panel). Representative non-adhesive and adhesive FD curves (retraction trace only) collected (middle panel). AFM measurement of the binding frequency between rotavirus virion and SA, before and after injection of Neu5Ac (right panel). Images adapted from Yang, J., et al.92. Schematics of left panel were created in BioRender.com. C AFM images from a mechanical fatigue assay showing disassembly of adenovirus particles. Different disassembly states are represented in each panel (together with the number of the individual frame), from intact (frame No. 1) to collapsed (frame No. 46) capsid. Scale bar is 37 nm. Images reproduced from Martín-González, N., et al.172 D HS-AFM images of detergent-resistance membrane (DRM) domains and MNV. The top panel of successive images represents raw DRMs on a mica surface, while the middle panel represents the dynamics of MNV particles on a mica surface. The images in the bottom panel represent MNV infected DRM fractions. Images reproduced from Aybeke, E.N., et al.99. E 3D reconstruction of high-resolution AFM topography images of adenovirus, before (top) and after (bottom) UVC irradiation. Images reproduced from Xue, Y., et al.100.
Understanding the physical properties of different virus types is also crucial for developing effective inactivation and mitigation strategies across the food supply chain. AFM can be applied to investigating the effectiveness of potential new inactivation strategies or antiviral treatments, contributing to the combat of foodborne viral infections. Grape seed extract, for example, showed irreversible virucidal effect against MNV95. Notably, in this study, AFM experiments revealed physical changes in the viral particles and in the formation of viral particle aggregates, which was confirmed to render MNV non-infectious in mice95. A variation of AFM, namely high-speed AFM (HS-AFM), arose to overcome a significant limitation of AFM: low imaging rate. HS-AFM provides high temporal resolution (10-20 fps for protein molecules) that enables capturing, for example, the assembly dynamics of viral capsids96,97,98. HS-AFM was used in a study from Aybeke et al.99 to investigate the dynamics of murine norovirus (MNV). The AFM data indicated the presence of an interaction between MNV and the detergent-resistant membrane domains (Fig. 3D)99. In another study, from Xue, Y., et al.100, AFM was used to assess the effect of UV irradiation on adenovirus, by measuring the topography of single virions before and after treatment (Fig. 3E). Also, AFM experiments on living Vero cells with ultraviolet C showed that the cells lost the capability of reproduction and normal metabolism, either by the infection or the irradiation100. This suggests that high-resolution methods, such as AFM, could offer valuable insights into viral impairment mechanisms, aiding the evaluation of inactivation techniques and their impact on food quality.
Beyond host cells, AFM can play a role in food safety by characterizing adhesion of viruses to food-related matrix or surfaces. A study using MS2 as an enteric virus surrogate, has applied AFM to quantifying viral adhesion to commonly used food processing or preparation surfaces. Their findings suggest viruses bind more strongly to polyvinyl chloride (PVC) than to glass, which was attributed to both intrinsic chemical characteristics and the substrate surface porosity101. Due to the resilience of foodborne viruses in aquatic environments, it is also crucial to deepen our understanding of their biophysical properties in these settings. Studies have been carried out by combining different biophysical methods, such as AFM. For example, interactions of rotavirus and Suwannee River natural organic matter was investigated, giving detailed insight into deposition mechanism and aggregation kinetics of rotavirus depending on divalent cation complexation102.
Even though AFM offers exceptionally high resolution and probing of interactions under physiologically relevant conditions, it comes with inherent limitations; it is a surface scanning technique therefore providing minimal information on internal structures, samples need to be immobilized, and it has a small field of view with limited vertical range. In addition, interaction of the probe with delicate biological samples can displace and potentially damage the samples during imaging and probing. The long imaging and data analysis times associated with AFM also make it less suitable for a practical and rapid screening of viral contamination in food. These challenges, together with low throughput, make it impractical for detecting and quantifying viral contamination in food production environments, where large-scale screening is required. Nevertheless, AFM has been employed as a molecular detector for a target viral protein captured form a biological fluid, in what was called an AFM-based fishing analysis103 and has also been proposed as a feasible virus detection method in combination with other techniques104. Despite its inherent limitations, AFM remains a powerful tool for fundamental research on foodborne viruses, particularly for investigating virus-surface interactions, environmental persistence, and the impact of inactivation treatments. AFM’s versatility is evident in its ability to not only image surface topography, but also measure various surface properties simultaneously.
AFM in Combination with Other Techniques
Since no single approach can characterize the complex processes of biological systems, AFM has been combined with other microscopy techniques, such as confocal and fluorescence microscopy, allowing high-resolution imaging of living cells simultaneous to AFM probing105. Through this combination, it is possible to identify and correlate relevant cellular structures from optical microscopy with the correlative AFM topography and adhesion maps. Other super-resolution fluorescence microscopy techniques have been combined with AFM as well, such as STimulated Emission Depletion (STED), Stochastic Optical Reconstruction Microscopy (STORM), and Photo-Activated Light Microscopy (PALM)106. Another combined technique, atomic force-electrochemical microscopy (AFM-SECM), offers potential in nanoscale imaging. The combination of AFM and scanning electrochemical microscopy (SECM) allows simultaneous measurements of topography and electrochemical activity107. This technique has been applied to probe redox functions and resolve the position of immune complexes on the virus surface, using the lettuce mosaic virus108. The characterization and identification of viral particles has also been achieved before by tip-enhanced Raman spectroscopy (TERS)109,110. Raman microscopy and AFM can be integrated to work simultaneously, which allows linking the chemical composition of the sample to its surface characteristics, providing structural, mechanical, and chemical information about a biological sample111,112,113.
Advances in virology have been made not only by simultaneous combination, but also by complementing data from different techniques. For example, the biophysical properties of hepatitis B virus (HBV) have been investigated by AFM and mass spectrometry (MS). By using complementary data from these techniques, knowledge was gained on the molecular composition and physical stability of HBV capsids114, which holds considerable interest in relating virus structure with its stability in the environment. In the context of foodborne viruses, a study has combined AFM nanoindentation experiments with MS (Fig. 4A) to provide complementary insights into mechanical properties and composition of particles of NVLPs (Fig. 4B, C). This study describes strengthening of the icosahedral viral particle, providing data on structural stability of particles that could be of interest in the study of other foodborne viruses as well115. A study by Bally et al.116 investigated if galactosylceramides (GalCer) could potentially be a receptor for NoV infection, using NVLPs. In this study, AFM was complemented by other methods, including fluorescence microscopy (Fig. 4E-D) and quartz crystal microbalance with dissipation monitoring (QCM-D). AFM was used to visualize and characterize the topography of the supported lipid bilayers (Fig. 4F), allowing further identification and analysis of lipid domains containing GalCer. This multidisciplinary approach revealed a specific interaction between NVLPs and GalCer in lipid domains116.
A Representation of mass spectrometry (MS) technique, which can be combined with AFM to provide a characterization of viral particles. B, C Images adapted from Baclayon et al.115, to show: (B) native nanoelectrospray mass spectra for NVLPs (0.2 μM capsid concentration) and (C) AFM images and height profiles of NVLP (top left panel; solid line) and mutant CT303 particle (bottom left panel; dashed line)115. D Representaion of fluorescence microscopy, that has been used in the study of Bally et al.116 as a complementary technique to AFM in the study of NoV (E). E, F Images adapted from Bally et al.116 showing (E) a correlation of NVLP binding with ___domain features given by fluorescence micrographs of GalCer bilayers, as well as (F) an AFM topography image of a bilayer GalCer ___domain, with corresponding height profile (white line in the image)116. G Representation of MD simulations technique, which has been combined with AFM in the study of Arkhipov et al.120 to perform both experiments and simulations of AFM indentation (H). H Schematics of AFM nanoidentation of a HBV capsid, in which an AFM tip is pushed against a viral capsid anchored on a substrate surface (I, J) Images adapted from Arkhipov et al.120 showing (I) Force-indentation curves obtained from experiments (gray) and simulations in different pushing directions (colored). Curves are obtained as measurement of the AFM tip position and the force experienced by the tip. Taking into account initial capsid height, the AFM tip position can be translated into indentation depth. Data represents average of multiple simulations or experiments. Error bars are RMSD values. J Exemplary force-indentation curves from individual simulations, each in a different color120. Schematics in panels A, D, G, and H were created in BioRender.com.
Other AFM-related techniques also open up new possibilities for nanoscale characterization of biological samples. AFM-based infrared spectroscopy (AFM-IR) combines the spatial resolution of AFM with the chemical analysis capability of infrared (IR) spectroscopy117, and has been used to image viruses inside cells through their IR signature118. To complement topographic HS-AFM data, surface-enhanced Raman spectroscopy (SERS) analysis was used to shown that lipid raft domains are involved in MNV internalization and that this interaction is likely cholesterol dependent99. These biophysical methods can all be applied to investigating different characteristics of viruses and often provide complementary data, which makes them useful tools in broadening our view on foodborne viral diseases.
It is also of note that the development of bioinformatics technologies, such as molecular dynamics (MD) simulations, sets in silico approaches as promising tools in the development of vaccines and antiviral treatments. The application of bioinformatics techniques can predict drug targets and ligand-receptor interactions, reducing the large number of screen processes119. Even though computational modeling shows great potential in revealing molecular-level features of viruses, MD simulations (Fig. 4G) using full viral particles are hindered by the size and complexity of the particles. In a study by Arkhipov et al.120, modeling AFM nanoindentation (Fig. 4H) is achieved through shape-based coarse-grained (SBCG) MD simulations, which balances computational efficiency with molecular detail. In this study, both AFM experiments and SBCG simulations (Fig. 4I, J) are performed on the empty capsid of the human hepatitis B virus (HBV) to investigate capsid deformation upon AFM nanoindentations. Although HBV is not a foodborne virus, the methodology presented in this study demonstrates how AFM simulations can bridge experimental and computational data. Overall, the results showed a close agreement between experiment and simulation, although a larger discrepancy between simulated and experimental curves was observed for increasing deformations (Fig. 4I)120. These techniques provide valuable insights into viral capsid mechanics, which can be applied to the study of foodborne viruses as well. An additional bioinformatics approach, called AFM-Assembly pipeline, is also a promising tool that aims to build model structures using high-resolution AFM topographic images and more structural data from other sources121. An interesting review on AFM-Assembly pipeline has been written by Pellequer121, in which perspectives and challenges of integrating AFM in modelling platforms are presented.
All the previous studies help portray that combining AFM with other techniques is highly valuable in expanding our knowledge of viral infections. In some cases, direct correlation of data from AFM experiments and other techniques is still challenging. Also, current limitations of combining these techniques include sample preparation compatibility, time scale discrepancies, and resolution mismatch. Nevertheless, using these biophysical methods as complementary techniques helps overcome the limitations of each technique and offers a more comprehensive understanding of complex biological processes.
Other Biophysical Methods in Studying Foodborne Viruses
Overall, the true incidence of foodborne viral transmission is still undetermined, though likely underestimated, for all of the mentioned foodborne viruses. Traditional viral detection methods mostly relied on electron microscopy (EM) and cultural isolation. Currently, immunological methods and molecular detection technology, such as PCR-based and isothermal amplification methods, have been widely used in the detection of foodborne viruses. However, each of these techniques still present limitations, such as lack of cell culture models, complicated procedures, low sensitivity, poor portability, or need for specialized professionals and equipment. Therefore, the development of sensitive, widely spread, and user-friendly detection tests is required for estimating the extent of foodborne viral infections, as well as the incidence and prevalence of their associated diseases122.
Structural biology methods, such as x-ray crystallography, EM, and nuclear magnetic resonance (NMR), have greatly contributed to the current knowledge of virus shell structures and capsid proteins123,124,125. Even though studies on human norovirus are hindered by the lack of a cell culture system, models that include the murine norovirus (MNV)99,126 and norovirus-like particles (noroVLPs)127 can be used to study norovirus infection. NMR is a nuclear method in solid state physics and provides information on the molecular structure and biomolecular interactions, allowing the identification of small molecules that bind to a known binding site on a virus surface. The binding of norovirus VLPs to host attachment factors has been investigated by several NMR studies, including saturation transfer difference (STD) NMR experiments. This technique, along with other ligand-based NMR studies, helped elucidate the binding of HBGAs and sialoglycans to human NoV and MNV capsid proteins, providing relevant insight into how glycans modulate norovirus infection128,129,130. Mass spectrometry (MS) is also an important tool in structural virology and has been applied to gather information on the constituents of viruses, the stoichiometry of viral structural proteins, and virus assembly131,132,133. A study by Uetrecht et al.134 used MS to reveal structural features of HBV and NoV assembly intermediates, providing valuable insights into the poorly understood mechanisms of viral self-assembly134. The ability to rapidly verify the structure of VLPs is important for vaccine development, as an incorrect structure may impact the immunological response. MS can also be used to characterize VLP samples and determine what species are present. Recently, the emerging approach of charge detection MS (CD-MS) provided fast and highly accurate information on noroVLP structures present in solution, making it a useful tool for vaccine development and quality control135. MS has also been used in combination with other proteomics approaches to identify host factors that regulate the replication of HEV, providing insight into potential therapeutic targets for intervention136. Additionally, hydrogen/deuterium exchange MS (HDX-MS) has been applied to investigating cell attachment by NoV, by shedding light into glycan-induced protein changes of NoV strains137. However, these techniques are not the most suited for capturing dynamic aspects of viruses and their interactions with the host, and are generally limited either on the size of the sample or on the requirement of complex sample preparation. For the measurement of thermodynamic properties of virus-host interactions, surface plasmon resonance (SPR) has been widely used, which relies on label-free and real-time measurements of biomolecule binding138. SPR is then a valuable tool in studying viral attachment139 and can be used to develop detection or diagnostic strategies for viruses140. Similarly, biolayer interferometry (BLI) provides characterization of binding interactions, including binding kinetics and affinity, and has been used to detect specific antibodies for noroVLPs, offering perspectives for clinical applications140,141. BLI was also used to assess and quantify binding between a human astrovirus capsid protein and neutralizing antibodies, revealing a high-affinity interaction and providing relevant information for the development of a vaccine for human astroviruses142. While these techniques are advantageous for high-throughput kinetic studies and large-scale screening, they only provide the average behavior of a large population. Within single-molecule techniques, besides AFM, optical and magnetic tweezers are also important tools in monitoring individual bond formation and dissociation, with the ability of detecting very low forces, although with lower spatial resolution in comparison to AFM143,144.
Viral dynamics can be investigated not only by experimental methods, but also by computational modelling. While MD simulations have been integrated with AFM to investigate viral physical properties, as described in the previous section, they have also proven valuable as an independent technique for exploring viral biophysics. A preprint study (Yarmohammadi, H. et al.145), using an in silico approach, has identified a relevant viral protein subunit that can be explored to develop a bivalent vaccine candidate that concurrently protects against rotavirus and HAV, which highlights the potential of these rapidly emerging technologies145. However, MD simulations are significantly limited by computational costs and simulation time scales, often leading to a compromise between fast computations and enough molecular detail. Nevertheless, increasing computational prowess and further developing of in silico approaches allow MD simulations using complete viral particles to become more feasible and an attractive tool for virus studies146, as interestingly summarized in a review by Borkotoky et al.147. MD simulations have been applied to studying foodborne viruses, including for the identification of inhibitors of NoV infection148, and to construct a vaccine candidate for preventing HEV infection149. The recent rapid development of these tools brings exciting perspectives for their application in combating foodborne viral diseases.
Challenges and Future Directions
Food harbors a big risk for future pandemics in introducing and transferring viruses. Foodborne viruses are known for their stability in various physical environments and their limitations in identification and mitigation strategies. Thus, further research in all of these areas is needed to develop new strategies applicable for the complete food-chain and to evaluate their effects when combined.
A challenge today is to be prepared to identify and characterize new infectious agents that can arise from unexpected sources150. Dirks et al.151 evaluated a scenario of a pandemic foodborne virus similar to NoV with a high disease burden. Their calculations reported that, while with a realistic sampling plan most infected batches would not be detected, sampling would still detect stronger changes and large data sets might enable trend analyses151. Emerging foodborne viruses were not the focus of this review, but need to be taken into account for pandemic preparedness. In particular, zoonotic viruses with a possible foodborne route of infection, including nipah viruses, ebola viruses, avian influenza viruses, aichi virus, tick-borne encephalitis virus, and coronaviruses need to be monitored and the focus of further research28. This emphasizes the need to understand and monitor viral infections in animals and contain them before they acquire the ability to spread in humans.
Detecting viruses in food matrices with high specificity, sensitivity and reproducibility is essential for ensuring food safety. However, it remains a significant challenge due to issues such as interference from non-target pathogens, low concentration of target viruses, and difficulties in sampling. Foodborne virus detection advancements could be made by strengthening research on virus interactions with food matrices, improving large-scale and multiplex detection methods, and exploring non-targeted detection approaches. Therefore, bridging the gap between laboratory research and practical application is essential to develop rapid, sensitive, and user-friendly strategies for foodborne virus detection122,152. In addition, there is a need for highly specific and sensitive methods of detection that go beyond simply assessing the infectivity of viruses using cell culture systems that support viral replication or animal models. For many foodborne viruses, these systems are lacking and research is often conducted using cultivable surrogate viruses151. More suitable cell culture systems and models for foodborne viruses are not only relevant for detection methods, but also to expand our understanding of host–virus interactions and disease pathology. A major development to overcome these limitations is the use of human intestinal enteroid (HIE) cultures to model the human gastrointestinal epithelium. This system recapitulates the natural intestinal epithelium and has been successfully used for replication of rotavirus and NoV, providing a valuable tool for studying the infection mechanisms of foodborne viruses44,153.
Despite efforts to reduce foodborne viruses in the food industry by developing several inactivation strategies, innovative technologies that maintain the sensory and nutritional characteristics of the food products are still needed. The diversity of virus types and emergence of new strains, together with different sensitivities toward inactivation strategies, pose major challenges in developing strategies to inactivate all foodborne viruses. Therefore, it is crucial to develop innovative control technologies tailored to each specific virus type and strain, adaptable to diverse sectors of the food industry, and effective either as standalone solutions or in combination with other techniques65.
Overall, AFM stands out as a single-molecule tool with unique advantages over other biophysical methods, especially in its ability to probe individual molecular interactions and providing unparalleled insight into biomolecular behavior. When combined with other techniques, such as optical microscopy, AFM’s capabilities are further enhanced, offering a more complete picture of molecular structures and behaviors of foodborne viruses111. Also, other methods fall short when it comes to the level of detail AFM can provide about single viruses, a capability made possible by its wide range of specialized techniques and sophisticated data analysis tools.
The use of AFM-based techniques in combination with other microscopy methods, together with integrating them with artificial intelligence (AI) and machine learning tools, are positioned to be highly impactful in developing strategies to overcome foodborne viral infections154. Although there are no reports on combining AFM with AI to study foodborne viruses yet, AI is being used to improve analysis of AFM images155 and machine learning approaches have been combined with AFM to not only enhance its performance, but also increase data analysis efficiency, as reviewed in Masud et al.156. Another review article, by Pregowska et al.157, describes artificial intelligence and quantum computing as holding great potential in supporting scanning probe microscopy techniques, such as AFM157. Concurrently, AI is already used in studying viral infections, with valuable contributions in identifying new drug targets, unveiling virus-cell interactions, predicting viral protein structures, and boosting vaccine design, as described by Elste et al.158. For example, AlphaFold 3 has been used to offer insights into the structure of the Nipah virus polymerase complex, advancing our understanding of its replication process159. Despite inherent drawbacks of AI, such as lack of quality data to train the algorithms, the rapid development of these tools will most likely result in its widespread use in biophysical research, with its applications being extended to foodborne viruses.
Conclusion
Foodborne viruses are a significant cause of foodborne illnesses worldwide due to their low infectious doses, long-term survival and severe lack of specific and efficient inactivation strategies. Fundamental research on foodborne viruses, such as their interactions with the food matrix, their biological mechanisms, and structural properties, is of highest importance in the development of new strategies to combat foodborne illnesses.
AFM’s ability to probe virus-surface and virus–host interactions on a single-molecule level has made it especially relevant for understanding the infection process of foodborne viruses, such as noroviruses.
Insight into adhesion to surfaces can guide the development of surface treatments, coatings, and sanitization protocols aimed at preventing viral spread. AFM also allows the identification of key viral proteins and host cell receptors involved in virus attachment and entry, which offers valuable information for the development of effective antiviral treatments. Lastly, this technique allows for the quantification of binding forces and dynamics, which is critical for identifying viral antigens that trigger immune responses, with great potential in enhancing vaccine design. Thus, the use of AFM alone and in combination with various other complementary techniques offer innovative approaches for a comprehensive view on foodborne viral infections and for future studies that aim at designing novel therapeutic interventions.
It is also of note that emerging computational modelling approaches and the use of AI are expected to become major contributors to expanding our fundamental understanding of foodborne viruses. Ultimately, the application of the biophysical methods described here can be extended to other virus families as well, paving the way to a deeper knowledge on viral infection.
Data availability
No datasets were generated or analysed during the current study.
References
(WHO), W. H. O. WHO estimates of the global burden of foodborne diseases: foodborne diseases burden epidemiology reference group 2007-2015, https://www.who.int/publications/i/item/9789241565165 (2015).
Linscott, A. J. Food-borne illnesses. Clin. Microbiol. Newsl. 33, 41–45 (2011).
Pal, M. & Ayele, Y. Emerging role of foodborne viruses in public health. Biomed. Res. Int. 5, 1–4 (2020).
Acheson, D., Bresee, J. S., Widdowson, M.-A., Monroe, S. S. & Glass, R. I. Foodborne viral gastroenteritis: challenges and opportunities. Clin. Infect. Dis. 35, 748–753 (2002).
Goodgame, R. Norovirus gastroenteritis. Curr. Gastroenterol. Rep. 8, 401–408 (2006).
Ahmed, S. M. et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 725–730 (2014).
Fallucca, A., Restivo, V., Sgariglia, M. C., Roveta, M. & Trucchi, C. Hepatitis A vaccine as opportunity of primary prevention for food handlers: a narrative review. Vaccines 11, 1271 (2023).
Sánchez, G. Processing strategies to inactivate hepatitis A virus in food products: a critical review. Compr. Rev. Food Sci. Food Saf. 14, 771–784 (2015).
Schmid, D. et al. Foodborne outbreak of hepatitis A, November 2007–January 2008, Austria. Eur. J. Clin. Microbiol. Infect. Dis. 28, 385–391 (2009).
Ryu, W.-S. Molecular virology of human pathogenic viruses. (Academic Press, 2016).
Di Cola, G., Fantilli, A. C., Pisano, M. B. & Ré, V. E. Foodborne transmission of hepatitis A and hepatitis E viruses: a literature review. Int. J. food Microbiol. 338, 108986 (2021).
Pexara, A. & Govaris, A. Foodborne viruses and innovative non-thermal food-processing technologies. Foods 9, 1520 (2020).
Zhao, L. & Yang, H. In Fundamentals and Application of Atomic Force Microscopy for Food Research, 161–187 (Elsevier, 2023).
Bhunia, A. K. General mechanism of pathogenesis for foodborne pathogens. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis, 93–112 (2008).
Guo, A. Unraveling the Factors Affecting Virus Adhesion to Food Contact Materials and Virus-Virus Interaction: A Nanoscopic Study. (Illinois Institute of Technology, 2020).
Le Guyader, F. S., Atmar, R. L. & Le Pendu, J. Transmission of viruses through shellfish: when specific ligands come into play. Curr. Opin. Virol. 2, 103–110 (2012).
Biel, S. & Gelderblom, H. Electron microscopy of viruses. Cell culture: a practical approach. Oxford University Press, Oxford, England, 111–147 (1999).
De Pablo, P. & Schaap, I. Atomic force microscopy of viruses. Phys. Virol.: Virus Struct. Mech., 159–179 (2019).
Rusnati, M., Chiodelli, P., Bugatti, A. & Urbinati, C. Bridging the past and the future of virology: Surface plasmon resonance as a powerful tool to investigate virus/host interactions. Crit. Rev. Microbiol. 41, 238–260 (2015).
Lo Giudice, C., Dumitru, A. C. & Alsteens, D. Probing ligand-receptor bonds in physiologically relevant conditions using AFM. Anal. Bioanal. Chem. 411, 6549–6559 (2019).
Mateu, M. G. Mechanical properties of viruses analyzed by atomic force microscopy: a virological perspective. Virus Res. 168, 1–22 (2012).
Ray, A. et al. From viral assembly to host interaction: AFM’s contributions to virology. J. Virol. 99, e00873–00824 (2025).
Bosch, A. et al. Foodborne viruses: detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 285, 110–128 (2018).
Li, D., Zhao, M. Y. & Tan, T. H. M. What makes a foodborne virus: comparing coronaviruses with human noroviruses. Curr. Opin. food Sci. 42, 1–7 (2021).
De Graaf, M., van Beek, J. & Koopmans, M. P. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 14, 421–433 (2016).
Flynn, T. G., Olortegui, M. P. & Kosek, M. N. Viral gastroenteritis. Lancet 403, 862–876 (2024).
Ku, M.-S., Sheu, J.-N., Lin, C.-P., Chao, Y.-H. & Chen, S.-M. Clinical characteristics and outcome in norovirus gastroenteritis. Indian J. Pediatrics 81, 1321–1326 (2014).
Olaimat, A. N. et al. Common and potential emerging foodborne viruses: a comprehensive review. Life 14, 190 (2024).
Appleton, H. Control of food-borne viruses. Br. Med. Bull. 56, 172–183 (2000).
Burnett, E., Parashar, U. D. & Tate, J. E. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children< 5 years old: 2006–2019. J. Infect. Dis. 222, 1731–1739 (2020).
Hallowell, B. D., Chavers, T., Parashar, U. & Tate, J. E. Global estimates of rotavirus hospitalizations among children below 5 years in 2019 and current and projected impacts of rotavirus vaccination. J. Pediatr. Infect. Dis. Soc. 11, 149–158 (2022).
Emerson, S. U. & Purcell, R. H. Hepatitis E virus. Rev. Med. Virol. 13, 145–154 (2003).
Kamar, N., Dalton, H. R., Abravanel, F. & Izopet, J. Hepatitis E virus infection. Clin. Microbiol. Rev. 27, 116–138 (2014).
Joon, A., Rao, P., Shenoy, S. M. & Baliga, S. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J. Med. Microbiol. 33, S102–S105 (2015).
Organization, W. H. Viruses in food: Scientific advice to support risk management activities: meeting report. (World Health Organization, 2008).
Wißing, M. H., Brüggemann, Y., Steinmann, E. & Todt, D. Virus–host cell interplay during hepatitis E virus infection. Trends Microbiol. 29, 309–319 (2021).
Das, A. et al. Cell entry and release of quasi-enveloped human hepatitis viruses. Nat. Rev. Microbiol. 21, 573–589 (2023).
Ji, H. et al. The different replication between nonenveloped and quasi-enveloped hepatitis E virus. J. Med. Virol. 93, 6267–6277 (2021).
Walker, C. M. Adaptive immune responses in hepatitis A virus and hepatitis E virus infections. Cold Spring Harb. Perspect. Med. 9, a033472 (2019).
Li, Z., Baker, M. L., Jiang, W., Estes, M. K. & Prasad, B. V. Rotavirus architecture at subnanometer resolution. J. Virol. 83, 1754–1766 (2009).
Gómez-López, V. M. et al. Inactivation of foodborne viruses by UV light: a review. Foods 10, 3141 (2021).
Goswami, B. & Kulka, M. Pathogenic mechanisms of foodborne viral disease. Food consumption and disease risk, 343 (2006).
Zhan, X., Li, Q., Tian, P. & Wang, D. The attachment factors and attachment receptors of human noroviruses. Food Microbiology, 104591 (2024).
Ettayebi, K. et al. Replication of human noroviruses in stem cell–derived human enteroids. Science 353, 1387–1393 (2016).
Prasad, B. V. et al. Norovirus replication, host interactions and vaccine advances. Nature Reviews Microbiology, 1-17 (2025).
Kato, K. & Ishiwa, A. The role of carbohydrates in infection strategies of enteric pathogens. Tropical Med. health 43, 41–52 (2015).
Todd, E. C. & Greig, J. D. Viruses of foodborne origin: a review. Virus Adaptation and Treatment, 25-45 (2015).
Lopman, B. et al. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2, 96–102 (2012).
Koopmans, M., von Bonsdorff, C.-H., Vinjé, J., de Medici, D. & Monroe, S. Foodborne viruses. FEMS Microbiol. Rev. 26, 187–205 (2002).
Cook, N., Knight, A. & Richards, G. P. Persistence and elimination of human norovirus in food and on food contact surfaces: A critical review. J. food Prot. 79, 1273–1294 (2016).
Biziagos, E., Passagot, J., Crance, J.-M. & Deloince, R. Long-term survival of hepatitis A virus and poliovirus type 1 in mineral water. Appl. Environ. Microbiol. 54, 2705–2710 (1988).
Takuissu, G. et al. Hepatitis E virus in water environments: a systematic review and meta-analysis. Food Environ. Virol. 14, 223–235 (2022).
Wolff, A., Günther, T., Albert, T. & Johne, R. Effect of sodium chloride, sodium nitrite and sodium nitrate on the infectivity of hepatitis E virus. Food Environ. Virol. 12, 350–354 (2020).
Baert, L., Debevere, J. & Uyttendaele, M. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. food Microbiol. 131, 83–94 (2009).
Richards, G. P., Watson, M. A., Meade, G. K., Hovan, G. L. & Kingsley, D. H. Resilience of norovirus GII. 4 to freezing and thawing: implications for virus infectivity. Food Environ. Virol. 4, 192–197 (2012).
Shaffer, M., Huynh, K., Costantini, V., Bibby, K. & Vinjé, J. Viable norovirus persistence in water microcosms. Environ. Sci. Technol. Lett. 9, 851–855 (2022).
Chandran, S. & Gibson, K. E. Improving the detection and understanding of infectious human norovirus in food and water matrices: a review of methods and emerging models. Viruses 16, 776 (2024).
Esseili, M. A., Meulia, T., Saif, L. J. & Wang, Q. Tissue distribution and visualization of internalized human norovirus in leafy greens. Appl. Environ. Microbiol. 84, e00292–00218 (2018).
Razafimahefa, R. M., Ludwig-Begall, L. F. & Thiry, E. Cockles and mussels, alive, alive, oh—The role of bivalve molluscs as transmission vehicles for human norovirus infections. Transbound. Emerg. Dis. 67, 9–25 (2020).
Johne, R., Scholz, J. & Falkenhagen, A. Heat stability of foodborne viruses–Findings, methodological challenges and current developments. International Journal of Food Microbiology, 110582 (2024).
Cook, N. & Van der Poel, W. H. Survival and elimination of hepatitis E virus: a review. Food Environ. Virol. 7, 189–194 (2015).
Stunnenberg, M. et al. Thermal inactivation of hepatitis E virus in pork products estimated with a semiquantitative infectivity assay. Microorganisms 11, 2451 (2023).
Monini, M. et al. Persistence of hepatitis E virus (HEV) subtypes 3c and 3e: Long-term cold storage and heat treatments. Food Microbiol. 121, 104529 (2024).
Ezzatpanah, H. et al. New food safety challenges of viral contamination from a global perspective: Conventional, emerging, and novel methods of viral control. Compr. Rev. Food Sci. Food Saf. 21, 904–941 (2022).
Han, S. et al. Innovative nonthermal technologies for inactivation of emerging foodborne viruses. Compr. Rev. Food Sci. Food Saf. 22, 3395–3421 (2023).
Wang, F. et al. Reactive species of plasma-activated water for murine norovirus 1 inactivation. Food Res. Int. 194, 114877 (2024).
Molina-Chavarria, A., Félix-Valenzuela, L., Silva-Campa, E. & Mata-Haro, V. Evaluation of gamma irradiation for human norovirus inactivation and its effect on strawberry cells. Int. J. Food Microbiol. 330, 108695 (2020).
Falcó, I., Randazzo, W. & Sánchez, G. Antiviral Activity of Natural Compounds for Food Safety. Food Environ. Virol., 1–17 (2024).
Hakim, M. S., Gazali, F. M., Widyaningsih, S. A. & Parvez, M. K. Driving forces of continuing evolution of rotaviruses. World J. Virol. 13 (2024).
Hinterdorfer, P. & Dufrêne, Y. F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3, 347–355 (2006).
Cardoso-Lima, R. et al. Physical virology: how physics is enabling a better understanding of recent viral invaders. Biophys. Rev. 15, 611–623 (2023).
Lostao, A., Lim, K., Pallarés, M. C., Ptak, A. & Marcuello, C. Recent advances in sensing the inter-biomolecular interactions at the nanoscale–A comprehensive review of AFM-based force spectroscopy. Int. J. Biol. Macromol.238, 124089 (2023).
Dedecker, P., Hofkens, J. & Hotta, J.-i Diffraction-unlimited optical microscopy. Mater. Today 11, 12–21 (2008).
Gadegaard, N. Atomic force microscopy in biology: technology and techniques. Biotech. Histochem.81, 87–97 (2006).
Viljoen, A. et al. Force spectroscopy of single cells using atomic force microscopy. Nat. Rev. Methods Prim. 1, 63 (2021).
Zhang, H. et al. Atomic force microscopy for two-dimensional materials: A tutorial review. Opt. Commun. 406, 3–17 (2018).
Dumitru, A. C. & Koehler, M. Recent advances in the application of atomic force microscopy to structural biology. J. Struct. Biol. 215, 107963 (2023).
Gavara, N. A beginner’s guide to atomic force microscopy probing for cell mechanics. Microsc. Res. Tech. 80, 75–84 (2017).
Bhushan, B. Springer handbook of nanotechnology. (Springer, 2017).
Dufrêne, Y. F. et al. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 12, 295–307 (2017).
Koehler, M. et al. Control of ligand-binding specificity using photocleavable linkers in AFM force spectroscopy. Nano Lett. 20, 4038–4042 (2020).
Guo, A., Shieh, Y. C. & Wang, R. R. Features of material surfaces affecting virus adhesion as determined by nanoscopic quantification. Colloids Surf. A: Physicochem. Eng. Asp. 602, 125109 (2020).
Dupres, V., Verbelen, C. & Dufrêne, Y. F. Probing molecular recognition sites on biosurfaces using AFM. Biomaterials 28, 2393–2402 (2007).
Paiva, T. O., Geoghegan, J. A. & Dufrêne, Y. F. High-force catch bonds between the Staphylococcus aureus surface protein SdrE and complement regulator factor H drive immune evasion. Commun. Biol. 6, 302 (2023).
Alsteens, D. et al. Nanomechanical mapping of first binding steps of a virus to animal cells. Nat. Nanotechnol. 12, 177–183 (2017).
dos Santos Natividade, R. et al. Deciphering molecular mechanisms stabilizing the reovirus-binding complex. Proc. Natl. Acad. Sci. USA 120, e2220741120 (2023).
Rankl, C. et al. Multiple receptors involved in human rhinovirus attachment to live cells. Proc. Natl. Acad. Sci. USA 105, 17778–17783 (2008).
Kolbe, W., Ogletree, D. & Salmeron, M. Atomic force microscopy imaging of T4 bacteriophages on silicon substrates. Ultramicroscopy 42, 1113–1117 (1992).
Roos, W. H. in Seminars in Cell & Developmental Biology. 145–152 (Elsevier).
Cuellar, J., Meinhoevel, F., Hoehne, M. & Donath, E. Size and mechanical stability of norovirus capsids depend on pH: a nanoindentation study. J. Gen. Virol. 91, 2449–2456 (2010).
Feng, Y. et al. Fucose binding cancels out mechanical differences between distinct human noroviruses. Viruses 15, 1482 (2023).
Yang, J. et al. Rotavirus Binding to Cell Surface Receptors Directly Recruiting α2 Integrin. Adv. NanoBiomed Res. 1, 2100077 (2021).
Jimenez-Zaragoza, M. et al. Biophysical properties of single rotavirus particles account for the functions of protein shells in a multilayered virus. Elife 7, e37295 (2018).
de Pablo, P. J. & San Martín, C. Seeing and touching adenovirus: Complementary approaches for understanding assembly and disassembly of a complex virion. Curr. Opin. Virol. 52, 112–122 (2022).
Kudkyal, V. R., Matsuura, I., Hiramatsu, H., Hayashi, K. & Kawahara, T. Phenol derivatives obtained from grape seed extract show virucidal activity against murine Norovirus. Molecules 27, 7739 (2022).
Ando, T. High-speed atomic force microscopy and its future prospects. Biophys. Rev. 10, 285–292 (2018).
Buzón, P. et al. Virus self-assembly proceeds through contact-rich energy minima. Sci. Adv. 7, eabg0811 (2021).
Le, D. T. & Müller, K. M. In vitro assembly of virus-like particles and their applications. Life 11, 334 (2021).
Aybeke, E. N. et al. HS-AFM and SERS analysis of murine norovirus infection: involvement of the lipid rafts. Small 13, 1600918 (2017).
Xue, Y. et al. Identification and measurement of biomarkers at single microorganism level for in situ monitoring deep ultraviolet disinfection process. IEEE Trans. NanoBiosci. (2023).
Shim, J. et al. Differential MS2 interaction with food contact surfaces determined by atomic force microscopy and virus recovery. Appl. Environ. Microbiol. 83, e01881–01817 (2017).
Gutierrez, L. & Nguyen, T. H. Interactions between rotavirus and Suwannee River organic matter: aggregation, deposition, and adhesion force measurement. Environ. Sci. Technol. 46, 8705–8713 (2012).
Pleshakova, T. O. et al. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 10, 129 (2019).
Bukasov, R., Dossym, D. & Filchakova, O. Detection of RNA viruses from influenza and HIV to Ebola and SARS-CoV-2: a review. Anal. Methods 13, 34–55 (2021).
Newton, R. et al. Combining confocal and atomic force microscopy to quantify single-virus binding to mammalian cell surfaces. Nat. Protoc. 12, 2275–2292 (2017).
Miranda, A. et al. How did correlative atomic force microscopy and super-resolution microscopy evolve in the quest for unravelling enigmas in biology? Nanoscale 13, 2082–2099 (2021).
Shi, X., Qing, W., Marhaba, T. & Zhang, W. Atomic force microscopy-Scanning electrochemical microscopy (AFM-SECM) for nanoscale topographical and electrochemical characterization: Principles, applications and perspectives. Electrochim. Acta 332, 135472 (2020).
Anne, A., Chovin, A., Demaille, C. & Michon, T. Redox-immunofunctionalized potyvirus nanoparticles for high-resolution imaging by AFM-SECM correlative microscopy. Virus-Derived Nanoparticles for Advanced Technologies: Methods and Protocols, 455-470 (2018).
Hermann, P. et al. Evaluation of tip-enhanced Raman spectroscopy for characterizing different virus strains. Analyst 136, 1148–1152 (2011).
Olschewski, K. et al. A manual and an automatic TERS based virus discrimination. Nanoscale 7, 4545–4552 (2015).
Bhat, S. V., Price, J. D. & Dahms, T. E. AFM-based correlative microscopy illuminates human pathogens. Front. Cell. Infect. Microbiol. 11, 655501 (2021).
Khadem, H., Mangini, M., Farazpour, S. & De Luca, A. C. Correlative Raman imaging: development and cancer applications. Biosensors 14, 324 (2024).
Prats-Mateu, B. & Gierlinger, N. Tip in–light on: Advantages, challenges, and applications of combining AFM and Raman microscopy on biological samples. Microsc. Res. Tech. 80, 30–40 (2017).
Uetrecht, C. et al. High-resolution mass spectrometry of viral assemblies: molecular composition and stability of dimorphic hepatitis B virus capsids. Proc. Natl. Acad. Sci. USA 105, 9216–9220 (2008).
Baclayon, M. et al. Prestress strengthens the shell of Norwalk virus nanoparticles. Nano Lett. 11, 4865–4869 (2011).
Bally, M. et al. Norovirus GII. 4 virus-like particles recognize galactosylceramides in domains of planar supported lipid bilayers. Angew. Chem. (Int. ed. Engl.) 51, 12020 (2012).
Dazzi, A. & Prater, C. B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 117, 5146–5173 (2017).
Dazzi, A. et al. Chemical mapping of the distribution of viruses into infected bacteria with a photothermal method. Ultramicroscopy 108, 635–641 (2008).
Zheng, J., Haseeb, A., Wang, Z. & Wang, H. Network pharmacology, computational biology integrated surface plasmon resonance technology reveals the mechanism of ellagic acid against rotavirus. Sci. Rep. 14, 7548 (2024).
Arkhipov, A., Roos, W. H., Wuite, G. J. & Schulten, K. Elucidating the mechanism behind irreversible deformation of viral capsids. Biophys. J. 97, 2061–2069 (2009).
Pellequer, J. L. Perspectives Toward an Integrative Structural Biology Pipeline With Atomic Force Microscopy Topographic Images. J. Mol. Recognit. 37, e3102 (2024).
Yin, L. et al. Detection methods for foodborne viruses: Current state-of-art and future perspectives. J. Agric. Food Chem. 71, 3551–3563 (2023).
Jung, J. et al. High-resolution cryo-EM structures of outbreak strain human norovirus shells reveal size variations. Proc. Natl. Acad. Sci. USA 116, 12828–12832 (2019).
Rossmann, M. G. Structure of viruses: a short history. Q. Rev. Biophys.46, 133–180 (2013).
Takahashi, D., Kim, Y., Chang, K.-O., Anbanandam, A. & Prakash, O. Backbone and side-chain 1 H, 15 N, and 13 C resonance assignments of Norwalk virus protease. Biomolecular NMR Assign. 6, 19–21 (2012).
DiCaprio, E., Ma, Y., Hughes, J. & Li, J. Epidemiology, prevention, and control of the number one foodborne illness: human norovirus. Infect. Dis. Clin. 27, 651–674 (2013).
Carmona-Vicente, N., Allen, D. J., Rodríguez-Díaz, J., Iturriza-Gómara, M. & Buesa, J. Antibodies against Lewis antigens inhibit the binding of human norovirus GII. 4 virus-like particles to saliva but not to intestinal Caco-2 cells. Virol. J. 13, 1–12 (2016).
Creutznacher, R. et al. NMR experiments shed new light on glycan recognition by human and murine norovirus capsid proteins. Viruses 13, 416 (2021).
Fiege, B. et al. Molecular details of the recognition of blood group antigens by a human norovirus as determined by STD NMR spectroscopy. Angew. Chem. 124, 952–956 (2012).
Rademacher, C. et al. Targeting norovirus infection—multivalent entry inhibitor design based on NMR experiments. Chem.–A Eur. J. 17, 7442–7453 (2011).
Ashcroft, A. E. Mass spectrometry-based studies of virus assembly. Curr. Opin. Virol. 36, 17–24 (2019).
Shoemaker, G. K. et al. Norwalk virus assembly and stability monitored by mass spectrometry. Mol. Cell. Proteom. 9, 1742–1751 (2010).
Morton, V. L., Stockley, P. G., Stonehouse, N. J. & Ashcroft, A. E. Insights into virus capsid assembly from non-covalent mass spectrometry. Mass Spectrom. Rev. 27, 575–595 (2008).
Uetrecht, C., Barbu, I. M., Shoemaker, G. K., Van Duijn, E. & Heck, A. J. Interrogating viral capsid assembly with ion mobility–mass spectrometry. Nat. Chem. 3, 126–132 (2011).
Miller, L. M., Draper, B. E., Wang, J. C.-Y. & Jarrold, M. F. Charge Detection Mass Spectrometry Reveals Favored Structures in the Assembly of Virus-Like Particles: Polymorphism in Norovirus GI. 1. Anal. Chem. 96, 13150–13157 (2024).
Ju, X. et al. The PRMT5/WDR77 complex restricts hepatitis E virus replication. PLoS Pathog. 19, e1011434 (2023).
Dülfer, J. et al. Glycan-induced protein dynamics in human norovirus P dimers depend on virus strain and deamidation status. Molecules 26, 2125 (2021).
Suenaga, E., Mizuno, H. & Penmetcha, K. K. Monitoring influenza hemagglutinin and glycan interactions using surface plasmon resonance. Biosens. Bioelectron. 32, 195–201 (2012).
De Rougemont, A. et al. Qualitative and quantitative analysis of the binding of GII. 4 norovirus variants onto human blood group antigens. J. Virol. 85, 4057–4070 (2011).
Murali, S., Rustandi, R. R., Zheng, X., Payne, A. & Shang, L. Applications of surface plasmon resonance and biolayer interferometry for virus–ligand binding. Viruses 14, 717 (2022).
Auer, S. et al. In 45 th R 3 Nordic Symposium. 85.
Ricemeyer, L. et al. Structures of two human astrovirus capsid/neutralizing antibody complexes reveal distinct epitopes and inhibition of virus attachment to cells. J. Virol. 96, e01415–e01421 (2022).
Vilfan, I. D., Lipfert, J., Koster, D., Lemay, S. & Dekker, N. Magnetic tweezers for single-molecule experiments. Handbook of single-molecule biophysics, 371-395 (2009).
Sánchez, W. N. et al. Determination of protein–protein interactions at the single-molecule level using optical tweezers. Q. Rev. Biophys.55, e8 (2022).
Yarmohammadi, H., Sepahi, A. A., Hamidi-fard, M., Aghasadeghi, M. & Bahramali, G. Development of a Novel Bivalent Vaccine Candidate against Hepatitis A Virus and Rotavirus Using Reverse Vaccinology and Immunoinformatics. (2024).
Tarasova, E. et al. All-atom molecular dynamics simulations of entire virus capsid reveal the role of ion distribution in capsid’s stability. J. Phys. Chem. Lett. 8, 779–784 (2017).
Borkotoky, S., Dey, D., Hazarika, Z., Joshi, A. & Tripathi, K. Unravelling viral dynamics through molecular dynamics simulations-A brief overview. Biophys. Chem. 291, 106908 (2022).
Obaid, R. J. et al. In silico screening and molecular dynamics simulation studies in the identification of natural compound inhibitors targeting the human norovirus RdRp Protein to fight gastroenteritis. Int. J. Mol. Sci. 24, 5003 (2023).
Kumar, A., Sahu, U., Agnihotri, G., Dixit, A. & Khare, P. A novel multi-epitope peptide vaccine candidate targeting hepatitis E virus: an in silico approach. J. Viral Hepat. 31, 446–456 (2024).
Todd, E. Vol. 17 5129 (MDPI, 2020).
Dirks, R. A. et al. Preparedness for the transmission of pandemic viruses in the food chain. Food Control 156, 110138 (2024).
Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 11, 1111 (2023).
Crawford, S. E. et al. Rotavirus infection. Nat. Rev. Dis. Prim. 3, 1–16 (2017).
Ismail, B. B., Wang, W., Ayub, K. A., Guo, M. & Liu, D. Advances in microscopy-based techniques applied to the antimicrobial resistance of foodborne pathogens. Trends in Food Science & Technology, 104674 (2024).
Paruchuri, A., Wang, Y., Gu, X. & Jayaraman, A. Machine learning for analyzing atomic force microscopy (AFM) images generated from polymer blends. Digital Discov. 3, 2533–2550 (2024).
Masud, N., Rade, J., Hasib, M. H. H., Krishnamurthy, A. & Sarkar, A. Machine learning approaches for improving atomic force microscopy instrumentation and data analytics. Front. Phys. 12, 1347648 (2024).
Pregowska, A., Roszkiewicz, A., Osial, M. & Giersig, M. How scanning probe microscopy can be supported by artificial intelligence and quantum computing? Microsc. Res. Tech. 87, 2515–2539 (2024).
Elste, J., Saini, A., Mejia-Alvarez, R., Mejía, A., Millán-Pacheco, C., Swanson-Mungerson, M. & Tiwari, V. Significance of Artificial Intelligence in the Study of Virus-Host Cell Interactions. Biomolecules 14, 911 (2024).
Balıkçı, E. et al. Structure of the Nipah virus polymerase complex. EMBO J., 1–24 (2024).
Guu, T. S. et al. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. USA 106, 12992–12997 (2009).
Stuart, D. I., Ren, J., Wang, X., Rao, Z. & Fry, E. E. Hepatitis A virus capsid structure. Cold Spring Harb. Perspect. Med. 9, a031807 (2019).
Tami, C. et al. Immunoglobulin A (IgA) is a natural ligand of hepatitis A virus cellular receptor 1 (HAVCR1), and the association of IgA with HAVCR1 enhances virus-receptor interactions. J. Virol. 81, 3437–3446 (2007).
Sánchez, G. et al. Capsid region involved in hepatitis A virus binding to glycophorin A of the erythrocyte membrane. J. Virol. 78, 9807–9813 (2004).
Lin, K.-Y. et al. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: a review. World J. Gastroenterol. 23, 3589 (2017).
Miyazaki, N., Taylor, D. W., Hansman, G. S. & Murata, K. Antigenic and cryo-electron microscopy structure analysis of a chimeric sapovirus capsid. J. Virol. 90, 2664–2675 (2016).
Pitkänen, O., Vesikari, T. & Hemming-Harlo, M. The role of the sapovirus infection increased in gastroenteritis after national immunisation was introduced. Acta Paediatrica 108, 1338–1344 (2019).
Arias, C. F. & DuBois, R. M. The astrovirus capsid: a review. Viruses 9, 15 (2017).
Haga, K. et al. Neonatal Fc receptor is a functional receptor for classical human astrovirus. Genes to Cells (2024).
Gallardo, J., Pérez-Illana, M., Martín-González, N. & San Martín, C. Adenovirus structure: what is new? Int. J. Mol. Sci. 22, 5240 (2021).
Zhang, Y. & Bergelson, J. M. Adenovirus receptors. J. Virol. 79, 12125–12131 (2005).
Mohammed, D. et al. Altered glycan expression on breast cancer cells facilitates infection by T3 seroptype oncolytic reovirus. Nano Lett. 21, 9720–9728 (2021).
Martín-González, N. et al. Long-range cooperative disassembly and aging during adenovirus uncoating. Phys. Rev. X 11, 021025 (2021).
Acknowledgements
We thank the Leibniz Lab “Pandemic Preparedness”, its coordinator Prof. Dr. Gülşah Gabriel and all its members for the stimulating discussions during the inauguration, which contributed significantly to this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.K.; Writing—original draft preparation: R.N. and B.D; Preparing figures: R.N. and M.K.; Writing—reviewing and editing: M.K. and V.S.; all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
dos Santos Natividade, R., Danzer, B., Somoza, V. et al. Atomic force microscopy at the forefront: unveiling foodborne viruses with biophysical tools. npj Viruses 3, 25 (2025). https://doi.org/10.1038/s44298-025-00107-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44298-025-00107-y