Abstract
The irradiated tumor itself represents an opportunity to establish endogenous in situ vaccines. However, such in situ cancer vaccination (ISCV) triggered by radiation therapy (RT) alone is very weak and hardly elicits systemic anticancer immunity. In this study, we develop two-dimensional risedronate-manganese nanobelts (RMn-NBs) as an adjuvant for RT to address this issue. RMn-NBs exhibit good T2 magnetic resonance imaging performance and enhanced Fenton-like catalytic activity, which induces immunogenic cell death. RMn-NBs can inhibit the HIF-1α/VEGF axis to empower RT and synchronously activate the cGAS/STING pathway for promoting the secretion of type I interferon, thereby boosting RT-triggered ISCV and immune checkpoint blockade therapy against primary and metastatic tumors. RMn-NBs as a nano-adjuvant for RT show good biocompatibility and therapeutic efficacy, presenting a promising prospect for cancer radiotherapy and immunotherapy.
Similar content being viewed by others
Introduction
Recent researches from preclinical and clinical trials suggest that radiation therapy (RT) of tumors can inherently generate in situ cancer vaccination (ISCV), potentially triggering systemic anticancer adaptive immune response1,2,3. RT-triggered ISCV employs localized treatment at the lesion site to induce immunogenic cell death (ICD), striving to release tumor-associated antigens (TAAs) and immune adjuvants to initiate systemic anticancer immunnity4,5,6. Unfavorably, RT often exacerbates intrinsic hypoxia with the overexpression of hydrogen peroxide (H2O2) and glutathione (GSH) in tumor tissue, which results in a significantly reduced release of TAAs, thereby hindering systemic anticancer immune response7,8,9. Moreover, tumor cells are inclined to upregulate hypoxia-inducible factor 1α (HIF-1α) for stimulating the expression of vascular endothelial growth factor A (VEGF-A) to promote tumor growth and metastasis in the process of RT10,11. Targeting inhibition of HIF-1α/VEGF axis can relieve tumor hypoxia and suppress tumor angiogenesis, thus promoting the release of TAAs to improve the immunogenicity of tumor cells and make them easy to be attacked by immune system12,13,14,15. However, this strategy can not effectively induce the secretion of type I interferon (IFN) that is involved in ICD and indispensable for cross-presentation of TAAs to promote protective antitumor CD8+ T cell immunity, acting as a bridge molecule in linking innate and adaptive immune responses to prime ISCV16,17,18,19. Cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway can directly drive the secretion of type I IFN under the stimulation of damaged DNA to promote the cross-presentation of TAAs and activate systemic anti-tumor immune responses20,21. Therefore, constructing a cancer nanoadjuvant with the versatility of both inhibiting HIF-1α/VEGF axis and activating cGAS-STING pathway is expected for enhanced RT-triggered ISCV.
Self-assembly strategy based on clinical drugs and functional trace metal ions provides an avenue to construct visual in situ cancer nanoadjuvant22,23,24. This strategy can not only avoid the use of nanocarriers to potentially accelerate their clinical transformation, but also reduce toxicity of free clinical drugs and metal ions, improve their pharmacokinetic behaviors and physiochemical properties, resulting in multifunctional theranostics22,23,24,25,26,27. Risedronate as the first-line treatment drug for osteoporosis has been recently proved to be capable of alleviating hypoxia and inhibiting tumor angiogenesis by targeting inhibition of HIF-1α/VEGF signaling pathway28,29. Additionally, recent studies have shown that Mn2+ released from membrane-enclosed organelles during viral infections can directly bind to cGAS for triggering cGAS-STING pathway to improve type I IFN secretion, which can induce maturation of DCs and enhance TAAs presentation for promoting anticancer immune responses30,31. Mn2+ can also be used for T2 magnetic resonance imaging (MRI)32. However, free risedronate and Mn2+ exhibit severe acute toxicity and poor tumor accumulation capabilities, which hinder their efficacy in inhibiting HIF-1α/VEGF and activating cGAS-STING signaling pathway, respectively28,29,30,31. Furthermore, free Mn2+ in vivo always gives unsatisfactory MRI performance32. Self-assembly of risedronate and Mn2+ to form a self-delivering and tumor microenvironment (TME)-responsive visual nanoadjuvant should be able to efficiently inhibit HIF-1α/VEGF axis and activate cGAS-STING pathway with the guide of its enhanced MRI for promoting RT-triggered ISCV.
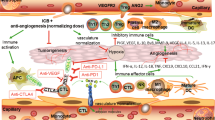
In this work, risedronate-manganese nanobelts (RMn-NBs) are constructed through coordination-driven self-assembly of risedronate and Mn2+ as a self-delivery visual nanoadjuvant to enhance RT-induced ISCV (Fig. 1a). RMn-NBs with negative surface charges and ultrathin nanobelt morphology exhibit excellent T2 MRI characteristics with a transverse relaxivity (r2) value of 11.25 mM−1 S−1 at 7.0 Tesla, slightly surpassing that of commercial Fe3O4 T2-MRI agents (r2 = 11.17 mM−1 S−1, 7.0 Tesla). The good T2-MRI performance of RMn-NBs grants them to be used for visually studying their own in vivo pharmacokinetic behaviors for precise RT. Moreover, RMn-NBs present the TME-responsive release of risedronate, which specifically inhibits HIF−1α/VEGF axis to substantially promote the release of TAAs triggered by RT. RMn-NBs also demonstrate excellent Fenton-like catalytic property, enabling them to amplify RT-mediated oxidative stress for inducing potent ICD. The released Mn2+ from RMn-NBs can further activate the cGAS-STING pathway, promoting the secretion of type I IFN and boosting the effects of RT-triggered ISCV. RMn-NB-sensitized RT as an ISCV approach can efficiently augment the efficacy of CD8+ T cell-dependent antitumor immune responses and immune checkpoint blockade (ICB) therapy against primary and metastatic tumors (Fig. 1b).
a Schematically illustrating the preparation of RMn-NBs. b The mechanism of RMn-NBs as a visual nanoadjuvant for in situ vaccination of RT. Reactive oxygen species (ROS), glutathione (GSH), calreticulin (CRT), high mobility group protein B1 (HMGB1), adenosine triphosphate (ATP), dendritic cells (DCs), interferon-beta (IFN-β), interferon-gama (IFN-γ), hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), cyclic GMP-AMP synthase (cGAS), cyclic GMP-AMP (cGAMP), stimulator of interferon genes (STING). Created in BioRender. Luo, Z. (2024) BioRender.com/u37f450.
Results
Preparation and characterization of RMn-NBs
Manganese chloride (MnCl2) and isomolar risedronate sodium (RANa) are reacted under room temperature to form nanosized risedronate-manganese coordination complex, referred as RMn-NBs. RMn-NBs aqueous suspension exhibits a zeta potential of −13.86 ± 0.88 mV (Supplementary Fig. 1a). A clear Tyndall effect can be observed for RMn-NBs aqueous suspension (Fig. 2a), suggesting that RMn-NBs exist as a nanoscale form in aqueous solution. Transmission electron microscope (TEM) imaging in Fig. 2b shows that RMn-NBs have an obvious nanobelt morphology with the width of ~5 nm and the length of ~180 nm. The average particle size of RMn-NBs is evaluated to be 177.1 ± 16.2 nm by dynamic light scattering (DLS) (Supplementary Fig. 1b), aligning with the length observed from TEM. The changes of their average particle sizes with different time indicate the good stability of RMn-NBs in deionized water (25 °C), phosphate buffered saline (PBS, 25 °C), and culture medium containing 10% fetal bovine serum (FBS, 37 °C) (Supplementary Fig. 1c). The ultraviolet-visible (UV-Vis) spectra in Supplementary Fig. 1d shows that RANa has a characteristic absorption peak at ~260 nm, which red-shifts to ~270 nm after coordination with Mn2+. Fourier transform infrared (FTIR) spectra of RMn-NBs in Supplementary Fig. 1e shows the characteristic absorption peaks of MnCl2 (~1130 cm−1) and RANa (~993, ~1053, and ~1635 cm−1)33,34, confirming the coordination interaction of Mn2+ and RANa to form RMn-NBs. The Powder X-ray diffraction (PXRD) analysis of RMn-NBs reveals distinct diffraction peaks, suggesting potential polymorphism in these supramolecular nanobelts (Fig. 2c). This result indicates that the assembly process of RMn-NBs is systematic and orderly. Energy dispersive spectroscopy (EDS) mapping (Fig. 2d) confirms the presence of N, O, P, and Mn elements within RMn-NBs. High-resolution Mn 2p X-ray photoelectron spectroscopy (XPS) spectrum shows two binding energies at 639.97 and 651.45 eV attributed to Mn2+, and two binding energies at 638.56 and 643.39 eV can be attributed to the formation of lower reduction-state Mn species due to self-assembly of Mn2+ with RANa (Fig. 2e and Supplementary Fig. 1f)35. Two binding energies at 529.95 and 531.38 eV in the high-resolution O 1 s XPS spectrum of RANa (Supplementary Fig. 1g) respectively redshift to 530.48 and 531.92 eV (Fig. 2f) after self-assembly of RANa with Mn2+ to form RMn-NBs, suggesting the formation of Mn-O bonds36. Similarly, two binding energies at 398.62 and 400.68 eV in the high-resolution N 1 s XPS spectrum of RANa respectively blueshift to 397.18 and 399.23 eV after self-assembly of RANa with Mn2+ to form RMn-NBs (Fig. 2g and Supplementary Fig. 1h), providing strong evidence for the formation of Mn-N bonds37. The self-assembly molar ratio of Mn2+ and RANa in RMn-NBs is about 1:1 by XPS elemental analysis of lyophilized RMn-NBs powder (Supplementary Table 1).
a Tyndall effect of RMn-NBs aqueous suspension. b TEM imaging of RMn-NBs, scale bar = 50 nm. This experiment is repeated twice independently with similar results. c PXRD of MnCl2, RANa, RMn-NBs. This experiment is repeated twice independently with similar results. d EDS mapping of RMn-NBs, scale bar = 50 nm. This experiment is repeated twice independently with similar results. High-resolution XPS spectra of (e) Mn 2p, (f) O 1 s, and (g) N 1 s of RMn-NBs (n = 3 independent samples). Arbitrary units (a. u.). h Electron spin resonance (ESR) spectra of ROS detection for different treatments, arbitrary units (a. u.). 2,2,6,6-tetramethyl-4-piperidinone (TEMP) is used as a trap for ROS (n = 3 independent samples). i Time-dependent GSH consumption after treating with RMn-NBs in a simulated tumor microenvironment (n = 3 independent samples). j Release profiles of RANa from RMn-NBs in different N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid (HEPES) buffer solutions (pH = 7.4, 6.5, 5.5) containing 10% serum in vitro, respectively (n = 3 independent samples). All data are shown as mean ± SD. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
Hydroxyl radical (•OH) is the major cytotoxic reactive oxygen species (ROS) generated from Fendon-like reaction38. RMn-NBs group shows stronger catalytic activity than MnCl2 group (Supplementary Fig. 1i), which can be attributed to electron-donating effect derived from coordination self-assembly of RANa with Mn2+ 39. The yield of •OH from RMn-NBs at different pH values is evaluated. As shown in Supplementary Fig. 1j, RMn-NBs can hardly produce •OH in PBS (pH = 7.4), but present evident yield of •OH in PBS (pH = 6.5 and 5.5), indicating that RMn-NBs can selectively kill tumor cells in the weakly acidic TME (Supplementary Fig. 1k). Moreover, electron spin resonance (ESR) spectroscopy not only validated the generation of •OH by RMn-NBs but has also confirmed the production of singlet oxygen (1O2) and superoxide anion radicals (•O2−) during RMn-NBs-mediated RT (Fig. 2h). The GSH scavenging capacity of RMn-NBs is measured at different time points. As shown in Fig. 2i, the clearance rate of GSH in the RMn-NBs group reaches 40.54% at 3 h, while the clearance rate of GSH in the MnCl2 and control groups only reaches 17.28% and 8.53%, respectively. The pH-responsive release curves of RMn-NBs are examined in HEPES buffers (pH = 7.4, 6.5, 5.5) containing 10% serum. RMn-NBs can scarcely release RANa in HEPES (pH = 7.4), but the release speed is obviously accelerated in HEPES (pH = 6.5 and 5.5), suggesting that RMn-NBs can responsively release RANa and Mn2+ when they are distributed in TME or endocytosed within lysosomes (Fig. 2j and Supplementary Fig. 1l).
Radiosensitization of RMn-NBs in vitro
Indocyanine green (ICG) is a fluorescent dye approved by the U.S. Food and Drug Administration (FDA) for in vivo applications in the first near-infrared region40. It exhibits excitation and emission wavelengths at around 785 nm and 810 nm, respectively, enabling deeper tissue penetration for in vivo imaging41. To evaluate the intracellular distribution of RMn-NBs, ICG is encapsulated in RMn-NBs during the coordination process, yielding ICG@RMn-NBs. Confocal laser scanning microscopy (CLSM) imaging of CT26 tumor cells treated with ICG@RMn-NBs (red fluorescence), DAPI (blue fluorescence), and LysoTracker (green fluorescence) reveals that ICG@RMn-NBs are efficiently endocytosed by CT26 tumor cells and primarily distributed in lysosomes (Supplementary Fig. 2a). Thiol Tracker Violet staining is used to measure GSH clearance in CT26 tumor cells. As shown in Fig. 3a, RMn-NBs can efficiently scavenge intracellular GSH (Fig. 3b and Supplementary Fig. 2b). The radiosensitization effect of RMn-NBs is examined in vitro by detecting intracellular ROS production using 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA). As shown in Fig. 3c, the green fluorescence of H2DCFDA in CT26 tumor cells treated with RMn-NB-sensitized radiation is obviously brighter than that in CT26 tumor cells treated with radiation alone, indicating that RMn-NBs can promote ROS generation through Fenton-like reaction (Fig. 3d and Supplementary Fig. 2c). These results suggest that RMn-NBs can amplify RT-mediated oxidative stress to enhance tumor cell killing. We next investigate the cytotoxicity of RMn-NBs at different concentrations on 3T3 mouse embryonic fibroblast cells and CT26 cells, respectively. As illustrated in the Fig. 3e, the half-maximal inhibitory concentration (IC50) of RMn-NBs against 3T3 cells and CT26 cells are 222.8 μM and 24.3 μM, respectively. The therapeutic cytotoxicity of RMn-NBs with or without irradiation against CT26 tumor cells is evaluated in vitro. It can be observed from Fig. 3f that RANa, MnCl2, and RMn-NBs groups do not cause significant cytotoxicity to CT26 tumor cells at lower concentrations. When combined with RT, RMn-NBs exhibit better inhibitory effect towards CT26 tumor cells than free RANa and MnCl2 at the same concentration (Fig. 3g), indicating that RMn-NBs can efficiently promote ROS production during RT to synergistically inhibit tumor cell proliferation.
a GSH levels in CT26 cells detected by fluorescence thiol probe (green fluorescence) merged with DAPI, scale bar = 20 μm. b The percentage of GSH+ cells detected by flow cytometry (FCM) (n = 3 cell samples). c Intracellular ROS generation in CT26 cells detected by H2DCFDA (green fluorescence) merged with DAPI, scale bar = 20 μm. d The percentage of ROS+ cells detected by FCM (n = 3 cell samples). e Half-maximal inhibitory concentration (IC50) of RMn-NBs against 3T3 and CT26 cells (n = 3 cell samples). The cytotoxicity of PBS, RANa, MnCl2, and RMn-NBs against CT26 cells (f) without and (g) with X-ray irradiation (6 Gy × 1) (n = 3 cell samples). All data are shown as mean ± SD. All experiments are repeated twice independently with similar results. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
As illustrated in the Supplementary Fig. 3a, the flow cytometry (FCM) results of propidium iodide (PI) and Annexin V staining demonstrate that RMn-NB-sensitized RT promotes more apoptosis, suggesting that the RMn-NBs significantly enhance the efficacy of RT in killing tumor cells (Supplementary Fig. 3b–d). We next perform ex vivo experiments to explore the direct effects of RMn-NBs on immune cells including tumor-associated macrophages (TAMs), bone marrow-derived dendritic cells (BMDCs), and spleen-derived T cells (T cells). As shown in Supplementary Fig. 3e, the inhibitory effect of RMn-NBs on RAW264.7 cells (TAMs) is further enhanced as the concentration of RMn-NBs increases, indicating that RMn-NBs can directly inhibit the survival of TAMs. However, the survival rate of BMDCs and spleen T cells respectively remain above 84.8% and 85.3% even that the concentration of RMn-NBs is 50 μg mL−1, demonstrating the good biocompatibility of RMn-NBs with BMDCs and spleen T cells (Supplementary Fig. 3f, g). These results indicate that RMn-NBs directly inhibit the survival of TAMs, but do not directly interfere with the activity of BMDCs and T cells under the therapeutic concentration range (0–50 μM). Additionally, an X-ray dose-dependent RT effect is observed when treating CT26 cells with saline, MnCl2, RANa or RMn-NBs. The RMn-NBs + RT group exhibits the most pronounced cell destruction among all groups under same X-ray irradiation doses (Supplementary Fig. 4a). The cell cloning assay to detect RT-induced long-term cytotoxicity is performed. As shown in Supplementary Fig. 4b, c, there are only a few viable cell colonies (122) in the RMn-NBs + RT group, while the tumor cell colonies are 506, 388, and 221 in Saline, RMn-NBs and Saline + RT groups, respectively. These results indicate that RMn-NBs can effectively sensitize radiation to prevent tumor cell proliferation.
Then, RMn-NBs are embedded with indocyanine green (ICG) as a fluorescent probe (ICG@RMn-NBs). The CT26 three-dimensional (3D) spheroid treated with ICG@RMn-NBs displays higher red fluorescence in its center after 6 h of co-incubation, and brightness reaches a maximum at the depth of 80 µm, suggesting the good tissue penetration of RMn-NBs (Supplementary Fig. 5a, b). The therapeutic cytotoxicity of RMn-NB-sensitized RT is investigated by Calcein AM and PI staining on CT26 3D spheroid. As shown in Supplementary Fig. 5c, d, the RMn-NBs + RT group produces considerably more PI+ dead tumor cells within 3D spheroid than the PBS and PBS + RT groups due to the good penetrating of RMn-NBs into CT26 3D spheroid and their ability of Fenton-like reaction-mediated ROS production. RMn-NBs exhibit a strong radiosensitization effect to sustain the best tumor growth suppression throughout the period of 10 consecutive days (Supplementary Fig. 5e, f).
MRI and pharmacokinetics of RMn-NBs
Chelation compound of paramagnetic Mn2+ has been frequently employed as a kind of MRI contrast agents in preclinical research42. We thus explore the T1-weighted and T2-weighted MRI performance of RMn-NBs in vitro, respectively. As shown in Fig. 4a, the longitudinal relaxation rates (r1) of RMn-NBs and commercial Fe3O4 contrast agent under a magnetic field of 7.0 Tesla (T) are evaluated to be 0.5394 mM−1 s−1 and 0.3393 mM−1 s−1, respectively. The transverse relaxation rates (r2) of RMn-NBs and commercial Fe3O4 contrast agent under a magnetic field of 7.0 Tesla (T) are evaluated to be 11.25 mM−1 s−1 and 11.17 mM−1 s−1, respectively (Fig. 4b). These longitudinal and transverse relaxation rates of RMn-NBs are very close to those of commercial Fe3O4 contrast agent. The ratio of r2 to r1 (r2/r1) of RMn-NBs is determined to be 20.86, suggesting that RMn-NBs can be an excellent T2-weighted MRI contrast agent. As shown in Fig. 4c, T2-weighted MRI performance of RMn-NBs can be comparable to that of commercial Fe3O4 contrast agent, which is expectant to be used for studying their own pharmacokinetic behaviors in vivo. RMn-NBs are used for MRI in vivo of ectopic CT26 tumor and orthotopic 4T1 breast cancer models. As shown in Fig. 4d (CT26 model) and Supplementary Fig. 6a (4T1 model), the MRI signal in tumor reaches a maximum after intravenous injection for 8 h, and gradually decreases in the following 16–72 h. The intensity of MRI signal in the kidney gradually increases within 24 h after intravenous injection of RMn-NBs, and gradually decays in the following 24–72 h (Fig. 4e and Supplementary Fig. 6b).
a Measuring the longitudinal relaxation rates (r1) of RMn-NBs and commercial Fe3O4 contrast agent (n = 3 independent samples). b Measuring the transverse relaxation rates (r2) of RMn-NBs and commercial Fe3O4 contrast agent (n = 3 independent samples). c T2-weighted MRI of RMn-NBs and commercial Fe3O4 contrast agent (n = 3 independent samples). d Dynamic MRI after intravenous injection of RMn-NBs (10 mM × 0.2 mL) in CT26 tumor. The doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively (n = 3 mice). e Relative MRI signal intensities of tumor and kidney regions in the 4T1 tumor-bearing mice at various time points (n = 3 mice). The dynamic concentrations of Mn2+ accumulated within (f) tumor and (g) kidney in the 4T1 tumor-bearing mice determined by inductively coupled plasma optical emission spectrometer (ICP-OES), respectively (n = 3 mice). h Pharmacokinetic profiles of free RANa and RMn-NBs in orthotopic 4T1 tumors. The dynamic concentrations of RANa accumulated within (i) tumor and (j) kidney of 4T1 tumor-bearing mice determined by HPLC, respectively (n = 3 mice). All data are shown as mean ± SD. Source data are provided as a Source Data file.
Relative MRI signal intensity of tumor and kidney regions at various time points (Fig. 4e) shows that the concentration of RMn-NBs in the tumor tissues reaches a maximum value ([Mn] = 21.7 μg g−1 tumor) after intravenous injection of RMn-NBs for 8 h and maintains a high value ([Mn] = 16.7 μg g−1 tumor) even after intravenous injection of RMn-NBs for 16 h. However, the maximum concentration of accumulative Mn2+ from MnCl2 in the tumor tissues is relatively low ([Mn] = 7.2 μg g−1 tumor) after intravenous injection of MnCl2 for 8 h (Fig. 4f). The concentration of RMn-NBs in the kidney reaches a maximum value ([Mn] = 29.4 μg g−1 kidney) after intravenous injection of RMn-NBs for 24 h and becomes scarce in 72 h (Fig. 4g). We further compare the in vivo pharmacokinetics of free RANa and RMn-NBs in orthotopic 4T1 tumors at various time points (0.5, 1, 2, 6, 12, 24, 48 h). The blood elimination half-life (t1/2) of free RANa is ~1.5 ± 0.1 h, while RMn-NBs exhibit a significantly prolonged half-life of ~5.8 ± 0.4 h (Fig. 4h and Supplementary Table 2). RANa or RMn-NBs accumulation in tumor tissues is evaluated. As depicted in Fig. 4i, RMn-NB-treated tumors exhibit the highest concentration of accumulated RANa (48.7 µg g−1 tumor) at 8 h post-administration. However, free RANa has relative low accumulation concentration (21.1 µg g−1) at 2 h post-administration. The concentration of RMn-NBs in the kidney reaches a maximum value ([RANa] = 57.6 μg g−1 kidney) post-injection for 24 h and becomes scarce in 72 h (Fig. 4j). Based on this pharmacokinetics of RMn-NBs, we conduct a detailed analysis of their biodistribution in major organs by using HPLC and ICP-OES, respectively. As depicted in the Supplementary Fig. 6c, d, the highest concentration of RMn-NBs is observed in tumor tissues ([RANa] = 48.7 μg g−1, [Mn] = 24.3 μg g−1) at 8 h post-administration, followed by the distribution of RMn-NBs in renal tissues ([RANa] = 20.3 μg g−1, [Mn] = 13.6 μg g−1), with smaller quantities of RMn-NBs detected in the heart, liver, spleen and lung. These results indicate that the self-assembly of Mn2+ and RANa to form RMn-NBs can significantly improve the in vivo pharmacokinetic behavior of free drugs and better accumulate into tumor tissues to trigger ISCV.
Radiosensitization of RMn-NBs in CT26 tumors
The therapeutic effect of RMn-NBs is assessed in BALB/c mice bearing CT26 tumor. All mice are assigned into 10 groups at random when the average tumor volume grows to ~100 mm3, including Saline, Saline + RT, RANa, RANa + RT, MnCl2, MnCl2 + RT, RANa + MnCl2, RANa + MnCl2 + RT, RMn-NBs and RMn-NBs + RT groups. As shown in the Supplementary Fig. 7a, all the unirradiated groups show almost no tumor growth inhibition compared to the Saline group on day 15. The RANa + RT, MnCl2 + RT, and RANa + MnCl2 + RT groups do not show any synergistically therapeutic effects (Supplementary Fig. 7b). However, the RMn-NBs + RT group shows the highest tumor inhibition among all groups, indicating that RMn-NBs can be effectively accumulated at tumor sites and react with H2O2 for amplifying intracellular oxidative stress through combination with RT. The most visibly diminished weights of excised tumors on day 18 for the RMn-NBs + RT group provide further evidence of the tumor growth suppression of RMn-NBs combined with RT (Supplementary Fig. 7c).
The production of γ-H2Aχ is a crucial indicator of double-strand DNA breakage in the process of RT. As shown in Supplementary Fig. 7d, e, the Saline + RT, MnCl2 + RT, RANa + RT, and MnCl2 + RANa + RT groups present scarce pink fluorescence, while the RMn-NBs + RT group displays evident pink fluorescence, indicating that RMn-NB-sensitized RT can cause the effective breakage of dsDNA. TUNEL staining images show that the RMn-NBs + RT group has more apoptotic tumor cells than other groups (Supplementary Fig. 7d, f), suggesting that intracellular oxidative stress for tumor cell inhibition may be greatly amplified by RMn-NBs. Immunohistochemical (IHC) staining for tumor malignant proliferation marker (Ki67) demonstrates that hyperproliferative tumor cells are remarkably diminished after RMn-NB-sensitized RT (Supplementary Fig. 7d, g). These biological analysis results indicate that RMn-NBs, as a kind of RT nanoadjuvant, can effectively inhibit the proliferation and growth of tumor cells through their strong ISCV. Besides, Supplementary Fig. 8a shows that the body weight of mice in the MnCl2 + RT, RANa + RT and MnCl2 + RANa + RT groups gives a downward trend, while the body weight of mice in Saline, Saline + RT, and RMn-NBs + RT groups exhibits no significant change, preliminarily indicating the good biological safety of RMn-NBs throughout therapy. Analysis of serum biochemical parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), and urinary anine (UREA) shows that free MnCl2 or RANa causes certain damages to the function of liver and kidney, but there is no statistically significant difference between the RMn-NBs and Saline groups (Supplementary Fig. 8b). Histological examination (H&E) of major organs demonstrates that RMn-NBs have no statistically significant difference as compared to the Saline group (Supplementary Fig. 8c). The results of serum biochemical and H&E analysis further confirm the good biosafety of RMn-NBs.
RNA sequencing of CT26 tumor tissues after various treatments
To gain deep insights into the potential biological mechanisms on the synergistic treatment of tumor cells with RMn-NBs and RT, we conduct comprehensive genome-wide RNA expression sequencing (RNA-seq) analysis on CT26 tumor tissues subjected to various treatments. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses are performed43. The interrelation of gene expressions among different groups is effectively visualized through a Venn plot. The central overlapping region shows 77 genes with similar expression profiles between different groups, suggesting that there may be common regulatory pathways or biological functions (Fig. 5a). As shown in the Fig. 5b, c, RT alone or RMn-NB-sensitized RT can cause genetic changes to a certain extent. Specifically, Volcano plots show prominent down-regulation of Bmp7, Hjv, Cdkn2b, mapk13 and Pla2g4f genes, which are key constituents of HIF-1α/VEGF axis44. Notably, up-expression of Il1b, Cxcl3, and Ccl19 genes, which are related to the cGAS-STING pathway, can be observed in the RMn-NBs + RT group but not in the Saline + RT group (Fig. 5d)45.
a Venn plot indicates the gene expression relationship among groups (n = 3 mice). Differential gene volcano plots between (b) Saline and Saline + RT groups (n = 3 mice), (c) RMn-NBs and RMn-NBs + RT groups (n = 3 mice), (d) Saline + RT and RMn-NBs + RT groups (n = 3 mice), indicating the number of up- or down-regulated gene expressions. e GO enrichment analysis between Saline + RT and RMn-NBs + RT groups (n = 3 mice). f Differential gene clustering heatmap between Saline + RT and RMn-NBs + RT groups (n = 3 mice). Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (BioProject: PRJCA024822 and GSA: CRA019291).
GO enrichment analysis of Saline + RT and RMn-NBs + RT groups demonstrates that the RMn-NB-sensitized RT exhibits a substantial influence on relevant signaling pathways, such as T cell receptor-antigen processing and presentation, and cytokine-cytokine receptor interaction (Fig. 5e). The transcriptional down-regulation of Cd79b, Pik3r1 and Cdkn2aip genes is evident for the RMn-NBs + RT group in response to inflammatory stimuli and intercellular immunity46. Conversely, the transcriptional up-regulation of Cxc13, Hspb1, Cdkn2b, Cxc15, and Hspb1 genes indicates a heightened induction of apoptosis and ICD in the RMn-NBs + RT group compared to the Saline + RT group44,45,46. This discernible disparity suggests the superior efficacy of RMn-NB-sensitized RT in provoking ICD. Differential gene clustering heatmap between Saline + RT and RMn-NBs + RT groups in Fig. 5f reveals distinct transcriptional changes in response to different treatments, which further elucidates the enhanced capability of RMn-NB-sensitized RT to trigger ISCV.
HIF-1α/VEGF inhibition and cGAS-STING activation-enhanced ICD for ISCV
Guided by the cues from RNA sequencing, we next investigate immune response driven by HIF-1α/VEGF inhibition, cGAS-STING activation, and ICD induced by RMn-NB-sensitized RT. As shown in Fig. 6a, RT can exacerbate tumor hypoxia and result in up-expression of HIF-1α, while RMn-NB-sensitized RT is able to significantly improve tumor hypoxia and downregulate HIF-1α expression (Fig. 6b). The secreted levels of VEGF-A in the CT26 tumor tissues are examined by ELISA kit. As shown in Fig. 6c, a decreased level of VEGF-A is detected in CT26 tumor tissues harvested from RMn-NBs and RMn-NBs + RT groups versus Saline and Saline + RT groups. PicoGreen fluorescence staining is employed to explore the damage of dsDNA on CT26 tumor cells by RMn-NB-sensitized RT and determine whether broken DNA fragments infiltrate into the cytoplasm of tumor cells. As shown in Fig. 6d, few damaged DNA fragments can be found in the cytoplasm of tumor cells in the Saline, RMn-NBs, and Saline + RT groups, but a certain amount of damaged DNA fragments can be observed in the cytoplasm of tumor cells in the RMn-NBs + RT group after RMn-NB-sensitized RT for 24 h (Fig. 6e and Supplementary Fig. 9a).
a Immunofluorescent images of tumor slices stained with anti-HIF−1α antibody, scale bar = 20 μm (n = 3 mice). b Relative fluorescence intensity of HIF−1α after various treatments (n = 3 mice). c Detection of cytoplasmic VEGF-A by ELISA kit (n = 3 mice). d Representative images of PicoGreen and DAPI co-stained CT26 cells after treatments with Saline, RMn-NBs, Saline + RT (6 Gy × 1) and RMn-NBs + RT (6 Gy × 1), scale bar = 20 μm (n = 3 cell samples). The white arrows indicated damaged DNA fragments. e Relative fluorescence intensity of cytoplasmic DNA in PicoGreen-stained CT26 cells at 24 h post X-ray irradiation (n = 3 cell samples). f Western blot of IRF3, p-IRF3, STING, p-STING, and β-actin (an internal reference protein). g Detection of cytoplasmic IFN-β by ELISA kit (n = 3 cell samples). h Immunofluorescence of CT26 cells stained with anti-CRT antibody, scale bar = 20 μm. i Relative fluorescence intensity of CRT after various treatments (n = 3 cell samples). j Detection of cytoplasmic HMGB1 by ELISA kit (n = 3 cell samples). k Detection of extracellular ATP by Luciferin-based ATP assay kit (n = 3 cell samples). l FCM analysis of DCs maturation (CD80+ and CD86+ gated within CD11c+) in tumor-draining lymph nodes (TDLNs) (n = 6 mice). All data are shown as mean ± SD. All experiments are repeated twice independently with similar results. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
It is suggested that RMn-NBs can sensitize RT, cause mitotic impairment in tumor cells, and release damaged DNA fragments into the cytoplasm of tumor cells for recognition by immune cells. The release of Mn2+ can enhance the sensitivity of dsDNA sensor cGAS and trigger the production of the second messenger cGAMP, thereby enhancing the affinity between STING and cGAMP to induce IFN-β production and promote the activation of CD8+ T cells for inhibiting tumor growth47,48. Activation of cGAS-STING pathway by RMn-NB-sensitized RT to secrete type I IFN is investigated. As shown in Fig. 6f, the phosphorylation levels of interferon regulate factor 3 (IRF-3) and STING in the co-cultured CT26 tumor cells and RAW264.7 cells are increased in the RMn-NBs + RT group (Supplementary Fig. 9b, c). Concurrently, the release of IFN-β by immune cells is also improved in the RMn-NBs + RT group (Fig. 6g), indicating the strong synergistic effect between RMn-NBs and RT. ICD induced by RMn-NB-sensitized RT is studied by detecting classical ICD markers, including CRT, HMGB-1, ATP, and IFN-β16–19. When ICD occurs in tumor cells, CRT is exposed on the surface of cell membrane as a “eat-me” signal to promote the maturation of DCs16. As shown in Fig. 6h, administering saline or RMn-NBs alone has little effect on the exposure of CRT on the surface of cell membrane, while CRT translocation can be clearly observed on the surface of tumor cells treated with RMn-NB-sensitized RT (Fig. 6i and Supplementary Fig. 9d). Extracellular HMGB1 can bind to the pattern recognition receptors (PRRs) on the surface of immune cells, thereby activating the immune response16. Compared with the Saline, Saline + RT, and RMn-NBs groups, the RMn-NBs + RT group shows remarkably promoted release of HMGB1 from CT26 cells (Fig. 6j), which acts as a “find-me” signal to promote antigen-presenting cells (APCs) to phagocytose apoptotic cells and thus enhance specific anticancer immune killing16. Immunofluorescence images showcasing CRT translocation and HMGB1 in vivo are shown in the Supplementary Fig. 9e, f. Administering saline or RMn-NBs alone has little effect on the exposure of CRT on the surface of tumor cells, while CRT translocation can be clearly observed on the surface of tumor cells treated with RMn-NB-sensitized RT. Compared with the Saline, Saline + RT, and RMn-NBs groups, the RMn-NBs + RT group shows obviously promoted release of HMGB1 from CT26 cells (Supplementary Fig. 9g, h). As shown in Fig. 6k, RMn-NB-sensitized RT dramatically increases the secretion of ATP from CT26 tumor cells, indicating their superior ability to trigger powerful ICD. Maturation of DCs is a critical downstream feature of robust ICD induction, which can effectively initiate systemic anticancer immune responses. Tumor-draining lymph nodes (TDLNs) are collected from CT26-bearing mice with different treatments for FCM analysis. As shown in Fig. 6l and Supplementary Fig. 9i, the proportion of mature DCs (CD80+ CD86+ CD11c+) does not alter in the RMn-NBs or Saline + RT group. Compared with the ratio of mature DCs in the RMn-NBs group and Saline + RT group, the ratio of mature DCs in the RMn-NBs + RT group is dramatically increased. It is indicated that RMn-NBs can significantly increase radiation sensitivity to enhance the immunogenicity of treated tumor cells, which is very beneficial for inducing ISCV.
ISCV of RMn-NB-sensitized RT
Since CT26 tumor cells treated with RMn-NB-sensitized RT exhibit superior potency in inducing ICD and maturation of DCs, we speculate that the treated CT26 tumor cells with plentiful immunogenicity will potentiate systemic anticancer immune responses. The abscopal effect as a “live” responder to RT-induced ISCV, is a key indicator for the effectiveness of ISCV4,5,6. The abscopal effect caused by RMn-NB-sensitized RT is evaluated by using the CT26 bilateral tumor model. As shown in Fig. 7a, b, it is found that RT alone has little impact on the distant tumors. However, RMn-NB-sensitized RT significantly reduces the development of distant tumors, which is attributed to the activation of systemic anticancer immunity by RMn-NB-sensitized RT. To explore the mechanism of triggering ISCV with RMn-NB-sensitized RT, CD8+ T cells are immunodepleted by using anti-CD8a antibody (αCD8a). αCD8a greatly diminishes the therapeutic benefits of RMn-NB-sensitized RT in primary and distant tumors. Conversely, ICB inhibitor (αPD-L1) evidently boosts the ISCV of RMn-NB-sensitized RT in distant tumors. The synergistic effect of RMn-NB-sensitized RT with αPD-L1 is supported by the most prominently decreased weight of excised tumor tissues (Fig. 7c, d) and the most serious suppression growth curve of individual tumors in the RMn-NBs + RT + αPD-L1 group (Supplementary Fig. 10a, b). It is reasonably proposed from above results that RMn-NB-sensitized RT profoundly facilitates CD8+ T cell-mediated systematic immunity against primary and distant tumors.
a Primary and (b) distant tumor growth curves of CT26 colorectal bilateral tumor-bearing mice treated with Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1. The doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively. X-ray irradiation is performed after intravenous injection of RMn-NBs (10 mM × 0.2 mL) for 6 h. Treatments are performed with 3 fractions (total 6 Gy) on days 0, 3, and 6, and only primary tumors receive RT (black arrow). αPD-L1 (10 mg kg−1) and αCD8a (10 mg kg−1) are intraperitoneally injected respectively after RT for 6 h, and treatments are performed on days 0, 3, 6, and 9 (red arrow, n = 6 mice). c Primary and (d) distant tumor weights from mice with various treatments (n = 6 mice). The percentages of CD3+ CD4+ T cells infiltrated within (e) primary and (f) distant tumor tissues detected by FCM (n = 6 mice). The percentages of CD3+ CD8+ T cells infiltrated within (g) primary and (h) distant tumor tissues detected by FCM (n = 6 mice). The percentages of FoxP3+ T cells (Tregs) among CD3+ CD4+ T cells infiltrated within (i) primary and (j) distant tumor tissues detected by FCM (n = 6 mice). Relative content of IFN-γ in the (k) primary and (l) distant tumor tissues detected by ELISA kit (n = 6 mice). All data are shown as mean ± SD. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
Systemic anticancer immunity activated by RMn-NB-sensitized RT
To study the mechanism of systemic anticancer immunity elicited by RMn-NBs, we analyze infiltrated pro-inflammatory immune cells and anti-inflammatory immune cells by FCM49. As shown in Fig. 7e–h and Supplementary Fig. 11a–c, 12a–c, the RMn-NBs + RT group has higher levels of infiltrated CD4+ and CD8+ T cells than the Saline and Saline + RT groups. The RMn-NBs + RT + αCD8a group presents the effective depletion of CD8+ T cells, resulting in their poor therapeutic outcomes. However, the RMn-NBs + RT + αPD-L1 group gives the greatest levels of infiltrating CD4+ and CD8+ T cells, indicating the synergistic effect of RMn-NB-sensitized RT with αPD-L1 to activate systemic anticancer immunity. IHC staining analysis for tumor-infiltrating CD4+ and CD8+ T cells (Supplementary Fig. 13a, b) further supports these findings. FCM analysis results (Fig. 7i, j and Supplementary Fig. 14a–c) demonstrate that Tregs predominate among the tumor-infiltrating CD4+ T cells in the tumor tissues of the Saline and Saline + RT groups. Moreover, the total number of T cells per gram of tumor weight further confirms that RMn-NB-sensitized RT effectively activate systemic anticancer immunity (Supplementary Fig. 15a–f). The RMn-NBs + RT and RMn-NBs + RT + αPD-L1 groups exhibit the reduced proportion of Tregs, while the RMn-NBs + RT + αCD8a group obviously restores the proportion of Tregs to a higher level. The level of IFN-γ secretion in primary and distant tumors is evaluated by ELISA kit. As shown in Fig. 7k, l, RMn-NB-sensitized RT can promote the secretion of IFN-γ, which is further augmented by αPD-L1 treatment.
Enhanced radioimmunotherapy with RMn-NBs in B16F10-OVA tumor models
The therapeutic effect of RMn-NBs is assessed in B16F10-OVA tumor-bearing C57BL/6 mice. As shown in the Fig. 8a, RMn-NB-sensitized RT can activate systemic anticancer immunity and significantly reduce the development of B16F10-OVA tumors. αCD8a greatly diminishes the therapeutic benefits of RMn-NB-sensitized RT in B16F10-OVA tumors, while αPD-L1 evidently boosts the ISCV of RMn-NB-sensitized RT (Fig. 8b). The proportion of TAMs in the whole tumor tissue of RMn-NBs + RT group (3.28%) is significantly reduced in comparison with that of Saline (8.25%) and Saline + RT (8.03%) groups (Fig. 8c and Supplementary Fig. 16a, b). These results indicate that TAMs contribute to the anti-tumor effects mediated by RMn-NBs + RT, which is consistent with previous reports that nitrogen-containing bisphosphonates can inhibit the mevalonate pathway by blocking post-translational protein isoprenylation, thereby inducing apoptotic cell death in macrophages50. TAMs as the primary immunosuppressive cells can infiltrate tumors and secrete a range of suppressive cytokines, such as VEGF-A, to promote tumor metastasis and hinder recruitment, maturation, and migration of DCs51. A large proportion of CD11c+ H2Kb-SIINFEKL+ DCs observed in the RMn-NBs + RT and RMn-NBs + RT + αPD-L1 groups is higher than that in the Saline, Saline + RT, and RMn-NBs + RT + αCD8a groups (Fig. 8d and Supplementary Fig. 17a–c). This finding reinforces the concept that RMn-NBs can enhance the efficacy of RT and synergistically promote ISCV, potentially amplifying systemic anti-tumor immune responses. Notably, mice treated with RMn-NB-sensitized RT exhibits a 36.27% population of mature DCs (CD80+ and CD86+ gated on CD11c+), which is significantly higher than the percentages observed in the Saline (10.51%) and Saline + RT (11.13%) groups (Fig. 8e and Supplementary Fig. 17d). It is suggested that RMn-NB-sensitized RT elicits synergistic therapeutic effects and promotes the activation of DCs, thereby enhancing systemic anti-tumor immunity.
a Tumor growth curves of B16F10-OVA tumor-bearing C57BL/6 mice treated with Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1. Treatments are performed with 3 fractions on days 0, 2, and 4 (black arrow). αPD-L1 and αCD8a are intraperitoneally injected respectively after RT for 6 h (red arrow, n = 6 mice). b Tumor weights from mice with various treatments (n = 6 mice). c F4/80+ and CD11b+ macrophages in B16F10-OVA tumor-bearing C57BL/6 mice with different treatments detected by FCM (n = 6 mice). d FCM analysis of CD11c+ H2Kb-SIINFEKL+ DCs in tumor-draining lymph nodes from mice treated with Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (n = 6 mice). e Mature DCs (CD80+ and CD86+ gated on CD11c+) in B16F10-OVA tumor-bearing C57BL/6 mice with different treatments detected by FCM (n = 6 mice). f The percentages of CD3+ CD4+ T cells infiltrated within tumor tissues detected by FCM (n = 6 mice). g The percentages of CD3+ CD8+ T cells infiltrated within tumor tissues detected by FCM (n = 6 mice). h Percentages of effector memory T cells (TEM) in the spleen analyzed by FCM. The spleens in different groups are collected on day 10 (n = 6 mice). i The quantification of antigen-specific T cells by IFN-γ ELISpot (n = 3 mice). j The representative images of antigen-specific T cells by IFN-γ ELISpot. This experiment is repeated twice independently with similar results. All data are shown as mean ± SD. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
To further investigate the impact of RMn-NBs on T cell infiltration, we analyze immune cell infiltration using FCM. As depicted in Fig. 8f, g, the proportion of T cells in tumor tissues treated with RMn-NB-sensitized RT exhibits a marked increase. The RMn-NBs + RT group demonstrates higher levels of infiltrated CD3+ CD4+ (3.16%) and CD3+ CD8+ (1.84%) T cells compared to the Saline (1.26% of CD4+ T cells and 0.77% of CD8+ T cells), Saline + RT (1.53% of CD4+ T cells and 0.83% of CD8+ T cells), and RMn-NBs + RT + αCD8a (2.85% of CD4+ T cells and 0.65% of CD8+ T cells) groups. Additionally, the RMn-NBs + RT + αPD-L1 group exhibits the highest levels of infiltrating CD4+ (4.36%) and CD8+ (2.67%) T cells, indicating the synergistic effect of RMn-NB-sensitized RT combined with αPD-L1 in activating systemic anticancer immunity. To confirm the immunological memory responses induced by RMn-NBs + RT, we analyze TEM (CD3+ CD8+ CD44+ CD62L-) and TCM (CD3+ CD8+ CD44+ CD62L+) in the excised spleens of various groups52. The percentage of TEM after RMn-NBs + RT treatment is significantly higher than that in mice treated with Saline or Saline + RT. Additionally, αCD8a markedly reduces the percentage of TEM, while αPD-L1 significantly increases the percentage of TEM in the spleens of mice treated with RMn-NB-sensitized RT (Fig. 8h and Supplementary Fig. 18a, b). These results indicate that RMn-NB-sensitized RT can induce long-term immunological memory, potentially synergizing with ICB to eradicate residual tumors. IFN-γ ELISpot (enzyme-linked immune absorbent spot) assay is used to further assess the systemic anti-tumor immunity induced by RMn-NB-sensitized RT. As depicted in Fig. 8i, j, the number of OVA257-264 peptide-specific CD8+ T cells in the splenocytes of mice treated with RMn-NBs + RT is much higher than that in the Saline, RT, or RMn-NBs group. These findings further substantiate that RMn-NB-sensitized RT is effective in inducing ISCV and initiating antigen-specific anti-tumor immune responses.
In vivo long-term immune memory induced by RMn-NB-sensitized RT
Considering the excellent ability of RMn-NBs + RT-treated tumor cells to induce ICD, we hypothesize that these cells with increased immunogenicity will further enhance systemic antitumor immune responses. To test this hypothesis, we conduct prophylactic vaccination experiments in vivo using CT26 tumor cells. After treatment with Saline + RT or RMn-NBs + RT, CT26 cell fragments are obtained by cryo-shocking in liquid nitrogen to eliminate their tumorigenic capacity while preserving their major structures. Subsequently, these tumor cell fragments are subcutaneously inoculated into the left side of healthy mice, and the immunization process is repeated 3 times following the procedures outlined in Fig. 9a. Live CT26 cells are inoculated on the contralateral side of the mice on day 9, and tumor growth is monitored over the following period. As illustrated in Fig. 9b–d, the prophylactic vaccination induced by RMn-NBs + RT treatment significantly inhibits CT26 tumor growth, resulting in 83.3% (5/6) tumor-free survival, which is markedly different from the two control groups. To further confirm the immunological memory responses induced by RMn-NBs + RT treatment, TEM (CD3+ CD8+ CD44+ CD62L-) and TCM (CD3+ CD8+ CD44+ CD62L+) in the excised spleens of various groups are analyzed by FCM52. The percentage of TEM after RMn-NBs + RT treatment is much higher than that in mice treated by Saline or Saline + RT (Fig. 9e and Supplementary Fig. 19a, b). It is indicated that RMn-NB-sensitized RT can induce long-term immunological memory to synergize with ICB for potentially eradicating residual tumors.
a Schematic illustration of in situ vaccination mediated by RMn-NB-sensitized RT. Created in BioRender. Luo, Z. (2024) BioRender.com/n85g329. b Tumor growth curves, (c) isolated tumor photographs, and (d) tumor weights of BALB/c mice immunized with Saline + RT and RMn-NBs + RT-treated CT26 tumor cells, respectively (n = 6 mice). e Percentages of TEM in the spleen analyzed by FCM. The spleens in different groups are collected on day 30 (n = 6 mice). f Tumor growth curves of 4T1 breast tumor-bearing mice treated with Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (n = 6 mice). The doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively. X-ray irradiation is performed after intravenous injection of RMn-NBs (10 mM × 0.2 mL) for 6 h. Treatments are performed with 3 fractions (total 6 Gy) on days 0, 3, and 6. αCD8a (10 mg kg−1) and αPD-L1 (10 mg kg−1) are respectively administered via intraperitoneal injection after RT for 6 h, and treatments are performed on days 0, 3, and 6 (n = 6 mice). g Weights of 4T1 breast tumor after various treatments (n = 6 mice). h Survival curves of 4T1 breast tumor-bearing mice (n = 6 mice). i Quantification of metastatic lesions in the lungs (n = 6 mice). j Average size of each nodule in the lungs (n = 6 mice). All data are shown as mean ± SD. Statistical analysis is performed using two-tailed Student’s t-test for comparing two groups, and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance, while p < 0.05 indicate statistical significance. Source data are provided as a Source Data file.
Given the well-established ability of RMn-NB-sensitized RT to induce ISCV and stimulate CD8+ T cell-mediated long-term anticancer immune responses, we proceed to assess their potential in inhibiting metastasis by using a spontaneous 4T1 metastatic breast cancer model (Supplementary Fig. 20a). As shown in Fig. 9f, g and Supplementary Fig. 20b, RMn-NB-sensitized RT can improve the in situ tumor therapeutic outcome as compared to RT alone. Combination of αPD-L1 with RMn-NB-sensitized RT displays the best tumor suppression. When αCD8a is used to immunodeplete CD8+ T cells, the anticancer effect of RMn-NB-sensitized RT is greatly weakened, indicating that CD8+ T cells play a dominant role in the anti-metastatic therapy. Detecting body weights of treated groups can reflect the therapy effectiveness of different treatments. As shown in Supplementary Fig. 21a, the body weights of mice in the Saline, Saline + RT and RMn-NBs + RT + αCD8a groups dramatically drop, indicating their low therapy effectiveness to result in the death of treated mice. However, the body weights of mice in the RMn-NBs + RT and RMn-NBs + RT + αPD-L1 groups remain stable, suggesting their effective therapy towards 4T1 metastatic breast cancer. The RMn-NBs + RT group has the 150-day long-time survival rate of 33.3% (2/6), which is significantly greater than those of the Saline (0%), Saline + RT (0%) and RMn-NBs + RT + αCD8a (0%) groups. Furthermore, the long-term survival rate of RMn-NBs + RT + αPD-L1 group can reach 66.7% (4/6) in 150 days (Fig. 9h). The RMn-NBs + RT and RMn-NBs + RT + αPD-L1 groups have occasional metastatic lesions inside the lung tissues, whereas other groups have significant tumor burdens. H&E staining analysis reveals that all groups except the RMn-NBs + RT + αPD-L1 group have widespread metastatic lesions with agminated nuclei in the lungs (Supplementary Fig. 21b and Fig. 9i, j). These findings suggest that RMn-NB-sensitized RT can induce ISCV and synergize with ICB to inhibit both in situ and metastatic tumor growth.
Discussion
Enhancing RT-triggered ISCV has been proved to be a viable strategy for promoting adaptive immune responses and boosting clinical abscopal effects against tumors1,2,3. Nevertheless, RT-triggered ISCV has been significantly impeded by the overexpression of HIF-1α and VEGF within tumor tissues and RT-exacerbated hypoxia9,10,11. Targeting HIF-1α/VEGF inhibition strategy has been introduced in preclinical investigations to ameliorate tumor hypoxia and suppress angiogenesis for sensitizing RT12,13,14,15. A phase II clinical trial has demonstrated that combining VEGF-A inhibitor bevacizumab (Avastin®) with RT can significantly enhance the prognosis of recurrent glioblastoma patients53. Although targeted inhibition of HIF-1α/VEGF axis can enhance the efficacy of local RT, the released adjuvants are very limited to induce ISCV12,13,14,15. It is imperative to explore combination strategies for promoting RT-induced ICD and subsequent ISCV. Type I IFN serves as an important adjuvant for cross-antigen presentation, which plays a crucial role in priming ISCV of RT17,18,19. Recent researches indicate that cGAS-STING-dependent DNA-sensing pathway is crucial for the transmission of type I IFN signaling induced by RT20,21. For instance, combining RT with cGAS-STING agonists demonstrates a remarkable type I IFN-induced antitumor effect27. Hence, the strategy of synergistically inhibiting the HIF-1α/VEGF axis and activating the cGAS-STING pathway to promote the secretion of type I IFN holds promise for enhancing RT-triggered ISCV. We thus construct a self-delivering nanomedicine (RMn-NBs) by coordination-driven self-assembly of clinically available RANa and MnCl2. RMn-NBs can effectively alleviate tumor tissue hypoxia and suppress the formation of abnormal tumor vasculature by inhibiting HIF-1α/VEGF axis during RT. Fenton-like reaction of RMn-NBs generates •OH to result in extensive oxidative DNA damage. Additionally, Mn2+ released from RMn-NBs can interact with a large amounts of damaged DNA fragments in the cytoplasm or extracellular space, effectively activating cGAS-STING pathway and promoting secretion of type I IFN (Fig. 6d–g). Our experimental results have shown that RMn-NB-sensitized RT can synergistically induce translocation of CRT to cell surface and improve the release of HMGB1 and ATP, leading to robust ICD for promoting the phagocytosis of DCs (Fig. 6h–l). The TAAs phagocytosed by DCs are further degraded and cross-presented by MHC-I, which activates CD8+ T cell-mediated cytotoxicity54. Therefore, the concurrent induction of ICD and type I IFN secretion can significantly enhance RT-triggered ISCV against primary and metastatic tumors (Figs. 7–9).
RANa as a primary therapeutic agent for postmenopausal osteoporosis in women has gained considerable clinical usage for tumor treatment at recent years. Nonetheless, the free RANa presents notable limitations including acute toxicity and poor tumor accumulation capabilities, thereby curbing its efficacy in cancer therapy28,29. Self-assembly of RANa and Mn2+ to form RMn-NBs can improve the pharmacokinetic attributes of RANa. The responsive release behavior of RMn-NBs in the TME markedly mitigates the toxicity of excessive RANa. Considering the release behavior of RMn-NBs in a pH-dependent manner, it is speculated that the coordination state of Mn2+ in RMn-NBs is closely linked to pKa of RANa (pKa4 = 7.25, pKa3 = 6.12) (Supplementary Fig. 22a)55. RMn-NBs theoretically maintain a relatively stable particulate state at pH > 7.25. As the pH value gradually decreases, the phosphate partially becomes mono-protonated (6.12 < pH ≤ 7.25) and RMn-NBs still maintain their particulate or coordination state. When the pH value is below 6.12, free RA- and Mn2+ may be progressively released from RMn-NBs owing to the further protonation of phosphate groups (Supplementary Fig. 22b). As shown in the Supplementary Fig. 22c, RMn-NBs exhibit good stability under physiological condition (pH = 7.4), while they gradually degrade when the pH value is reduced to 5.0, which is proved by HRTEM characterization (Supplementary Fig. 22d). In order to elucidate the self-assembly process of RMn-NBs, (3-Pyridinylmethyl) phosphonic acid (PPA) (analog of risedronate containing only one phosphate group) is used as a control ligand to coordinate with Mn2+ for exploring the self-assembly of RMn-NBs. As demonstrated in Supplementary Table 3, PPA and Mn2+ self-assemble to form amorphous nanoclusters but not nanobelts. It is attributed to the fact that the C-P bond connecting pyridine group with phosphate group in PPA molecule is not constrained by rotation, resulting in anisotropic coordination of PPA with Mn2+ and eventually self-assembling into amorphous nanoclusters. Conversely, the steric hindrance of hydroxyl group and another phosphate group in the risedronate molecule restricts the free rotation of C-P bond between pyridine and phosphate groups, thus maintaining a rigid conformation with the lowest energy (MM2 Calculation by Chem 3D) that leads to cooperative stacking and the formation of nanobelts56,57,58. Interestingly, RMn-NBs exhibit excellent T2-MRI performance, which can be comparable to commercial Fe3O4 contrast agents. Moreover, their unique nanobelt structure facilitates deep penetration of RMn-NBs into tumor tissues and enhances their efficient accumulation at tumor sites (Supplementary Fig. 5)59. The MRI capability of RMn-NBs provides significant convenience for visually studying their own intratumoral accumulation and in vivo metabolic dynamics (Fig. 4 and Supplementary Fig. 6). Safety evaluations including detection of mouse body weight, serum biochemical analysis and H&E staining sections of major organs (Supplementary Fig. 8) demonstrate that RMn-NBs have good biocompatibility and safety in the process of tumor therapy.
In conclusion, versatile RMn-NBs are developed by coordination-driven self-assembly of clinically available RANa and Mn2+. The obtained RMn-NBs demonstrate excellent MRI performance and significantly improved pharmacokinetic behaviors. The intrinsic Fenton-like reaction activity of RMn-NBs effectively amplifies oxidative stress to induce robust ICD. Significantly, RMn-NBs can inhibit HIF-1α/VEGF axis and activate cGAS-STING pathway to promote the secretion of type I IFN for boosting RT-triggered ISCV, which synergistically potentiates ICB against primary and metastatic tumors. The designed RMn-NBs with prominent MRI characteristics, good therapeutic effect and biosafety provide a perspective for radioimmunotherapy.
Methods
Ethical statement and confirmation
The protocols for animal experiments in this study undergo rigorous review and approval by the Institutional Animal Care and Use Committees of Nanjing University of Posts and Telecommunications (IACUC-2022004) and Nanjing University (IACUC-D2202156), and are conducted in strict accordance with the principles outlined by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All animal testing is carried out in full compliance with applicable local and national ethical regulations.
Materials
Manganese (II) chloride is purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. RANa is purchased from Shanghai Titan Co., Ltd. (China). PBS, methylene blue (MB) sodium hydrogen carbonate (NaHCO3), GSH, calcein-AM, PI, 4’,6-diamidino-2-phenylindole (DAPI), 5,5’-dithiobis(2-nitrobenzoic acid) (DTNB), 2’,7’-Dichlorodihydro-fluorescein diacetate (H2DCFDA) and Lyso-tracker Green are purchased from Sigma-Aldrich (USA). Cell counting kit-8 (CCK-8) is obtained from Dojindo Laboratories (Japan). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) are purchased from Gibco (USA). PicoGreen dsDNA quantitation reagent is purchased from Yeasen Biological Technology Co., Ltd (China). Mouse HMGB1, VEGF-A, IFN-γ, and IFN-β precoated ELISA Kit are obtained from Dakewe Biotech Co., Ltd. Anti-gamma H2Aχ (phospho S139) antibody [9F3] (Cat# ab26350, diluted 1:200 with 3% BSA), TUNEL Assay Kit - BrdU-Red (Cat# ab66110, diluted 1:200 with 3% BSA), Anti-Ki67 antibody (Cat# ab15580, diluted 1:500 with 3% BSA), Anti-Calreticulin antibody [EPR3924] - ER Marker (Alexa Fluor® 488) (Cat# ab196158, diluted 1:500 with 3% BSA), Anti-beta Actin antibody [mAbcam 8226] - Loading Control (Cat# ab8226, diluted 1:500 with 3% BSA), Recombinant Alexa Fluor® 488 anti-HIF-1 alpha antibody (Cat# ab208419, diluted 1:300 with 3% BSA), Anti-CD4 antibody [EPR19514] (Cat# ab183685, diluted 1:400 with 3% BSA), Anti-CD8 alpha antibody [EPR21769] (Cat# ab217344, diluted 1:400 with 3% BSA), Goat anti-rabbit IgG H&L (Cy3®) (Cat# ab6939, diluted 1:300 with 3% BSA), Goat anti-mouse IgG H&L (Alexa Fluor® 488) (Cat# ab150113, diluted 1:200 with 3% BSA), Recombinant Anti-STING antibody [EPR25090-107] (Cat# ab288157, diluted 1:500 with 3% BSA) and Recombinant Anti-IRF3 antibody [EPR2418Y] (Cat# ab68481, diluted 1:500 with 3% BSA) are supplied by Abcam (USA). Phospho-STING (Ser366) Polyclonal Antibody (Cat# PA5-105674, diluted 1:500 with 3% BSA) and Phospho-IRF3 (Ser386) Polyclonal Antibody (Cat# PA5-121307, diluted 1:500 with 3% BSA) are purchased from Thermofisher (USA). HRP conjugated Goat Anti-Rabbit IgG (H + L) (Cat# GB23303, diluted 1:300 with 3% BSA) is obtained from Servicebio (China). APC anti-mouse CD80 Antibody [16-10A1] (Cat# 104713, 1.0 μg per million cells in 100 μL volume), PE anti-mouse CD86 Antibody [GL-1] (Cat# 105007, 0.25 μg per million cells in 100 μL volume), FITC anti-mouse CD11c Antibody [N418] (Cat# 117306, 0.25 μg per million cells in 100 μL volume), APC anti-mouse CD3 Antibody [17A2] (Cat# 100236, 0.5 μg per million cells in 100 μL volume), PE anti-mouse CD4 Antibody [GK1.5] (Cat# 100408, 0.25 μg per million cells in 100 μL volume), FITC anti-mouse CD8a Antibody [53–6.7] (Cat# 100706, 1.0 μg per million cells in 100 μL volume), Alexa Fluor® 488 anti-mouse FoxP3 antibody [MF-14] (Cat# 126406, 0.25 µg per million cells in 100 µL volume), FoxP3 Fix/Perm buffer (4×) (Cat# 421401), APC anti-mouse H-2Kb Antibody [AF6-88.5] (Cat# 116506, 1.0 μg per million cells in 100 μL volume), PE anti-mouse CD3 Antibody [17A2] (Cat# 100205, 0.5 µg per million cells in 100 µL volume), APC anti-mouse/human CD44 Antibody [IM7] (Cat# 103011, 0.25 µg per million cells in 100 µL volume), PerCP/Cyanine5.5 anti-mouse CD62L Antibody [MEL-14] (Cat# 104432, 0.25 µg per million cells in 100 µL volume), PE anti-mouse F4/80 Antibody [BM8] (Cat# 123110, 1.0 µg per million cells in 100 µL volume), PerCP/Cyanine5.5 anti-mouse/human CD11b Antibody [M1-70] (Cat# 101227, 0.25 µg per million cells in 100 µL volume), True-Nuclear™ transcription factor buffer set (Cat# 421403), Purified anti-mouse IFN-γ Antibody [R4-6A2] (Cat# 505702, 2.0 μg mL−1) and Ultra-LEAF™ Purified anti-mouse CD8a Antibody [53–6.7] (Cat# 100764, 10.0 mg kg−1) are purchased from BioLegend (USA). In Vivo MAb anti-mouse PD-L1(B7-H1) [Clone: 10 F.9G2] (Cat# BE0101, 10.0 mg kg−1) is purchased from BioXcell (USA).
Experimental instruments
The morphologies, crystal structures, and elemental compositions are characterized by transmission electron microscopy (TEM) (Hitachi HT7700, Japan) and high-resolution TEM (HRTEM) combined with EDS (JEM-2100F, JEOL, Japan). High angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) images are acquired using a double Cs-corrected transmission electron microscope (Thermo Scientific Spectra 300) under 300 kV with 10–20 pA beam current. Powder X-ray diffraction (PXRD) patterns are obtained by a D8 ADVANCE XRD (Bruker, Germany) with Cu Kα radiation (λ = 1.54 Å). Raman spectra are acquired using a micro-Raman spectroscopy system (Renishaw, England) with a 532 nm laser. XPS spectra are measured by a PHI 5000 VersaProbe (Ulvac-Phi, Japan) with Al Kα radiation (hν = 1486.6 eV). UV-vis absorption spectra are recorded with a Shimadzu UV-vis spectrophotometer (UV3600, Japan). FTIR spectra are recorded with a FTIR spectrometer (Bruker, Vertex 80 v and Tensor 27, Germany). The MRI performance is measured with a magnetic resonance scanner (Biospec 7 T/20 USR, Germany). The amount of Mn2+ is confirmed by an inductively coupled plasma optical emission spectrometer (ICP-OES) (Perkin Elmer Optima 5300 DV, USA). The types of all immune cells are confirmed by FCM (BD, FACS Calibur, USA). The X-ray irradiation equipment is the Rad Source RS2000 X-ray irradiator (X-ray Irradiator RS2000, USA). The instrument’s technical specifications comprise a dose rate of 10 Gy min−1 for cells and 1.2 Gy min−1 for small animals. The irradiation plane is situated 25 cm away in the cone-shaped irradiation field. The operating specifications of the power supply are 220 volts, 60 Hz, 40 amps.
Cell lines
The mouse 3T3 (Cat# CRL-1658), CT26 (Cat# CRL-2638), RAW264.7 (Cat# SC-6005), B16F10-OVA (Cat# CRL-6323), and 4T1 (Cat# CRL-2539) cell lines are purchased and authenticated by the American Type Culture Collection (ATCC). All cell lines in this study get tested without mycoplasma contamination.
Animals
BALB/c mice are sourced from the medicine center of Yangzhou University in Yangzhou of China. Ethical considerations are a priority throughout the study, and all animal-related procedures receive proper scrutiny and approval from the Institutional Animal Care and Use Committees of Nanjing University of Posts and Telecommunications (IACUC-2022004) and Nanjing University (IACUC-D2202156). The research is conducted in strict accordance with the guidelines established by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). For the construction of CT26-bearing mice and B1610-OVA-bearing mice, male BALB/c mice and C57BL/6 mice aged 5–7 weeks are respectively used, while female BALB/c mice aged 6–8 weeks are employed for the construction of 4T1-bearing mice. Mice are housed in a well-equipped animal facility, where temperature is carefully maintained at 20–25 °C and humidity is controlled ranging from 30% to 70%. Additionally, mice are exposed to a consistent dark/light cycle of 12 h, ensuring optimal conditions for their well-being. The maximum tumor size allowed by the ethics committee or institutional review board was 2000 mm3, and we confirmed that the maximum tumor burden would not be exceeded in all experimental groups.
Preparation of RMn-NBs and ICG@RMn-NBs
10.0 mL of RANa aqueous solution (10 mM) and 10.0 mL of MnCl2 aqueous solution (10 mM) are carefully added into a 50 mL flask, and then stirred at room temperature for 1 h. After stirring for 6 h, the formed precipitates are collected by centrifugation at 8000 g for 30 min, and then is washed three times with distilled water to remove impurities. After that, the washed precipitates are dispersed in 10.0 mL of an aqueous solution containing 2% mouse serum albumin (MSA) as both emulsifier and aggregation inhibitor under sonication with a power of 650 W at 45% intensity for 12 min to obtain MSA-decorated RMn-NBs. To prepare ICG@RMn-NBs, a mixture of RANa (10.0 mL, 10 mM) and ICG (0.1 mL, 1.0 mM) is slowly dropped into MnCl2 aqueous solution (10.0 mL, 10 mM), and then the mixed solution is stirred for 1 h. After the coordination self-assembly process, the resulting precipitates are washed and collected by centrifugation (8000 g × 30 min). Subsequently, the collected precipitates are dispersed into 10.0 mL of an aqueous solution containing 2% MSA to obtain ICG@RMn-NBs.
Assessment of hydroxyl radical generation in vitro
UV colorimetric method is employed to detect the generation of hydroxyl radicals (•OH) under various pH values (7.4, 6.5, 5.5). Saline and RMn-NBs are mixed with MB aqueous solutions ([Mn2+] = 20 μM, [NaHCO3] = 50 μM, [H2O2] = 200 μM, [MB] = 15 μg mL−1) for 3 h. Subsequently, the absorption of MB at 664 nm is measured to observe the degradation of MB and assess the generation of •OH.
Measurement of GSH depletion
GSH can react with DTNB to produce yellow TNB (2-nitro-5-thiobenzoate) and GSSG. This reaction serves as a reliable method for the quantitative detection of GSH by measuring the absorbance of TNB at 412 nm. For the experiments, 10 mL of MnCl2 (10 μM) or RMn-NBs (10 μM) is incubated with GSH (0.5 mM), NaHCO3 (50 μM) and H2O2 (200 μM) for 2 h at pH = 5.5. Subsequently, 980 μL of the reaction solution is taken out and mixed evenly with 20 μL of DTNB (10 mM). The absorbances of mixed solution at 412 nm are recorded by UV-vis spectrometer to evaluate the content of GSH.
Cumulative release of RANa and Mn2+
The release of RANa and Mn2+ from RMn-NBs is studied at different pH values. 1 mL of RMn-NBs (1 mM) is packed into Solarbio (10 kD) dialysis bags and stirred in 10.0 mL HEPES buffers containing 10% serum (37 °C) at pH = 7.4, 6.5, 5.5, respectively. 100 μL of the sample is collected from the dialysates at 0, 2, 4, 8, 12, 24, 48, and 72 h, respectively. RANa concentration is determined by reverse-phase high performance liquid chromatography (RP-HPLC)60, and Mn2+ concentration is determined by ICP-OES.
Intracellular uptake behavior analysis of RMn-NBs
CT26 tumor cells are seeded in a glass-bottom cell culture dish (NEST, 20 mm) at a density of 2 × 105 cells per dish and incubated with ICG@RMn-NBs (100 μM) for 6 h. Subsequently, Lysotracker Green is added and co-incubated with CT26 tumor cells for 30 min under 37 °C. After co-incubation, CT26 tumor cells are washed twice with PBS (0.1 M, pH = 7.4), and stained with DAPI for fluorescent imaging with an Olympus FV3000 CLSM.
Intracellular ROS generation and GSH depletion by RMn-NBs
CT26 cells are seeded in a glass-bottom cell culture dish (NEST, 20 mm) at a density of 2 × 105 cells per dish and incubated with RMn-NBs (100 μM) for 6 h. Subsequently, CT26 cells are incubated with H2DCFDA (a fluorescent probe used to detect intracellular ROS generation) at a dilution of 1:1000 for 30 min under 37 °C, followed by exposure to X-ray irradiation (6 Gy × 1). Immunofluorescence images are captured with an Olympus FV3000 CLSM, and data interpretation is conducted using ImageJ Software.
IC50 and cytotoxicity of RMn-NBs
CT26, 3T3 or RAW264.7 cells are cultured in DMEM (Gibco) containing 10% FBS (Gibco), 100 μg mL−1 penicillin, 100 μg mL−1 streptomycin and 50 μg mL−1 gentamicin under a humidified atmosphere with 5% CO2 at 37 °C. BMDCs and spleen T cells are isolated and cultured according to the method reported in the literatures61,62. To investigate the cytotoxicity of RMn-NBs with or without X-ray irradiation, CT26, 3T3, RAW264.7, BMDCs or spleen T cells are seeded in 96-well plates at a density of 8000 cells per well. After attachment, RMn-NBs aqueous suspensions with different concentrations (0, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 μM for IC50 experiments, 0, 3.12, 6.25, 12.5, 25.0, and 50 μM for cytotoxicity experiments) are added and incubated for 24 h. After treatments with or without X-ray irradiation (6 Gy × 1), CT26, 3T3, RAW264.7, BMDCs or spleen T cells are further cultured for an additional 24 h. The cell viability is then assessed by using CCK-8 (Dojindo, Japan) assay.
Penetration behaviors of RMn-NBs in 3D tumor spheroids
To evaluate penetration behaviors of RMn-NBs in 3D tumor spheroids, CT26 cells (3 × 103 per well) are seeded into ultralow attachment 96-well plates (Corning, 7007, USA) to form 3D CT26 tumor spheroids, which are then co-incubated with ICG@RMn-NBs for 8 h. The red fluorescence of ICG within 3D tumor spheroids is recorded with an Olympus FV3000 CLSM (Japan). To test the cytotoxicity of RMn-NB-sensitized radiation within 3D CT26 tumor spheroids, 3D CT26 tumor spheroids are incubated with RMn-NBs (50 μM) for 12 h and then subjected to X-ray irradiation (6 Gy × 1). After 24 h, Calcein-AM and PI are used to stain live and dead cells, respectively. The sizes of 3D CT26 tumor spheroids after various treatments are measured by Nikon Eclipse Ti microscope (Japan) at days 5 and 10.
Accumulation and MRI of RMn-NBs in vivo
All mice are obtained from Yangzhou University Medical Center (Yangzhou, China) and received care following the guidelines of the Institution Animal Care and Use Committee of and Nanjing University of Posts and Telecommunications (IACUC-2022004). To assess the accumulation of RMn-NBs in tumor tissues and kidneys, CT26 tumor-bearing and orthotopic 4T1 tumor-bearing mice (150–200 mm3) are treated with RMn-NBs (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively) via intravenous injection. The mice are anesthetized with isoflurane and secured in the animal groove. MRI is performed by using an MR scanner (Biospec 7 T/20 USR, Germany). The parameters for T2-weighted MRI are as follows: flip angle = 30o, TR = 850 ms, TE = 20.0 ms, FOV = 3 × 3, matrix = 256 × 256, slice thickness = 1.0 mm, averages = 3, slices = 12, NEX = 1. Multiple locations of MRI images are observed at different time points: 0, 2, 8, 16, 24, 48, and 72 h. The acquired images are analyzed by using ImageJ Software.
Biodistribution and pharmacokinetics of RMn-NBs in vivo
To evaluate the in vivo pharmacokinetics of RMn-NBs, CT26 tumor-bearing and orthotopic 4T1 tumor-bearing mice (100–150 mm3, n = 3 mice) are intravenously injected with free MnCl2 (the dose of Mn2+ is 5.5 mg kg−1) or RANa (the dose of RANa is 30.5 mg kg−1) or RMn-NBs (the dose of Mn2+ and RANa is 5.5 mg kg−1 and 30.5 mg kg−1, respectively). Tumor tissues are collected from the treated mice at different time (0, 2, 8, 16, 24, 48, 72 h), which are crushed and homogenized, followed by burning and nitrification. The resulting samples are then diluted with 1.0 mL of 1% HNO3, and the concentration of Mn2+ is quantified by ICP-OES. RANa concentration is determined by RP-HPLC60.
Radiosensitization of RMn-NBs in CT26 tumor model
When tumor volume reaches 80–100 mm3, mice are administered with different treatments. The treatment groups are including the Saline, Saline + RT, MnCl2, MnCl2 + RT, RANa, RANa + RT, MnCl2 + RANa, MnCl2 + RANa + RT, RMn-NBs and RMn-NBs + RT groups. The doses of Mn2+ and RANa in corresponding treatment groups are 5.5 mg kg−1 and 30.5 mg kg−1, respectively. Tumor volumes and body weights of mice are monitored daily. Tumor volumes are calculated as the equation of volume (V) = length × width2 × 1/2. Tumor tissues, hearts, livers, spleens, lungs, and kidneys are collected, fixed in 4% formalin, embedded in paraffin, sliced, and stained with Hematoxylin and Eosin (H&E) for histological examination. Residual tumor tissues of Saline, RMn-NBs, Saline + RT, and RMn-NBs + RT groups are homogenized, and the supernatants are collected. The levels of vascular endothelial growth factor-A (VEGF-A) are determined by mouse VEGF-A ELISA kit (Dakewe Biotech Co., Ltd.).
Immunofluorescence of γ-H2Aχ and HIF-1α, TUNEL staining and IHC analysis of Ki67
The CT26 tumor-bearing mice with a tumor volume of 150–200 mm3 are classified into the following groups: Saline, Saline + RT, MnCl2, MnCl2 + RT, RANa, RANa + RT, MnCl2 + RANa, MnCl2 + RANa + RT, RMn-NBs and RMn-NBs + RT groups. The doses of Mn2+ and RANa in corresponding treatment groups are 5.5 mg kg−1 and 30.5 mg kg−1, respectively. The X-ray irradiation dosage is 2 Gy × 3 (total 6 Gy). Saline, MnCl2, RANa, MnCl2 + RANa, and RMn-NBs are administered intravenously at 6 h before RT. After RT, tumor tissues are collected for evaluating γ-H2Aχ expression at 24 h of post-irradiation. Tumor tissues are sliced and stained with γ-H2Aχ mouse monoclonal primary antibody (diluted 1:200 with 3% BSA, Abcam, UK) and secondary antibody conjugated to Alexa Fluor 488 (Bioss, China) to detect DNA double-strand breaks. Tumor tissues of Saline, RMn-NBs, Saline + RT, and RMn-NBs + RT groups are sliced and stained with recombinant Alexa Fluor® 488 anti-HIF-1α antibody (diluted 1:300 with 3% BSA, Abcam, UK) to detect tumor hypoxia. To assess apoptosis and proliferation rates within the tumor tissues, tumors are collected at 48 h of post treatments for terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay and Ki67 staining according to the manufacturer’s protocol25.
RNA sequencing analysis
CT26 tumor-bearing mice with the average tumor size of ~100 mm3 are randomly divided into four groups: Saline, Saline + RT, RMn-NBs, RMn-NBs + RT (The doses of Mn2+ and RANa in corresponding treatment groups are 5.5 mg kg−1 and 30.5 mg kg−1, respectively) (n = 3 mice). X-ray irradiation is performed after intravenous injection of RMn-NBs for 6 h, and treatments are performed with 3 fractions (total 6 Gy) on day 0, 3 and 6. Mice are euthanized on day 7 after treatment, and tumor tissues are collected and digested. TRIzol is added to extract RNA from tumor cells for transcriptome analysis. RNA sequencing based on the BGISEQ-500 platform and RSEM is employed to quantify transcription levels. Genes are considered as differential expression if the p value is ≤0.001 and the fold change is ≥2. Volcano plots and heatmaps are generated by using Ggplot 2 and Pheatmap, respectively. KEGG pathway enrichment analysis is conducted by using Phyper. Enrichment of gene is considered as significance when p values are ≤0.05.
Detection of cytosolic DNA damages by RMn-NB-sensitized RT
To assess cytosolic DNA damages, CT26 cells are seeded in glass-bottom cell culture dishes (NEST, 20 mm) at a density of 2 × 105 cells per dish and incubated with RMn-NBs (50 μM), followed with or without X-ray irradiation. After that, the treated CT26 cells are incubated with PicoGreen dsDNA Quantitation Reagent (diluted 1:200 with PBS, Yeasen, CHINA) for 10 min at 37 °C, followed by washing with PBS (0.1 M, pH = 7.4) and staining with DAPI (Beyotime, China).
cGAS-STING activation and IFN-β secretion by RMn-NB-sensitized RT
CT26 cells are grown for 24 h in 6-well plates at a density of 5 × 105 cells per well, and then treated with RMn-NBs (50 μM). After incubation for additional 24 h, X-ray irradiation (2 Gy × 3) is performed and RAW264.7 cells are added to co-incubate for another 24 h. To assay the activation of cGAS-STING pathway, the proteins of mixed cells are isolated by using a mixture of nondenatured Tissue/Cell Lysate Kit (Solarbio) with a broad-spectrum protease inhibitor cocktail (EDTA-free, BOSTER) and a broad-spectrum phosphatase inhibitor cocktail (EDTA-free, BOSTER). Subsequently, 40 µL of isolated samples is used for western blotting analysis to confirm the key proteins involved in the cGAS-STING pathway (p-STING, STING, p-IRF3, IRF3, and β-actin). To detect the production of IFN-β, the treated CT26 cells are further co-incubated with RAW264.7 cells for another 24 h. The supernatant is collected by centrifugation (5000 g × 10 min) to be used for determining the content of IFN-β by using an ELISA assay kit (Biolegend, USA)63.
Detection of CRT exposure
CT26 cells are seeded in glass-bottom cell culture dishes (NEST, 20 mm) at a density of 2 × 105 cells per dish. After incubation for 24 h, the cells are treated with RMn-NBs (50 μM) and incubated for additional 24 h, followed by X-ray irradiation (6 Gy × 1). After RT, the cells are further incubated for 4 h and then washed three times with PBS (0.1 M, pH = 7.4), which are stained with CRT-antibody (diluted 1:500 with 1% BSA, Abcam Rb mAb to Calreticulin [EPR3924], UK) for 1 h. Subsequently, the tumor cells are washed three times with PBS (0.1 M, pH = 7.4) and stained with Alexa Fluor 647-conjugated goat anti-rabbit IgG (H&L, diluted 1:200 with 1% BSA, Zen-Bioscience, China) for another 1 h at 37 °C. Finally, the tumor cells are further stained with DAPI (Beyotime, China). Immunofluorescence images are acquired by using an Olympus FV3000 CLSM (Japan) and analyzed with ImageJ software.
Measurement of HMGB1 and ATP releases
Following the manufacturer’s protocol, the concentration of HMGB1 in the cytoplasm is measured by using an ELISA kit (Dakewe Biotech Co., Ltd) after subjecting tumor cells to different treatments. The concentration of ATP in the tumor cell supernatants after the specified treatment is determined by using ATP detection kit (Beyotime, China). Luminescence and absorbance are recorded by using a Microplate reader (VICTOR® Nivo, USA).
Gating strategy and methodology of FCM
All of FCM experiments are carried out with similar gating strategy. Forward Scatter (FSC) and Side Scatter (SSC) dot maps are established during the running process, and the voltage is adjusted to ensure that all events are visible on the dot maps. Gating is then performed to select events with appropriate FSC (200-600) and SSC (200-600) values, while events with low FSC/low SSC and low FSC/high SSC are excluded as they represent cell debris and air bubbles. Specific cell types are gated using fluorescently labeled antibodies, including DCs (CD80+ and CD86+ gated on CD11c+), CD4+ T cells (CD3+ and CD4+), CD8+ T cells (CD3+ and CD8+), OVA-specific DCs (CD11c+ H2Kb-SIINFEKL+), TAMs (F4/80+ and CD11b+), TEM (CD44+ CD62L− in CD3+ CD8+).
In vivo DCs maturation
To assess DCs maturation in TDLNs, TDLNs are harvested after different treatments (Saline, Saline + RT, RMn-NBs, and RMn-NBs + RT) for 5 days. The collected TDLNs are then processed to obtain single-cell suspensions by digestion and filtration through nylon mesh filters. Afterwards, the isolated cells are stained with PE/Cyanine7 anti-mouse CD11c, PE anti-mouse CD86, and APC anti-mouse CD80 antibodies (BioLegend, USA) for 30 min. FCM with BD Calibur is employed to detect and analyze the stained cells, providing valuable insights into the maturation status of DCs in the TDLNs.
ISCV of RMn-NB-sensitized RT
To investigate the function of RMn-NBs in eliciting the abscopal effect of RT, CT26 mouse model bearing both primary and distant tumors is established. CT26 cells are injected subcutaneously with 5 × 105 cells in the right and left lower flanks to form primary and distant tumors, respectively. After 10 days, CT26 tumor-bearing mice with the average tumor size of ~100 mm3 for primary tumors and ~20 mm3 for distant tumors are randomly divided into five groups: Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively). X-ray irradiation is performed after intravenous injection of RMn-NBs for 6 h, and treatments are performed with 3 fractions (total 6 Gy) on days 0, 3, and 6. αPD-L1 (10 mg kg−1 × 4 with fractions delivered 3 days apart, Clone: 10 F.9G2, BioXcell, USA) or Ultra-LEAFTM Purified αCD8a (10 mg kg−1 × 4 with fractions delivered 3 days apart, Clone: 53–6.7, BioLegend, America) are treated via intraperitoneal injection after RT for 6 h. Both primary and distant tumors are collected for analysis at day 18. To accurately assess the infiltration of immune cells, fresh tumor tissues are dissociated into single cells by using an enzyme mixture containing neutral protease (3 mg mL−1), collagenase II (6 mg mL−1) and hyaluronidase (75 μg mL−1). FCM analysis is performed after staining the cells with APC anti-mouse CD3, PE anti-mouse CD4, Alexa Fluor® 488 anti-mouse FoxP3, and FITC anti-mouse CD8a antibodies (BioLegend, USA). The IFN-γ secreted by active CD8+ T cells is detected by using ELISA assay kits (Dakewe Biotech Co., Ltd.) following the manufacturer’s instructions25.
IHC staining of CD4+ and CD8+ T cells infiltration
To further confirm the infiltration of CD4+ and CD8+ T cells after RMn-NB-sensitized RT, tumor tissues from various groups are harvested on the 18th day after treatment for IHC staining of CD4+ and CD8+ T cells. Firstly, paraffin sections of tumor tissues are incubated with primary anti-CD4 or anti-CD8 antibodies (Abcam, USA) overnight in a wet box at 4 °C. Subsequently, HRP-conjugated secondary antibodies are incubated with the sections marked by anti-CD4 or anti-CD8 antibodies for 60 min at 37 °C in darkness. Chromogenic reagents (Diaminobenzidine and Hematoxylin solutions) are then applied for nucleus staining of the above sections. IHC images are captured by using Nikon Eclipse Ti microscope (Japan) and analyzed with ImageJ Software.
Enhanced radioimmunotherapy with RMn-NBs in B16F10-OVA tumor models
The B16F10-OVA tumor-bearing mice with a tumor volume of 80–100 mm3 are classified into the following groups: Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively). X-ray irradiation is performed after intravenous injection of RMn-NBs for 6 h, and treatments are performed with 3 fractions (total 6 Gy) on days 0, 2, and 4. αPD-L1 (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 10 F.9G2, BioXcell, USA) or Ultra-LEAFTM Purified αCD8a (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 53–6.7, BioLegend, America) are administered via intraperitoneal injection after RT for 6 h. Tumors are collected for analysis at day 10. To accurately assess the infiltration of immune cells, fresh tumor tissues are dissociated into single cells by using an enzyme mixture containing neutral protease (3 mg mL−1), collagenase II (6 mg mL−1), and hyaluronidase (75 μg mL−1). FCM analysis is performed after staining the cells with APC anti-mouse CD3, PE anti-mouse CD4, FITC anti-mouse CD8a, PE anti-mouse CD3, APC anti-mouse/human CD44, PerCP/Cyanine5.5 anti-mouse CD62L, PE anti-mouse F4/80, PerCP/Cyanine5.5 anti-mouse/human CD11b antibodies (BioLegend, USA).
Antigen presentation of B16F10-OVA
To detect the impact of RMn-NBs + RT treatment on specific antigen presentation, B16F10-OVA subcutaneous tumor model is established on C57BL/6 mice. Initially, 5 × 105 B16F10-OVA cells are subcutaneously injected into the mice. When the average tumor size in the B16F10-OVA tumor-bearing mice reaches ~100 mm3 after 7 days, the mice are grouped randomly into five categories: Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively). X-ray irradiation is performed after intravenous injection of RMn-NBs for 6 h, and treatments are performed with 3 fractions (total 6 Gy) on day 0, 2 and 4. αPD-L1 (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 10 F.9G2, BioXcell, USA) or Ultra-LEAFTM Purified αCD8a (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 53–6.7, BioLegend, America) are administered via intraperitoneal injection after RT for 6 h. On day 10, TDLNs are harvested and processed into single-cell suspensions. The obtained cells are stained with FITC-CD11c and APC-H2Kb-SIINFEKL antibodies (BioLegend, USA) for 30 min. Subsequently, these cells are analyzed through FCM using the BD FACS Calibur system (USA)63.
IFN-γ ELISpot assay
To assess the effects of RMn-NBs + RT treatment in promoting antigen-specific CD8+ T cell activation, we establish B16F10-OVA subcutaneous tumor model upon C57BL/6 mice. B16F10-OVA (5 × 105) cells are subcutaneously injected into the left lower abdomen of C57BL/6 mice. When the average tumor size in B16F10-OVA tumor-bearing mice reaches 150–200 mm3, they are randomly divided into five groups: Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1 (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively). X-ray irradiation is performed after intravenous injection of RMn-NBs for 6 h, and treatments are performed with 3 fractions (total 6 Gy) on days 0, 2, and 4. αPD-L1 (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 10 F.9G2, BioXcell, USA) or Ultra-LEAFTM Purified αCD8a (10 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 53–6.7, BioLegend, America) are administered via intraperitoneal injection after RT for 6 h. Spleens are harvested for IFN-γ ELISpot assay (Dakewe Biotech, 2210005) on day 10. Spleens are grinded and lysized into single-cell suspensions by ACK lysis solution and filtering through a nylon mesh. For IFN-γ ELISpot assay, the pre-coated plates are activated by coculturing with 200 μL of 1640 culture medium supplemented with penicillin/streptomycin and 10% FBS for 10 min on the first day. Culture medium is then removed and spleen cells are seeded into plate with a density of 4 × 105 per well. Additionally, 10 μL of OVA peptide solution (50 ng mL−1) is added into each well. The culture plate is cultured overnight under 37 °C and 5% CO2. After 24 h, the chromogenic reaction is performed according to the manufacturer’s protocol63.
Long-time prophylactic vaccination in vivo
The CT26 cells are treated with Saline + RT and Saline + RT (the doses of Mn2+ and RANa are 5.5 mg kg−1 and 30.5 mg kg−1, respectively). After irradiation (0 or 6 Gy × 1) for 4 h, CT26 cells are collected and frozen in liquid nitrogen for 12 h. Subsequently, the cell fragments (3 × 105 cells per mouse) are inoculated into the left abdomen of healthy mice, and three consecutive immunizations are performed on days 0, 3, and 6, respectively. Mice are inoculated with untreated CT26 cells (3 × 105 cells per mouse) on the contralateral abdomen on day 9, and tumor growth is recorded over the following period.
Long-time inhibition of 4T1 tumor metastasis
To evaluate the efficacy of RMn-NB-sensitized RT in inhibiting 4T1 tumor metastasis, a female 4T1 tumor-bearing mouse model is established. Subcutaneous injection of 4T1 cells (5 × 105 cells per mouse) is performed in the right lower flank to induce tumor growth. After 10 days, when the tumor size reaches 80–100 mm3, the 4T1 tumor-bearing mice are randomly divided into 5 groups for different treatments: Saline, Saline + RT, RMn-NBs + RT, RMn-NBs + RT + αCD8a and RMn-NBs + RT + αPD-L1. The doses of Mn2+ and RANa in corresponding treatments are 5.5 mg kg−1 and 30.5 mg kg−1, respectively. Treatments are performed with 3 fractions (total 6 Gy) on days 0, 3 and 6. αPD-L1 (10.0 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 10 F.9G2, BioXcell, USA) or Ultra-LEAFTM Purified αCD8a (10.0 mg kg−1 × 3 with fractions delivered 3 days apart, Clone: 53–6.7, BioLegend, America) are administered via intraperitoneal injection after RT for 6 h. Tumor volumes and body weights of the treated mice are recorded daily in the period of 30-day treatment. After treatment, tumors in situ and surrounding epidermis are removed and sutured. The survival status and body weight of treated mice are recorded for a total of 150 days.
Statistics and reproducibility
No statistical method is used to predetermine sample size. No data are excluded from the analyses. The experiments are randomized and the investigators are not blinded to allocation during experiments and outcome assessment. Statistical analysis is performed by using two-tailed Student’s t test for two groups and one-way ANOVA analysis of variance for multiple groups. p > 0.05 represent nonsignificance and p < 0.05 represent statistically significant, *p < 0.05, **p < 0.01, ***p < 0.001. Error bars indicate the standard deviation (S.D.). Tumor size and weight of tumor-bearing mice are recorded with WPS Office 13.33.0. CasaXPS 2.3.25 is used to analyze XPS data. MDI Jade 6 is used to analyze XRD data. Gatan Digital Micrograph is used to analyze HRTEM. GraphPad Prism Version 9.0.2 is used to analyze statistical data. FlowJo (Version 10.8.1) is used to analyze FCM data. CaseViewer 2.4.0 and ImageJ Version 1.52 v are used to analyze immunofluorescent and immunochemical data. The pharmacokinetic parameters of RANa are analyzed by DAS 2.1.1 software. Figures 1b, 9a and Supplementary Fig. 20a, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en. The minimum value of the conformational energy (MM2) of (pyridin-3-ylmethyl) phosphonate and risedronate is calculated by Chem 3D 20.0. http://www.cloud.biomicroclass.com/CloudPlatform is used to analyze transcriptomic data and map.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (BioProject: PRJCA024822 and GSA: CRA019291) that are publicly accessible at https://bigd.big.ac.cn/gsa/browse/CRA019291. The authors declare that the remaining data supporting the findings of this study are available within the Article, and its Supplementary Information files. Source data are provided with this paper.
References
Petroni, G. et al. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat. Rev. Clin. Oncol. 19, 114–131 (2022).
Galluzzi, L. et al. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 20, 543–557 (2023).
Ngwa, W. et al. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 18, 313–322 (2018).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022).
Chen, J. et al. In situ cancer vaccination using lipidoid nanoparticles. Sci. Adv. 7, eabf1244 (2021).
Zhang, Y. et al. Multifunctional nanoparticle potentiates the in situ vaccination effect of radiation therapy and enhances response to immune checkpoint blockade. Nat. Commun. 13, 4948 (2022).
Guo, S. et al. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Sig. Transduct. Target. Ther. 8, 205 (2023).
Kirchhammer, N. et al. Combination cancer immunotherapies: emerging treatment strategies adapted to the tumor microenvironment. Sci. Transl. Med. 14, eabo3605 (2022).
Suwa, T. et al. Tumor microenvironment and radioresistance. Exp. Mol. Med. 53, 1029–1035 (2021).
Rankin, E. B. et al. Hypoxic control of metastasis. Science 352, 175–180 (2016).
Singleton, D. C. et al. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Semenza, G. et al. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Xie, T. et al. Vitexin, an inhibitor of hypoxia-inducible factor-1α, enhances the radiotherapy sensitization of hyperbaric oxygen on glioma. Clin. Transl. Oncol. 22, 1086–1093 (2020).
Timke, C. et al. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin. Cancer Res. 14, 2210–2219 (2008).
Fallah, J. et al. HIF inhibitors: status of current clinical development. Curr. Oncol. Rep. 21, 6 (2019).
Krysko, D. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Duong, E. et al. Type I interferon activates MHC class I-dressed CD11b+ conventional dendritic cells to promote protective anti-tumor CD8+ T cell immunity. Immunity 55, 308–323 (2022).
Perez-Shibayama, C. et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8+ T cells. Sci. Immunol. 5, eabb7066 (2020).
Zitvogel, L. et al. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Samson, N. & Ablasser, A. The cGAS-STING pathway and cancer. Nat. Cancer 3, 1452–1463 (2022).
Kwon, J. & Bakhoum, S. F. The Cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 10, 26–39 (2020).
Reker, D. et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. 16, 725–733 (2021).
Liu, C. et al. Drug self-delivery systems: molecule design, construction strategy, and biological application. Adv. Healthc. Mater. 12, 2202769 (2023).
Sun, X. et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021).
Huang, Z. et al. Nanoscale coordination polymers induce immunogenic cell death by amplifying radiation therapy mediated oxidative stress. Nat. Commun. 12, 145 (2021).
Huang, Z. et al. Zoledronic acid-gadolinium coordination polymer nanorods for improved tumor radioimmunotherapy by synergetically inducing immunogenic cell death and reprogramming the immunosuppressive microenvironment. ACS Nano 15, 8450–8465 (2021).
Wang, C. et al. Maintaining manganese in tumor to activate cGAS-STING pathway evokes a robust abscopal anti-tumor effect. J. Control Release 331, 480–490 (2021).
Tang, X. et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1α/VEGF signaling pathways in human breast cancer cells. Int. J. Cancer 126, 90–103 (2010).
Yuena, T. et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc. Natl Acad. Sci. USA 111, 17989–17994 (2014).
Wang, C. et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48, 675–687 (2018).
Lv, M. et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 30, 966–979 (2020).
Ndiaye, D. et al. Mn2+ Bispidine complex combining exceptional stability, inertness, and MRI efficiency. J. Am. Chem. Soc. 144, 22212–22220 (2022).
Leroi, G. E. et al. Infrared spectra of gaseous transition-metal dihalides. J. Chem. Phys. 36, 2879–2883 (1962).
Errassifi, F. et al. Infrared, Raman and NMR investigations of risedronate adsorption on nanocrystalline apatites. J. Colloid Interf. Sci. 420, 101–111 (2014).
Foord, J. S. et al. An X-ray photoelectron spectroscopic investigation of the oxidation of manganese. Philos. Mag. A 49, 657–663 (1984).
Sandomierski, M. et al. A long-term controlled release of the drug for osteoporosis from the surface of titanium implants coated with calcium zeolite. Mater. Chem. Front. 5, 5718–5725 (2021).
Yang, Y. et al. Buffering the local pH via single-atomic Mn-N auxiliary sites to boost CO2 electroreduction. Chem. Sci. 13, 13172–13177 (2022).
Satoh, A. Y. et al. Methylene blue dye test for rapid qualitative detection of hydroxyl radicals formed in a Fenton’s reaction aqueous solution. Environ. Sci. Technol. 41, 2881–2887 (2007).
Yang, G. et al. Ultrathin cobalt-manganese nanosheets: an efficient platform for enhanced photoelectrochemical water oxidation with electron-donating effect. Adv. Funct. Mater. 29, 1904622 (2019).
Perkinson, C. F. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA 115, 4465–4470 (2018).
Boni, L. et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 29, 2046–2055 (2015).
Geraldes, C. F. G. C. et al. Mn(III) porphyrins as potential MRI contrast agents for diagnosis and MRI-guided therapy. Coord. Chem. Rev. 445, 214069 (2021).
Haas, B. et al. Advancing RNA-Seq analysis. Nat. Biotechnol. 28, 421–423 (2010).
Palazon, A. et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32, 669–683 (2017).
Hopfner, K. P. et al. Molecular mechanisms and cellular functions of cGAS- STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Li, S. et al. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity 35, 647–648 (2011).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Gajewski, T. et al. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Rogers, M. J. et al. Bisphosphonates induce apoptosis in mouse macrophage-like cells in vitro by a nitric oxide-independent mechanism. J. Bone Miner. Res. 11, 1482–1491 (1996).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019).
Kaech, S. M., Wherry, E. J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Tsien, C. I. et al. NRG Oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus Bevacizumab alone as treatment for recurrent glioblastoma. J. Clin. Oncol. 41, 1285–1295 (2023).
Salimu, J. et al. Cross-presentation of the oncofetal tumor antigen 5T4 from irradiated prostate cancer cells-A key role for heat-shock protein 70 and receptor CD91. Cancer Immunol. Res. 3, 678–688 (2015).
Meloun, M. et al. Thermodynamic dissociation constants of risedronate using spectrophotometric and potentiometric pH-titration. Cent. Eur. J. Chem. 10, 338–353 (2012).
Covitz, F. & Westheimer, F. H. The hydrolysis of methyl ethylene phosphate: Steric hindrance in general base catalysis. J. Am. Chem. Soc. 85, 1773–1777 (1963).
Bott, G., Field, L. D. & Sternhell, S. Steric effects. A study of a rationally designed system. J. Am. Chem. Soc. 102, 5618–5626 (1980).
Pinter, B. et al. On the origin of the steric effect. Phys. Chem. Chem. Phys. 14, 9846–9854 (2012).
Fang, J., Islam, W. & Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 157, 142–160 (2020).
Subramaniam, A. T. et al. Development and validation of RP-HPLC/UV methods for the estimation of Risedronate sodium in pure and pharmaceutical dosage form. J. Pharm. Chem. 6, 5–8 (2021).
Sauter, M. et al. Protocol to isolate and analyze mouse bone marrow derived dendritic cells (BMDC). STAR Protoc. 3, 101664 (2022).
Grosjean, C. et al. Isolation and enrichment of mouse splenic T cells for ex vivo and in vivo T cell receptor stimulation assays. STAR Protoc. 2, 100961 (2021).
Huang, Z. et al. Chiral coordination polymer nanowires boost radiation-induced in situ tumor vaccination. Nat. Commun. 15, 3902 (2024).
Acknowledgements
We acknowledge the Natural Science Foundation of Jiangsu Province-Major Project (No. BK20212012, L. Wang), the National Natural Science Foundation of China (No. 22275096, Z. Luo; 62288102, W. Huang; 52002396, L. Wang; 32222090, Y. Dai and 32171318, Y. Dai), the Multi-Year Research Grant (MYRG) of University of Macau (No. MYRG2022-00011-FHS, Y. Dai), the Macao Young Scholars Program (No. AM2023029, Z. Huang), the Young Scientists Fund of the National Natural Science Foundation of China (No. 82404567, Z. Huang), the Young Scientists Fund of the Natural Science Foundation of Jiangsu Province (No. BK20240648, Z. Huang), the Key Research and Development Program of Jiangsu (No. BE2018732, L. Wang), Natural Science Key Fund for Universities in Jiangsu Province (No. 22KJA430007, Z. Luo), General project of natural science research in colleges and universities of Jiangsu Province (No. 22KJB350001, Z. Huang), Natural Science Research Start up Foundation of Recruiting Talents of Nanjing University of Posts and Telecommunications (No. NY221133, Z. Huang), and the Project of State Key Laboratory of Organic Electronics and Information Displays, Nanjing University of Posts and Telecommunications (No. GZR2022010033, Z. Huang). Special thanks to Professor Lixing Weng for providing genome-wide RNA expression sequencing analysis.
Author information
Authors and Affiliations
Contributions
Z. Huang, Y. Dai, Z. Luo, and L. Wang conceive the project, design the studies and analyze the results. Z. Huang, S. Huang, S. Song, Y. Ding, H. Zhou, S. Zhang, L. Weng, and Y. Zhang carry out the experiments. Z. Huang, Y. Dai, Z. Luo, and L. Wang write the manuscript. Y. Hu, A. Yuan, Y. Dai, Z. Luo, and L. Wang supervise and revise the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kaiting Yang, Jintao Zheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Z., Huang, S., Song, S. et al. Two-dimensional coordination risedronate-manganese nanobelts as adjuvant for cancer radiotherapy and immunotherapy. Nat Commun 15, 8692 (2024). https://doi.org/10.1038/s41467-024-53084-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53084-w
This article is cited by
-
Synergistic strategies for glioblastoma treatment: CRISPR-based multigene editing combined with immune checkpoint blockade
Journal of Nanobiotechnology (2025)
-
Enhancing radiotherapy-induced anti-tumor immunity via nanoparticle-mediated STING agonist synergy
Molecular Cancer (2025)