Abstract
Three-dimensional structured illumination microscopy (3D-SIM) provides excellent optical sectioning and doubles the resolution in all dimensions compared with wide-field microscopy. However, its much lower axial resolution results in blurred fine details in that direction and overall image distortion. Here we present 4Pi-SIM, a substantial revamp of I5S that synergizes 3D-SIM with interferometric microscopy to achieve isotropic optical resolution through interference in both the illumination and detection wavefronts. We evaluate the performance of 4Pi-SIM by imaging various subcellular structures across different cell types with high fidelity. Furthermore, we demonstrate its capability by conducting time-lapse volumetric imaging over hundreds of time points, achieving a 3D resolution of approximately 100 nm. Additionally, we illustrate its ability to simultaneously image in two colors and capture the rapid interactions between closely positioned organelles in three dimensions. These results underscore the great potential of 4Pi-SIM for elucidating subcellular architecture and revealing dynamic behaviors at the nanoscale.
Similar content being viewed by others
Main
Studying the behavior of living cells in three dimensions over time is essential in cell biology to provide a dynamic perspective on cellular processes and unveil fundamental elements that underpin life itself. Fluorescence microscopy is a widely used tool in this regard, owing to its molecular specificity and compatibility with living samples. However, its resolution is limited by the diffraction of light, preventing clear discernment of cellular features smaller than 200 nm. The emergence of various super-resolution techniques has substantially improved the achievable resolution of fluorescence microscopy, thus opening new avenues for studying nanoscale subcellular structures and providing valuable biological insights1,2,3. Among them, structured illumination microscopy (SIM) has several advantages over its peers, such as compatibility with conventional fluorophores, low phototoxicity and high imaging speed4,5,6,7,8,9,10,11,12,13,14.
In spatial frequency space, SIM enhances resolution by convolving the illumination optical transfer function (OTF), which is composed of delta functions located at the spots corresponding to the frequencies in the illumination pattern, with the detection OTF support, which sets the conventional resolution limit in frequency space. To further enhance the resolution of SIM, one can increase the illumination frequencies, expand the detection OTF support, or both (Extended Data Fig. 1). If only one objective is used for both illumination and detection, however, the detection OTF support is fundamentally constrained by the numerical aperture (NA) of the objective. Thus, previous efforts in such scenarios have mainly focused on increasing the highest frequencies in the illumination pattern. For example, three-dimensional SIM (3D-SIM) used three-beam interference to generate the highest illumination frequencies allowed by the objective’s NA in both the lateral and axial directions, effectively doubling the resolution (~120 nm laterally and ~300 nm axially) in each dimension, compared with widefield (WF) microscopy6,7,15,16,17. Four-beam SIM further enhanced the axial resolution to ~160 nm by retroreflecting the central illumination beam in 3D-SIM using a mirror, thus generating four-beam interference and producing illumination patterns with higher axial frequencies, although its resolution remains anisotropic18,19. This is understandable because, as illustrated in Extended Data Fig. 1, increasing the illumination frequencies alone without axially expanding the detection OTF support is insufficient for obtaining an isotropic resolution.
The most effective way to expand the detection OTF support, aside from radical changes in objective design, is by using multiple objectives in the form of an interferometer to simultaneously detect the same fluorescence wavefront from multiple angles. The axial resolution of such interferometric approaches, including 4Pi confocal20,21 and I5M microscopy22,23, referred to hereafter as 4Pi microscopy, has surpassed the lateral resolution by using two opposing objectives to combine interference in both illumination and detection. The integration of 4Pi microscopy with SIM led to the development of I5S, resulting in an isotropic 100-nm resolution in all three dimensions using oil-immersion objectives (1.4 NA)24. The greatly improved resolution isotropy of I5S can be attributed to three factors: (1) the interference of six beams, three from each objective, produces more higher-frequency components and finer illumination patterns in the axial direction; (2) the interference in fluorescence detection expands the detection OTF support axially; and (3) the use of partially coherent illumination further broadens and evens the OTF support in the axial direction (Extended Data Fig. 1 and Supplementary Fig. 1). However, despite being the only SIM technique offering isotropic optical resolution, the application and impact of I5S have been limited by its high complexity and incompatibility with living samples. To date, I5S has been demonstrated as a proof of concept only on fixed cellular samples in one color24, and its full potential in live-cell imaging has yet to be explored.
Here, we present 4Pi-SIM, a revamped implementation of I5S that expands its imaging capabilities to two-color live-cell imaging. Through imaging a variety of biological samples, we demonstrate that 4Pi-SIM achieves a 3D isotropic resolution of 100 nm using water-immersion objectives (1.27 NA), enabling the visualization of subcellular structures with excellent clarity and detail. In addition, we showcased its capabilities by conducting time-lapse volumetric imaging of living cells for more than 300 time points, with an acquisition speed reaching 0.7 Hz. Furthermore, we illustrated the feasibility of simultaneous two-color imaging using one excitation wavelength, allowing the examination of rapid interactions between organelles in close proximity. These results highlight the great potential of 4Pi-SIM for advancing the understanding of dynamic processes in living cells.
Results
Development and characterization of 4Pi-SIM
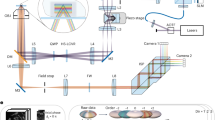
4Pi-SIM achieves isotropic 3D resolution by using interference between six illumination beams and between the fluorescence wavefronts detected by the two objectives (Fig. 1a). The interferometric nature of 4Pi-SIM renders it susceptible to thermal fluctuations, vibrations and refractive-index mismatch. Previous implementations of 4Pi single-molecule super-resolution microscopes have demonstrated that sufficient stability can be achieved to consistently attain a sub-20-nm 3D resolution on cellular samples25,26,27. We therefore incorporated the techniques behind these accomplishments into the development of the 4Pi-SIM microscope (Extended Data Figs. 2 and 3, Supplementary Figs. 2 and 3, and Supplementary Video 1) and made substantial improvements over the original I5S. Unlike the original I5S, our system features a symmetrical optical layout (Extended Data Fig. 2), with the core optomechanical components mounted on a single vertical aluminum plate (Extended Data Fig. 3), which minimizes the influence of thermal fluctuations and mechanical vibrations in the interferometric cavity (hereafter referred to as the 4Pi cavity) of the microscope. In addition, water-immersion objectives were used instead of oil-immersion objectives to match the refractive index of aqueous mounted samples, effectively reducing phase errors. Furthermore, we have completely reengineered the optical path length difference (OPLD) adjustment module to enable rapid and precise fine-tuning of the OPLD to near zero (Extended Data Fig. 4). This module is essential for producing the maximum axial fringe contrast in 4Pi-SIM. Finally, we have implemented a focus-lock module to ensure that the objectives remain precisely aligned in three dimensions throughout the imaging process (Supplementary Figs. 4 and 5). These design refinements have enabled stable imaging under optimal conditions over extended periods.
a, Simplified schematic of 4Pi-SIM (top). The illumination beams are shown in blue, and the fluorescence emission is shown in green. Six partially coherent illumination beams interfere at the sample plane. Two fluorescence emission beams are coherently combined at the NPBS to generate two phase-complementary images (the xy and xz cross-sections are shown). In the frequency ___domain, the illumination pattern of one lateral SIM orientation consists of 19 illumination components (bottom left), and the detection OTF extends axially (bottom middle). Convolution of the two components yields an effective 4Pi-SIM OTF for one lateral SIM orientation (bottom right). OBJ1 and OBJ2, objectives 1 and 2; L1–L4, lens 1 through lens 4; M1 and M2, mirrors 1 and 2; initial phase, φ0; π, constant phase difference between the two phase-complementary interference images. b, A lateral cross-section image of a field of 100-nm beads vertically divided into three sub-fields, showing the difference in lateral resolution of WF (top), 3D-SIM (middle) and 4Pi-SIM (bottom). c, Axial cross-sections along the vertical dashed line in b, showing the difference in axial resolution. d, Experimentally measured OTFs of WF (top), 3D-SIM (middle) and 4Pi-SIM (bottom). e, Axial intensity profiles of the bead highlighted by arrowheads in c, demonstrating the optical sectioning capability of 3D-SIM and 4Pi-SIM and the improved axial resolution. a.u., arbitrary units. f, Resolution quantification with 100-nm fluorescent beads. The FWHMs were measured on 150 beads in 3 independent experiments for each imaging mode. WF: 246.7 ± 4.3 nm laterally, 561.6 ± 10.9 nm axially; 3D-SIM: 107.7 ± 4.7 nm laterally, 322.9 ± 16.9 nm axially; 4Pi-SIM: 108.1 ± 4.0 nm laterally, 102.2 ± 4.6 nm axially. g, Resolution quantification with immunolabeled microtubules in fixed COS-7 cells. The FWHMs were measured at 75 locations in 3 independent experiments for each imaging mode. WF: 254.5 ± 18.7 nm laterally, 584.5 ± 29.1 nm axially; 3D-SIM: 105.4 ± 7.5 nm laterally, 320.7 ± 17.6 nm axially; 4Pi-SIM: 104.4 ± 5.0 nm laterally, 101.7 ± 6.2 nm axially. In f and g, statistical data are presented as mean ± s.d. Scale bars, 500 nm (a,c); 1 µm (b); 1/200 nm–1 (d). Whiskers: 75% and 25% percentiles; centerlines: medians. All cross-section slices are 30 nm thick.
The non-polarizing beamsplitter (NPBS) in the 4Pi cavity separates the three SIM excitation beams into six beams that pass through two objectives and that overlap and interfere with each other upon reaching the sample (Extended Data Fig. 2). Generating excitation patterns with high modulation contrast is crucial for SIM. A key step in enhancing the modulation contrast is to maintain s-polarization (that is, linearly polarized perpendicular to the plane of incidence) for the six input beams. This can be achieved using a combination of a high-speed liquid crystal variable retarder (HS-LCVR) and a quarter-wave plate (QWP)7 (Extended Data Fig. 5a). However, we observed that the large number of optical components in the illumination beam path retards the phase of s- and p-polarized light unequally. Consequently, linearly polarized light with orientations other than s- or p-polarization was degraded to elliptical polarization after reflection by multiple mirrors, especially at large incident angles, before reaching the sample plane. To address this issue, we aligned the fast axis of the HS-LCVR with the s-polarization of the mirrors and repositioned the QWP behind the last mirror, setting its fast axis at 45° relative to the s-polarization (Extended Data Fig. 5b). This configuration allowed the HS-LCVR to compensate for the phase difference introduced by the mirrors, effectively altering the polarization of the illumination light. To enhance HS-LCVR functionality, we incorporated a half-wave plate (HWP) to rotate the linearly polarized incident light by 45° with respect to the s-polarization.
As in 3D-SIM and I5S, 4Pi-SIM requires image reconstruction from raw images, which involves inverse filtering using an experimentally measured OTF (Extended Data Fig. 6). Generally, the experimental OTF is obtained by imaging a fluorescent bead at an OPLD of zero. To ensure successful 4Pi-SIM reconstruction, it is imperative to acquire raw images under conditions identical to those of the experimental OTF (that is, zero OPLD). Failing to do so would lead to a mismatched OTF during reconstruction, producing axial ringing artifacts. However, consistently maintaining the OPLD between two beam paths at zero is challenging, owing to air perturbations and thermal drift. One possible solution is to add extra infrared light for real-time monitoring and adjustment of the OPLD, as previously reported24. However, this method adds complexity to an already complicated system. We thus adopted a previously developed approach that handles this issue during post-processing, alleviating the stringent requirements on the OPLD28. The underlying principle is that, when fluorescence signals collected by the 4Pi cavity are combined interferometrically at the NPBS, the interference fringes exiting the two exit ports of the NPBS are phase-complementary28. The OPLD can be computationally estimated from these two images and incorporated into the subsequent reconstruction process (Extended Data Fig. 6 and Supplementary Fig. 6). To implement this approach, we reconfigured the detection pathway to simultaneously capture two complementary images at distinct regions on the camera (Extended Data Fig. 2). Additionally, this approach requires measuring the detection OTF when there is no illumination interface, or in the ‘I2M’ (image interference microscopy) mode23. For this purpose, we implemented a new I2M illumination module that enabled us to acquire an I2M OTF in a time-interleaved manner (Extended Data Fig. 2 and Supplementary Fig. 7). Subsequently, we synthesized a library of 4Pi-SIM OTFs corresponding to various OPLDs by convoluting the phase-modulated 4Pi-SIM illumination spectrum with the phase-modulated I2M OTF, which can be easily obtained by multiplying the complex phase factor by the sidebands of the I2M OTF, as described above. Finally, during image reconstruction, the synthetic OTF whose OPLD was closest to the estimated OPLD from the raw images was selected from the library for reconstruction. Although this approach has been previously demonstrated on a single fluorescent bead28, this study extends its application to both fixed and living biological samples (Extended Data Fig. 7 and Supplementary Fig. 8).
To assess the resolution of our 4Pi-SIM microscope, implementing the aforementioned improvements, we imaged 100-nm fluorescent beads and measured their full-width at half-maximum (FWHM) (Fig. 1b–f). A total of 15 images per plane (5 phases × 3 orientations) were captured, with an exposure time of 10 ms and an axial step size of 40 nm. For comparison, we also conducted 3D-SIM imaging on the same regions immediately after 4Pi-SIM, using an exposure time of 10 ms and a step size of 120 nm. As expected, the axial resolution of 3D-SIM (322.9 ± 16.9 nm, mean ± s.d., n = 150, where n refers to the number of beads or sub-regions in the microtubule images used for each statistic) was approximately twofold better than that of WF microscopy (561.6 ± 10.9 nm, mean ± s.d., n = 150), and the axial resolution of 4Pi-SIM (102.2 ± 4.6 nm, mean ± s.d., n = 150) was threefold better than that of 3D-SIM (Fig. 1f and Supplementary Table 1). The experimental OTFs of both 3D-SIM and 4Pi-SIM verified the improvement in the measured resolution (Fig. 1d). Next, we imaged immunolabeled microtubules in fixed COS-7 cells and measured the sizes of the tubules (axial value: 101.7 ± 6.2 nm, mean ± s.d., n = 75) (Fig. 1g, Supplementary Fig. 9 and Supplementary Table 1). The results confirmed that 4Pi-SIM maintained the same level of resolution in the biological samples.
4Pi-SIM achieved isotropic optical resolution in fixed cells
To demonstrate the capabilities of 4Pi-SIM, we first imaged the outer membrane of mitochondria in fixed HeLa cells (Fig. 2a and Supplementary Video 2). 4Pi-SIM successfully delineated the interconnected contour of the mitochondrial network, whereas 3D-SIM generated axially elongated outlines owing to its inferior axial resolution. The hollow nature of the outer mitochondrial membrane was completely obscured in WF microscopy (Fig. 2b).
a, 4Pi-SIM image (maximum intensity projection) of the immunolabeled mitochondrial outer membrane in a fixed HeLa cell (Supplementary Video 2). The inset shows the x–y cross-section at z = 2.64 μm. Color represents depth from the coverslip in all panels except m. b, The y–z and x–z cross-sections along the two dashed lines in a for 3D- and 4Pi-SIM. c, 4Pi-SIM image (maximum intensity projection) of immunolabeled vimentin in a fixed COS-7 cell (Supplementary Video 3). d, The y–z cross-sections along the dashed line in c for each imaging mode. e, Intensity axial profiles along the dashed line in d for WF, 3D- and 4Pi-SIM. f, 4Pi-SIM image (maximum intensity projection) of immunolabeled βII-spectrin in a fixed hippocampal neuron (Supplementary Video 4). g, Magnified cross-sections of the boxed region lableled ‘g’ in f for 3D- and 4Pi-SIM. The dashed lines indicate the planes of the axial sections. h, Magnified x–y cross-section at z = 1.62 μm of the boxed region labeled ‘h’ in f. i, Intensity profile along the dashed line in h. j, Fourier transform of the one-dimensional intensity distribution in i, showing a periodicity of 194 nm. k, A 3D rendering of the immunolabeled synaptonemal complex (SYCP3) in a fixed mouse spermatocyte imaged with 4Pi-SIM (Supplementary Video 5). The inset shows the maximum intensity projection in the x–y view. l, The y–z cross-sections along the white dashed line in k for 3D- and 4Pi-SIM. The white arrowheads indicate the double-line structures resolved in 4Pi-SIM but not in 3D-SIM. m, The x–y and y–z cross-sections of the boxed region in k, merged 4Pi-SIM (magenta) and 3D-SIM (green). Scale bars: 5 μm (c,f); 2 μm (a, projection of k, l); 1 μm (b,d); and 500 nm (g,h,m). The thickness of all cross-section slices is 30 nm. Representative results are shown from three to five independent experiments.
Next, we next imaged immunolabeled vimentin in fixed COS-7 cells, a more challenging sample (Fig. 2c and Supplementary Video 3). As a type III intermediate filament, vimentin forms a highly intricate fiber network that spans the entire cell. Using 4Pi-SIM, we could effectively distinguish the interwoven filaments, whereas with 3D-SIM, the different filaments were frequently misidentified as axially connected structures (Fig. 2d,e). These results highlight the significance of the isotropic resolution provided by 4Pi-SIM, particularly in analyzing complex 3D structures.
To further evaluate the applicability of 4Pi-SIM to a wider range of cytoskeletal proteins, we imaged cultured primary neurons immunolabeled with antibodies to βII-spectrin, a component of the membrane-associated periodic skeleton (Fig. 2f,g and Supplementary Video 4). 4Pi-SIM revealed that βII-spectrin forms a periodic structure with a spacing of 194 nm, consistent with a previous report29 (Fig. 2h–j).
We next assessed the performance of 4Pi-SIM using thicker samples by imaging the synaptonemal complexes in mouse spermatocyte nuclei in the pachytene phase of meiotic prophase, through the immunolabeling of the SYCP3 scaffold protein (Fig. 2k and Supplementary Video 5). 4Pi-SIM successfully revealed the twisted double-line feature of the SYCP3 substructures, regardless of their orientation or depth. By contrast, 3D-SIM erroneously merged the two chromosomes when aligned axially (Fig. 2l,m), highlighting 4Pi-SIM’s superior ability to preserve structural integrity across multiple dimensions.
Revealing subcellular structures in the secretory pathway
The isotropic 3D resolution of 4Pi-SIM has notable advantages in cell biology. To illustrate its ability to accurately depict intricate 3D subcellular architecture, we specifically imaged three important structures within the secretory pathway: the endoplasmic reticulum (ER), the Golgi apparatus and the plasma membrane (PM).
The ER is the primary site for protein synthesis and is the largest membranous structure in cells30,31. 4Pi-SIM accurately recaptured the complex tubular network of the ER in both the peripheral and perinuclear regions in fixed COS-7 cells (Fig. 3a–c and Supplementary Video 6). Notably, ER tubules spaced 132 nm or 182 nm apart axially were well resolved with 4Pi-SIM, but merged into one structure with 3D-SIM (Fig. 3b).
a, 4Pi-SIM image (maximum intensity projection) of immunolabeled ER in a fixed COS-7 cell (Supplementary Video 6). b, Left: magnified 3D rendering of the boxed region labeled ‘b’ in a. Right: the y–z and x–z cross-sections along the corresponding dashed lines, taken in the 3D- and 4Pi-SIM modes. The arrowheads indicate the positions where the axial intensity profiles were taken. The distances between peaks were obtained after fitting with multiple Gaussian functions. c, Left: magnified 3D rendering of the boxed region denoted ‘c’ in a. Right: the y–z and x–z cross-sections along the corresponding dashed lines, taken in the the 3D- and 4Pi-SIM modes. d–f, 3D renderings of the immunolabeled Golgi apparatus in fixed HeLa cells imaged with 4Pi-SIM (Supplementary Video 7). In d–f, antibodies labeled the cis-Golgi (GM130), medial-Golgi (giantin) and trans-Golgi (Golgin97). Insets: maximum intensity projection in the x–y view. Bottom: magnified cross-sections of 3D- and 4Pi-SIM along the dashed lines in the corresponding insets. g, A 3D rendering of the immunolabeled plasma membrane (WGA-Alexa Fluor 488) in a fixed HeLa cell imaged with 4Pi-SIM (Supplementary Video 8). h, Maximum intensity projection of g in the x–y view, and cross-sections along the dashed lines denoted (1), (2) and (3). Colors represent depth from coverslip, denoted by the color bars. Scale bars: 5 μm (a; projection in d, e, f, h); 1 μm (b (1,2), c (1,2), d(1,2), e (1,2), f (1,2), h (1,2,3)). The thickness of all cross-section slices is 30 nm. Representative results are shown from three to six independent experiments.
After leaving the ER, proteins are transported to the Golgi apparatus for further modification and packaging into vesicles. We imaged the Golgi apparatus in fixed HeLa cells by immunolabeling three proteins that represent different Golgi cisternae: cis-localized GM130, medial-localized giantin and trans-localized Golgin97 (Fig. 3d–f and Supplementary Video 7). Despite being components of the Golgi complex, 4Pi-SIM elucidated their distinct morphological and structural features: GM130 appeared as layered sheets, giantin appeared as rims and Golgin97 displayed discrete vesicle-like structures. These fine details along the axial direction were obscured when using 3D-SIM (cross-sections in Fig. 3d–f).
Finally, the vesicles from the Golgi apparatus are transported to the PM through dedicated cytoskeletal networks, wherein their contents are released into the extracellular space. We imaged the PM in fixed HeLa cells using fluorescently labeled wheat germ agglutinin (WGA) (Fig. 3g and Supplementary Video 8). The PM formed many thin membrane protrusions, typically 100–300 nm in diameter, called filopodia. 4Pi-SIM clearly resolved the hollow structures of these filopodia in both the lateral and axial directions. By contrast, such features of the filopodia were not resolved in the axial views of 3D-SIM (Fig. 3h).
Time-lapse isotropic-resolution imaging in living cells
By using water-immersion objectives that minimize spherical aberrations for aqueous mounted cell samples, we could challenge 4Pi-SIM for time-lapse volumetric isotropic imaging at 100-nm resolution in living cells—a formidable task for any super-resolution approach, owing to the need for high resolution, high imaging speed and low sample tolerance to phototoxicity and photobleaching. With 4Pi-SIM, these challenges are even more severe because (1) the number of raw images was increased by threefold compared with 3D-SIM, owing to the increase in axial resolution by the same factor, which decreases the overall imaging speed and worsens the phototoxicity and photobleaching situations; and (2) maintaining the long-term stability of the 4Pi cavity of 4Pi-SIM is not easy to achieve. Fortunately, our mechanical design ensured long-term preservation and stabilization of the interference pattern, and the focus-lock module guaranteed precise alignment of the two objectives. Moreover, our reconstruction algorithm accurately estimated the OPLD from the two complementary images and incorporated it into data analysis to produce images with minimal artifacts (Extended Data Fig. 7).
We first imaged mitochondria in live HeLa cells by co-expressing two outer membrane proteins, TOM20 and OMP25, each fused to the highly photostable fluorescent protein, oxStayGold32 (Fig. 4a and Supplementary Video 9). 4Pi-SIM clearly depicted the shell of the mitochondrial membrane and captured its dynamic behaviors (Fig. 4b). We examined a specific region of the membrane and observed the reorganization of a rod-shaped mitochondrion into a structure resembling a concave disk (Fig. 4c). Previous studies have reported that mitochondria can form tubular protrusions called mitochondrial nanotunnels33,34,35. The observed cell displayed numerous tubulation events, wherein a tubular mitochondrion generated nanotunnels from both ends, resulting in a reduction in diameter from approximately 600 nm to 150 nm (Fig. 4d). Furthermore, we visualized the dynamic fusion and fission processes of mitochondria (Supplementary Fig. 10).
a, 4Pi-SIM image (maximum intensity projection) of the mitochondrial outer membrane (TOM20-oxStayGold and oxStayGold-OMP25) in a live HeLa cell (Supplementary Video 9). b, An oblique view (–7 degrees along the z axis) of the boxed region denoted ‘b’ in a. The x–z and y–z cross-sections were along the dashed color lines in a. c, Time-lapse magnified views of the boxed region denoted ‘c’ in a, showing that a mitochondrion transitioned from rod-shaped to concave-disc-shaped. Right at each time point, corresponding x–z and y–z cross-sections along the colored dashed line. d, Left: time-lapse magnified views of the boxed region denoted ‘d’ in a, showing the mitochondria generating nanotunnels from both ends (dashed outline). Right: corresponding x–z cross-sections at positions labeled with colored arrowheads. e, 4Pi-SIM image (maximum intensity projection) of the microtubules (oxStayGold-Ensconsin) in a live COS-7 cell (Supplementary Video 10). f, Left: 3D rendering of the boxed region labeled ‘f’ in e. Right: time-lapse x–y and y–z cross-sections (along the microtubule denoted by the arrowheads), showing the growth of a microtubule whose tip is labeled with arrowheads. g, 4Pi-SIM image (maximum intensity projection) of the actin filaments (Lifeact-mStayGold) in a live COS-7 cell (Supplementary Video 11). h, Time-lapse magnified views of the boxed region labeled ‘h’ in g, showing that actin puncta structures that formed (colored arrowheads) and disappeared (dashed circles). i, Top: time-lapse magnified views of the boxed region labeled ‘i’ in g, showing that the bundle of actin filaments in the filopodia is redistributed and spread. Bottom: the x–z cross-sections along the dashed line. j, Left: time-lapse magnified views of the boxed region denoted ‘j’ in g, showing the retraction and merging of the actin filaments. Right: the y–z cross-sections at positions denoted by the arrowheads. Colors represent depth, denoted by the color bars. Scale bars: 2 μm (a,b,e,g); 1 μm (c,f,h,i); 1 μm (left) and 500 nm (right) (d,j). The thickness of all cross-section slices is 60 nm in f and 30 nm in all other panels. Representative results from four to six independent experiments are shown.
Next, we visualized the dynamics of the cytoskeleton in live COS-7 cells. Using oxStayGold-Ensconsin, a microtubule-binding protein, we observed the growth of a microtubule in the crowded yet organized tubular network (Fig. 4e,f and Supplementary Video 10). Additionally, by expressing Lifeact-mStayGold36 in the cells, we could capture the diverse remodeling and reorganization of actin filaments using 4Pi-SIM (Fig. 4g and Supplementary Video 11). Notably, several actin puncta structures formed and disappeared at the bottom of the cell (Fig. 4h), likely correlating with endocytosis events, as has been reported37. Furthermore, the dense bundle of actin filaments in the filopodia underwent rapid redistribution, transforming into loose, spreading structures (Fig. 4i). We also observed the retraction of actin filaments, in which two small bundles merged to form a larger bundle (Fig. 4j).
Revealing the dynamic behavior of the ER in living cells
In addition to its intricate network structure, the ER exhibits remarkable dynamism in living cells and undergoes continuous remodeling in three dimensions. However, capturing this dynamic nature poses a considerable challenge to live-cell imaging methods. In previous studies, various super-resolution techniques were used to visualize ER remodeling, albeit mostly in two dimensions31,38,39. With the exceptional spatial–temporal resolution of 4Pi-SIM, we aimed to dissect the structural dynamics of the ER isotopically in three dimensions.
We visualized the ER dynamics in COS-7 cells for hundreds of time points by co-expressing oxStayGold-Sec61β and oxStayGold-KDEL (Fig. 5a and Supplementary Video 12). We observed two distinct behaviors in specific areas in which the ER exhibited sheet-like structures. In the first scenario, a small hole appeared in the ER sheet and gradually expanded, evidenced by the abrupt disruption of the continuous ER membrane from the axial perspective (Fig. 5b). In the second scenario, the edges of the ER sheets shrank, leading to their transformation into tubular structures (Fig. 5c). In another region, we observed the formation of ER nanoholes (Fig. 5d), consistent with a previous report31. In a different cell, we visualized the formation of ER-sheet-like structures (Fig. 5e and Supplementary Video 13). These sheets could either originate from existing small sheets (Fig. 5f) or arise progressively from individual tubules (Fig. 5g).
a, Left: 4Pi-SIM image (maximum intensity projection) of the ER (oxStayGold-Sec61β and oxStayGold-KDEL) in a live COS-7 cell (Supplementary Video 12). Right: magnified views of the yellow boxed region at 8,462 s and 15,824 s, respectively. b–d, Top: time-lapse magnified views of the boxed regions denoted in a, showing that a small hole appeared and spread within the ER sheet (b), that an ER sheet transformed into tubules (c) and that an ER nanohole formed (d). Bottom: the x–z cross-sections at positions denoted by the arrowheads. e, Left: 4Pi-SIM image (maximum intensity projection) of ER in a live COS-7 cell. Right: magnified views of the yellow boxed region at 0 s and 9,084 s (Supplementary Video 13). f,g, Top: time-lapse magnified views of the boxed regions denoted in e, showing the formation of ER sheets either extending from pre-existing small sheets (f) or individual tubules (g). Bottom: the x–z cross-sections at positions denoted by the arrowheads. h, 4Pi-SIM image (maximum intensity projection) of ER at a volume rate of 0.7 Hz (Supplementary Video 14). i, Left: time-lapse magnified views of the boxed regions denoted in h, showing the retraction of an ER tubule. Right: the y–z cross-sections at positions denoted by the arrowheads. j,k, Top: time-lapse magnified views of the boxed regions denoted in h, showing the growth of ER tubules as they form stable connections (j) or tri-junctions (k) with other tubules. Bottom: the x–z cross-sections at positions denoted by the arrowheads. Color represents depth denoted by the color bars. Scale bars: 2 μm (a,e,h); 1 μm (b–d,f,g,j,k); and 500 nm (i). The thickness of all cross-section slices is 30 nm. Representative results are shown from four to six independent experiments.
To capture high-speed events, we used a 1-ms exposure time to image the ER, resulting in a volume rate of 0.7 Hz, covering a 1.2-μm thickness over 300 time points (Fig. 5h and Supplementary Video 14). In the captured images, we observed rapid growth of ER tubules beneath the ER sheets (Fig. 5i,j). In one instance, a tubule underwent retraction (Fig. 5i); in another, it established a stable connection with a different tubule (Fig. 5j). In yet another region, a rapidly growing ER tubule fused with another tubule, resulting in the formation of tri-junctions (Fig. 5k).
Simultaneous two-color imaging with one excitation laser
Multicolor imaging is essential for studying organelle interactions, but achieving optimal performance for multiple colors simultaneously with 4Pi-SIM is not a trivial task. This is because of the axial offset between the illumination pattern maxima and the detection focal plane, which varies for each wavelength and can be minimized only for a specific wavelength by checking the illumination pattern focus (IPF)40. The presence of such an offset would cause axial artifacts in 4Pi-SIM. This problem can be solved by using multiple cameras or by physically moving the camera between each wavelength19. The former adds cost and complexity to the system; the latter introduces a noticeable delay (several seconds) between each color. To address this issue, we adopted a different strategy by using dCyOFP2s, a large-Stokes-shift fluorescent protein that can be efficiently excited at 488 nm and emits fluorescent signals at 592 nm (ref. 41). By combining dCyOFP2s with a green fluorescent protein, such as oxStayGold, and a 488-nm illumination laser, simultaneous two-color imaging can be achieved (Supplementary Fig. 11a). Compared with sequential methods that switch lasers and/or filter wheels, this approach reduces phototoxicity by using a single excitation laser and enables faster imaging. To implement this approach, we introduced a dichroic mirror in the emission beam path, which allows the separate projection of fluorescent signals from the two proteins onto distinct regions of the camera (Supplementary Fig. 11b). This allowed us to optimize the IPF for both channels simultaneously to achieve the best performance.
To evaluate the performance of our two-color imaging approach, we visualized the interaction between ER and two different organelles. By co-expressing dCyOFP2s-KDEL and oxStayGold-OMP25 in live HeLa cells, we documented frequent interactions between the ER and mitochondria at specific regions, referred to as ER–mitochondria contact sites (ERMCSs) (Fig. 6a and Supplementary Video 15). Notably, we identified one such site at which ER tubules contacted a nanotunnel of mitochondria (Fig. 6b). This observation aligns with a previous study reporting that the ER marks the initiation sites of nanotunnels35. Moreover, we observed instances of coordinated spatial fusion and fission of mitochondria at these contact sites (Fig. 6c,d). Furthermore, we conducted experiments by co-expressing dCyOFP2s-KDEL and oxStayGold-Ensconsin in live COS-7 cells, which revealed the intricate intertwining of the ER tubular networks and microtubules, effectively creating dynamic interconnections that spanned the cytoplasm (Fig. 6e,f and Supplementary Video 16). These spontaneous, rapid interactions facilitated the rearrangement of both organelles (Fig. 6g,h).
a, Two-color image (maximum intensity projection) of the ER (dCyOFP2s-KDEL, magenta) and mitochondria (oxStayGold-OMP25, green) in a live HeLa cell (Supplementary Video 15). b, Top: magnified x–y views of the boxed region in a at the indicated time points, showing the ER touching a mitochondrial nanotunnel (denoted by the arrowhead). Bottom: x–z cross-sections along the dashed lines. Right: y–z cross-sections at the colored dashed lines drawn in the 116-s x–y view. c,d, Top: magnified x–y views of the boxed regions in a at the indicated time points, showing a mitochondrial fusion (c) and fission (d) event. Arrowheads indicate the fusion and fission sites. Bottom: x–z cross-sections along the dashed lines. e, Two-color image (maximum intensity projection) of the ER (dCyOFP2s-KDEL, magenta) and microtubules (oxStayGold-Ensconsin, green) in a live COS-7 cell (Supplementary Video 16). f, The y–z cross-section of the dashed line in e. g,h, Top: magnified x–y views of the boxed regions in e at the indicated time points. Arrowheads indicate the contact sites (g) and the coordinated motion of the ER and microtubules (h). Bottom: x–z cross-sections along the dashed lines. Scale bars: 2 μm (a,e) and 1 μm (b–d,f–h). The thickness of all cross-section slices is 30 nm. Representative results are shown from two to four independent experiments.
Discussion
Although the concept of interferometric microscopy using two opposing objectives and 3D-SIM was first demonstrated experimentally long ago24, 4Pi-SIM further enhances its capabilities and vastly expands its application, from fixed samples to live-cell and two-color imaging, with a greatly improved design, as demonstrated above. In a series of biological experiments, we have comprehensively illustrated that 4Pi-SIM achieved a 3D isotropic optical resolution of approximately 100 nm in fixed and living cells. This exceptional combination of resolution and isotropy enables the detailed visualization of a wide range of subcellular structures, ranging from the cytoskeleton to various membrane structures and beyond (Supplementary Table 2), exemplifying the broad applicability of 4Pi-SIM in diverse research contexts.
In this study, live-cell imaging was conducted on cells sandwiched between two coverslips at room temperature. The typical duration at which cell viability was successfully maintained was approximately 5–7 h. To enhance the robustness and broad applicability of 4Pi-SIM for imaging living cells under physiological conditions, it is essential to use a live-cell perfusion chamber with temperature and CO2 regulation. This facilitates extended imaging sessions, potentially spanning a day or longer. However, maintaining zero OPLD over such prolonged periods presents substantial challenges, leading to diminished interference contrast and degraded image quality. To address this issue, it is crucial to implement an active-feedback strategy, whereby the OPLD is constantly monitored and adjusted on the basis of real-time analysis of the complementary images acquired from the two exit ports of the NPBS.
We have conducted time-lapsed volumetric imaging in living cells for hundreds of volumes using StayGold and its variants, which are the most photostable fluorescent proteins to date. To demonstrate that conventional fluorescent proteins can be used with 4Pi-SIM, we used monomeric enhanced green fluorescent protein (mEGFP) and mScarlet-H42 fused to Lifeact to label and visualize the dynamics of actin filaments in live COS-7 cells (Extended Data Fig. 8a–d); we successfully captured up to 100 and 70 volumes for each constructed fusion, respectively. Furthermore, we used the self-labeling HaloTag43 combined with the live-cell compatible organic dye JFX554 (ref. 44) to label ER in COS-7 cells and recorded up to 600 volumes (Extended Data Fig. 8e,f). These results indicate that 4Pi-SIM is compatible with a variety of conventional fluorophores for live-cell imaging. The continuing development of highly photostable fluorescent probes will further enhance 4Pi-SIM’s performance in multi-color live-cell imaging.
The performance of current two-color imaging approaches is constrained by the limited choice and inferior characteristics of long-Stokes-shift fluorescent proteins. To extend the multi-color imaging capability of 4Pi-SIM, we successfully imaged the ER, microtubules and mitochondria in fixed cells, which were immunolabeled using antibodies conjugated with Alexa Fluor 488 and Alexa Fluor 568 sequentially using two excitation lasers (Extended Data Fig. 9). The image quality in both channels was excellent. However, this approach is not suitable for live-cell imaging because (1) the process is too slow, because the relative positions of the objectives to the sample must be adjusted to re-align the illumination beams when switching lasers, owing to chromatic aberrations, and (2) the current optical design cannot capture two complementary images for each color simultaneously, preventing automatic estimation of the OPLD. To overcome these issues, one could used multiple cameras that enable each channel to be focused on the cameras simultaneously. Another approach is to use a high-speed filter wheel along with a fast-focusing device, such as an electrically tunable lens45, to adjust the focal position of each channel sequentially. This strategy can be extended to imaging more than two colors in both fixed and live cells.
Currently, 4Pi-SIM is limited to imaging samples that are ideally less than 10 μm thick, primarily for two reasons: (1) it employs WF illumination that causes the out-of-focus background to overwhelm the in-focus signal and elevate the shot noise, substantially reducing image contrast and high-frequency information and thereby degrading the reconstruction quality. Light-sheet illumination46,47 and other optical-sectioning approaches have the potential to remove the out-of-focus background and increase imaging depth. (2) The dual-objective interferometric imaging nature renders it sensitive to refractive index heterogeneities in biological samples, which generate various optical aberrations that distort the illumination patterns, consequently degrading image quality and introducing artifacts (Extended Data Fig. 10). For fixed samples, tissue clearing48 or expansion49 techniques are effective methods to mitigate optical aberrations. For living samples, the integration of adaptive optics50 represents a promising avenue to improve the performance of 4Pi-SIM when imaging deep within samples.
Although we focused on the optical resolution of 4Pi-SIM, many recent advances in image processing, such as prior-knowledge-based Hessian deconvolution10 and the sparse deconvolution algorithm based on sparsity and continuity51, can be utilized to enhance the image quality of 4Pi-SIM. Moreover, we anticipate that the live-cell imaging capability of 4Pi-SIM can be further improved by utilizing deep-learning-based methods, such as denoising techniques52,53,54,55 or resolution-enhancement neural networks19,53. However, predicting missing high-frequency information beyond the OTF support through deep learning to improve resolution is challenging and prone to artifacts, necessitating specialized network architectures and high-fidelity training data. With the capability to optically generate high-quality, high-resolution images, 4Pi-SIM offers a unique opportunity for designing and optimizing the performance of deep-learning approaches.
Time-lapse volumetric isotropic super-resolution imaging in living cells has been a long-standing goal in cell biology. Limited by the NA of the objective, virtually all single-objective super-resolution microscopes produce an anisotropic resolution. Owing to the excellent axial resolution of interferometric microscopy, it has been successfully combined with nearly all super-resolution imaging methods, leading to the development of techniques such as I5S (ref. 24), 4Pi-SMLM25,26,27,56,57,58, 4Pi-STED59,60,61 and 4Pi-RESOLFT62. Among these techniques, to the best of our knowledge, live-cell imaging has previously been demonstrated only with 4Pi-RESOLFT, albeit with extremely low acquisition speeds (>100 min per cell) and for only a few time points, making it practically unsuitable for imaging dynamic behaviors in living cells. In comparison, the 4Pi-SIM technique demonstrated here represents a remarkable advancement in live-cell super-resolution imaging, thanks to its capability of high-quality volumetric imaging of living cells for hundreds of time points at a 3D isotropic 100-nm resolution. Thus, 4Pi-SIM holds great potential for elucidating subcellular dynamic behaviors at the nanoscale.
Methods
4Pi-SIM microscope
The detailed optomechanical design of the 4Pi-SIM microscope is shown in Extended Data Figs. 2 and 3, and it comprises the following main modules.
Illumination module
The module is illustrated in Extended Data Fig. 2. We used a 488-nm laser (Sapphire 488 nm LPX-500 mW, Coherent) as the light source; its power was modulated through an acousto-optical tunable filter (AOTF, AOTFnC-400.650-TN, AA Opto-Electronic). The laser beam was coupled into a multimode fiber (MMF, FG105LVA fiber core, Thorlabs, 0.9 mm sleeve, 10 m long) using a coupling lens (PAF2P-A10A, Thorlabs). A mechanical shaker (P1350/DES/001, Cairn Research) was attached to the MMF to adequately fill the mode space of the fiber and smooth out the speckle pattern of the laser output from the MMF due to spatial coherence. The diverging beam from the MMF output end was collimated by an objective (CFI Plan Apochromat Lambda D 2×, Nikon). Owing to the constraint of the pupil plane position of the objective, a pair of relay lenses L1 and L2 (f = 100 mm, PAC052AR.14, Newport) was added to maintain a perfect 4f system so that the fiber tip could be eventually projected onto the pupil plane of the imaging objectives. The beam was then redirected onto a phase-diffraction grating6,12 composed of a polarizing beam splitter (PBS, CCM1-PBS251/M, Thorlabs), an HWP (HWP1, AHWP25-VIS-A-M, LBTEK) and a ferroelectric liquid crystal spatial light modulator (SLM, QXGA-3DM, Forth Dimension Displays), which is capable of switching between a set of preprogrammed binary pixel patterns within a few milliseconds. The beam was diffracted by the grating on the basis of the pixel patterns on the SLM, which in our case were designed to generate a one-dimensional diffraction pattern. The diffracted beams were then refocused by the lens L3 (f = 300 mm, ACT508-300-A, Thorlabs), and a custom mask was placed at its focal plane to block any undesired diffraction orders and stray light. After passing through two more relay lenses (L4 (f = 180 mm, AC508-180-A, Thorlabs) and L5 (f = 200 mm, PAC064AR.14, Newport)) and being reflected off several mirrors and a custom dichromatic mirror (DM1, ZT405/488/561/640-phaseR, Chroma, polarization maintenance at wavelength (λ = 405, 488, 561 and 640 nm), the illumination beams entered the 4Pi cavity through a custom NPBS (50/50 BS cube, Chroma, polarization maintenance for λ = 450–750 nm, reflective and transmission rate 47.5% ± 5%, mean ± bound). The lens pairs L6–L8 (f = 125 mm PAC055AR.14 and f = 250 mm PAC067AR.14, Newport) and L7–L9 (identical to L6–L8) refocused each half of the split beams onto the back focal planes of the two water-immersion objectives OBJ1 and OBJ2 (CFI SR Plan Apo IR 60XC WI, ×60/1.27 NA, Nikon), respectively, and the six beams finally met and interfered at the sample plane to generate the desired illumination pattern. The objectives were mounted on an objective tower designed to divide the degrees of freedom between the objectives: XY adjustment for the lower objective by a 2D piezoelectric stage (P-733.2CD, Physik Instrumente) and Z adjustment for the upper objective by a linear piezoelectric stage (N-565.260, Physik Instrumente). The sample stage comprised a 2D (U-751.24, Physik Instrumente) and a linear (P-733.ZCD, Physik Instrumente) piezoelectric stage, enabling precise XY and Z positioning of the sample, respectively.
Polarization rotation module
To maximize the contrast of illumination pattern in SIM and thus optimize its resolution-extension capacity, SIM systems require a polarization rotation module to preserve the s-polarization of the illumination beams for all lateral SIM orientations6,7,12,15. For this purpose, both DM1 (at 405, 488, 561 and 640 nm) and NPBS (at 450–750 nm) were custom-made by Chroma Technology to maintain the polarization of the reflected light. However, DM2 was not custom-made. We found that transmission through DM2 notably alters the polarization state, constituting the primary source of the phase shift. Moreover, there are three dielectric mirrors (M4, M5, M6 and M7) in the illumination beam path, which introduce a noticeable amount of phase shift. Last, although silver mirrors effectively preserve the polarization state of p- or s-polarized light, they are unable to do so for arbitrary polarizations, particularly when several mirrors are involved. Consequently, the reflections or transmission of these optical elements could introduce unequal phase retardance between the s- and p-polarizations, resulting in elliptically polarized illumination light at the sample plane and thus reducing the modulation contrast (Extended Data Fig. 5a). To address this issue, we designed a new mechanism that can compensate for the unequal phase retardance experienced by the s- and p-polarizations (Extended Data Fig. 5b). An HWP (HWP2, AHWP25-VIS-A-M, LBTEK) rotated the polarization to 45° relative to the s-polarization before an HS-LCVR (HLC-200-VIS, Meadowlark Optics) with its fast axis at 0°. The QWPs (QWP1 and QWP2, AQWP25-VIS-A-M, LBTEK) were positioned at a 45° angle to the s-polarization of incident light and placed immediately before the objectives and after all mirrors. Owing to the symmetrical design of the 4Pi cavity and the custom NPBS that maintains the polarization between the reflected and transmitted light, the polarization of the illumination beams at the sample in both beam paths can be fine-tuned to the desired s-polarization by simply adjusting the HS-LCVR voltage.
Fluorescence-detection module
The module is illustrated in Extended Data Fig. 2. Fluorescence was collected simultaneously by the two objectives and then coherently combined at the NPBS. The OPLD within the 4Pi cavity could be adjusted either coarsely or finely using the OPLD module (see below). In addition, two electronic shutters (04RDI232, CVI Laser Optics) allowed for convenient manipulation of the light paths by toggling them on and off, facilitating observation and adjustment. For single-color imaging, phase-complementary interference images from both exit ports of the NPBS (see below) were relayed toward the imaging plane through a pair of lens triplets: L10–L12–L15 (f = 125 mm, PAC055AR.14, Newport; f = 125 mm, 47717, Edmund; f = 170 mm, DLB-30-170PM, OptoSigma, respectively) and L11–L13–L14 (identical to L10–L12–L15). A knife-edge right-angle prism protective silver mirror (PM3, MRAK25-P01, Thorlabs) directed the final images on two separate regions of an sCMOS camera (CAM1, ORCA-Fusion, C14440-20UP, Hamamatsu). A single-band filter (F1) (ET525/50m, Chroma) was mounted directly in front of the camera to ensure proper filtering.
4Pi-SIM uses more optical components (such as mirrors, lenses and dichroic mirrors) in the fluorescence-detection path than does a conventional 3D-SIM microscope. On the basis of the flatness information of the optics provided by the manufacturers, we performed simulations in Zemax and confirmed that the wavefront distortion was minimal and did not affect the performance of 4Pi-SIM (Supplementary Fig. 2). This was validated by numerous experimental data. Using the 552-nm laser of the I2M illumination, we experimentally measured a fluorescence loss of approximately 30%, excluding the transmission efficiency of the objective.
For two-color imaging, we used a single 488-nm laser to excite both normal (oxStayGold) and large-Stokes-shift (dCyOFP2s) fluorescent proteins (Supplementary Fig. 11b). To implement this strategy, a customized dichroic mirror DM5 (T565lpxr-5 mm, Chroma, anti-reflective coating for λ = 450–700 nm, flatness ≤ λ / 8) was introduced after L12 and L13 to separate fluorescence with different spectra. The upper detection path, equipped with a red filter F2 (ET600/50m, Chroma), collected red fluorescence; the lower path, equipped with a green filter F3 (ET525/50m, Chroma), collected green fluorescence (Supplementary Fig. 11a). Compared with using two excitation sources, this approach allowed us to capture two colors simultaneously in the same exposure time as in single-color detection and facilitated concurrent optimization of axial illumination contrast for both detection channels.
I2M illumination module
The module is illustrated in the lower left corner of Extended Data Fig. 2. A 473-nm laser (OBIS 473 nm LX-75 mW, Coherent) was coupled into a single-mode fiber (SMF1, PMJ-D-460, Thorlabs) through an AOTF. The laser beam was then collimated by lens L16 (f = 30 mm, AC254-030-A-ML, Thorlabs) and was directed through objective OBJ2 after passing through lens L17 (f = 400 mm, 49369, Edmund). This beam was reflected by a dichroic mirror DM3 (ZT473/546/633/700/780rpc, Chroma), providing WF illumination on the sample. The resulting fluorescence images (or I2M images), containing only the detection interference fringes, were then collected by the main camera (CAM1) and used later for OPLD estimation and compensation (see below).
Focus-lock module
The module is illustrated in Extended Data Fig. 2 (upper-left corner) and Supplementary Fig. 5. A 785-nm laser (OBIS 785 nm LX-100 mW, Coherent) was coupled into a single-mode fiber (SMF2, PMJ-D-630, Thorlabs). The laser beam exited from SMF2 and was collimated by lens L18 (f = 30 mm, AC254-030-AB-ML, Thorlabs), and then directed towards the pupil plane of OBJ1 through reflection from a dichroic mirror DM2 (ZT473/546/633/700/780rpc, Chroma), and passed through two objectives. The recollimated beam leaving OBJ2 was reflected by another dichroic mirror DM3 and focused by lens L17 and a cylindrical lens CY1 (LJ1144RM-B, Thorlabs) onto a CMOS camera CAM2 (UI-3240LE-NIR-GL, IDS). A short-pass dichroic mirror DM4 (ZT775sp-2p, Chroma) was used to differentiate the beams for I2M and focus-lock. This module was sensitive to the misalignment of the optical system. Any drift in the XY direction of the two objectives resulted in a lateral shift of the laser spot on camera CAM2. In addition, any drift in the Z direction altered the shape of the captured light spot owing to the astigmatism introduced by CY1. The position and shape variations of the light spot provided real-time feedback for the XY translation stage of the lower objective and the Z translation stage of the upper objective, enabling accurate compensatory adjustments of these stages. This feedback mechanism played a critical role in ensuring long-term stability, precise alignment and optimized imaging performance over extended periods.
OPLD adjustment module
Maximizing axial fringe contrast is crucial for the optimal performance of 4Pi-SIM, which requires precise adjustment of the OPLD between the two beam paths towards zero. In previous 4Pi-SMS microscopes, the entire sample stage was moved vertically using a pair of motorized stages to manipulate the OPLD25,26,27. However, this approach proved to be slow, unstable and unable to guarantee a zero OPLD owing to the insufficient resolution of the motorized stages. To address this issue, we designed a new OPLD-adjustment module consisting of a fine-tuning translation submodule (FTS) and a coarse-tuning translation submodule (CTS) located in the upper and lower optical arms, respectively (Extended Data Fig. 4). The two submodules shared a similar design: a compact optical delay line consisting of a fixed knife-edge right-angle prism protective silver mirror (PM1 and PM2, MRAK25-P01, Thorlabs) and a pair of silver mirrors (47717, Edmund) mounted on a translation stage (Extended Data Fig. 4b,c). However, there is a slight difference in the translation stage between the two. The CTS comprised a long-travel linear stage (9066-COM-M, Newport) and a Picomotor piezo linear actuator (8301NF, Newport), providing a 12.7 mm travel range and sub-μm resolution (Extended Data Fig. 4b,d). The CTS was used for coarsely adjusting the OPLD to be within 1 μm. By contrast, the FTS featured a linear piezoelectric stage (P621.1CD, Physik Instrumente) with a 100-μm travel range and sub-nm resolution (Extended Data Fig. 4c,e). The FTS allowed for precise tuning the OPLD towards zero.
To generate fluorescence interference, the OPLD within the 4Pi cavity must be less than the coherence length, which is typically a few micrometers. To achieve this, we moved the stage in the CTS (Extended Data Fig. 4d) at a speed of 1.5 μm s–1 and recorded the average intensity fluctuation of 20 fluorescent beads in the same view after ensuring that the images formed by both objectives overlap on the main camera. This brought the OPLD of the 4Pi cavity within the coherence length to allow fluorescence interference. Subsequently, we fine-tuned the FTS (Extended Data Fig. 4e) to precisely identify the optimal position of constructive fluorescence interference. The coarse tuning of the OPLD was necessary only during the initial alignment of the microscope. For daily fine-tuning, we relied on only the FTS, which facilitated rapid, precise adjustments. This approach guaranteed the adjustment of the OPLD without compromising the stability of the entire system.
Field of view
The field of view (FOV) of 4Pi-SIM is largely determined by the performance of the objectives, primary aberrations and magnification. By imaging fluorescence beads and examining their PSFs (point spread functions), we selected a pair of objectives exhibiting minimal aberrations with a magnification difference of 0.1%. To determine the FOV for fluorescence interference, we imaged fluorescence beads using the I2M illumination, which has a much larger illumination area. We found that the FOV is ~90 μm in diameter. However, owing to the need to project two images onto the same camera, the illumination FOV for 4Pi-SIM was designed to be ~60 μm in diameter. Under these conditions, the effective FOV for 4Pi-SIM is ~50 μm, comparable to the typical FOV of 3D-SIM (30–60 μm).
Structured illumination generation
The 4Pi-SIM system necessitates a polarization rotation module to preserve the s-polarization of the illumination beams across all three orientations (See Extended Data Fig. 5b). Specifically, for this study, orientations of 37°, 97° and 157° were used. An excitation angle of 60° was chosen to fill the gap in the 4Pi-SIM OTF, and the grating period was calculated accordingly. The grating constant was converted into periodicity on the SLM (pixel size = 8.2 µm). As the non-integer values were allowed in the chosen orientations, we adopted grating periods T488 = 10.31 pixels and T560 = 11.83 pixels, for 488 nm and 560 nm excitation wavelength, respectively. By modulating the grating duty cycle to 30%, the intensity of the diffraction order 0 was set to 75% of that of order ±1. This unequal intensity ratio slightly enhanced the highest lateral frequency spectrum15,19. Partially coherent illumination was introduced to slightly broaden the axial component of the OTF (see Supplementary Fig. 1)6,15. The fiber end was projected onto the back pupil plane with a diameter of 8.3% of the pupil’s diameter.
4Pi-SIM system alignment
The optical path alignment of the 4Pi-SIM is similar to that of a previously published protocol for 4Pi-SMS27. Like the 4Pi-SMS system, the 4Pi-SIM system was designed with either lateral (bottom objective) or axial (top objective) translational adjustments, but without any angle adjustment freedom for either objective, owing to stability concerns. The co-axial alignment of the two objectives was achieved through dedicated optomechanical design, precision machining and careful alignment. However, unlike 4Pi-SMS, 4Pi-SIM requires a near-perfect 4f system for optimal imaging quality. This involved conjugating the SLM to the sample plane, conjugating the sample plane to the camera plane and conjugating the fiber end to the pupil plane of the objective. By doing so, we could ensure the optimal alignment of the illumination z-modulation with the focal plane. We used the Fiji plugin ‘SIMcheck’ to visualize the axial stripes (that is, IPF) of a sample made of a thin layer of fluorescent beads40. A symmetrical pattern should be observed when six beams interfere with the best contrast at the focal plane (Supplementary Fig. 4a). Any misalignment in either the lateral or axial direction will result in a degraded axial stripe (Supplementary Fig. 4b).
For single-color imaging (488 nm/525 nm, or 560 nm/600 nm, excitation wavelength/emission wavelength), the lenses L14 and L15 were positioned to ensure that the two complementary images were simultaneously focused on the camera (Extended Data Fig. 2). In the case of two-color imaging in living cells using a single excitation laser (488 nm), the position of L15 (488 nm/525 nm) remained unchanged, whereas L14 was adjusted so that the image with constructive interference for 488 nm/600 nm was in focus on the camera (Supplementary Fig. 11). In the case of two-color imaging in fixed cells using two excitation lasers (488 nm and 560 nm), L14 was adjusted to focus the image with constructive interference for 560 nm/600 nm on the camera. Furthermore, the objectives were manually adjusted when switching lasers to align the illumination beams, compensating for the focal shift caused by chromatic aberrations.
For imaging biological samples, a minimal amount of fluorescent beads was introduced to the sample, to aid in alignment. Before imaging the target area, we positioned the sample stage to a nearby region containing fluorescent beads. These sparsely distributed beads served as fiducial markers to align the two objectives both laterally and axially. Then, the FTS was used to maximize fluorescence interference contrast and thus minimize the OPLD, before finally turning on the focus-lock module and repositioning the sample stage to the target area. This approach proved capable of achieving both precise alignment and minimal photobleaching during imaging preparation.
Hardware control and data acquisition
The image acquisition process for 4Pi-SIM followed the principles established for conventional 3D-SIM, with the primary difference being the reduction of the focal step from 120 nm to 40 nm to satisfy the Nyquist criterion. The 4Pi-SIM acquisition system was built around an NI PXIe-1083 chassis with an NI PXIe-7867 FPGA module inserted and programmed using LabVIEW 2022 (National Instruments) to ensure precise timing. The timing diagram of the system is shown in Supplementary Fig. 3. The SLM’s ‘LED_EN’ TTL output signal, with ON indicating that the desired pixel pattern was properly displayed, served as the primary trigger. The SLM was programmed using a series of binary pixel patterns, specifying both the patterns to be displayed and their durations. The onboard microcontroller ensured the switching and timing of displayed patterns and sent out the ‘LED_EN’ TTL signal, which then triggered the camera exposures and the AOTF to modulate the illumination. The change in the voltage input to the HS-LCVR was initiated after detecting five falling edges of the SLM TTL output, indicating the completion of one illumination orientation. Once the falling edge count reached 15, marking the end of the application of one cycle of illumination patterns on one focal plane, the sample z-stage moved to the next focal plane by a predefined step size. Once all planes were imaged, a reset signal was sent to the z-stage, returning it to its initial position.
For volumetric time-lapse imaging, we programmed a custom time interval between the acquisition of consecutive volumes. During this intermediate time, the camera, laser, HS-LCVR and sample stage were not triggered. The procedure for two-color imaging was identical to that for single-color imaging, because only one illumination wavelength was used.
For the acquisition of experimental OTFs, only five patterns in a single orientation were necessary, with a constant HS-LCVR voltage optimal for that orientation. After adjusting the system’s OPLD to zero, we captured an 8-μm image stack with a step size of 40 nm (120 nm for 3D-SIM) on a 100-nm fluorescent bead located near the center of the field of view.
To generate OTFs with different OPLDs, an additional I2M image was captured under I2M illumination using a 473-nm laser after acquiring the normal 4Pi-SIM images at each focal plane (Supplementary Fig. 7b).
Optical-path-length difference estimation
Maintaining the OPLD of the 4Pi cavity at zero during imaging is challenging owing to air perturbation and thermal drift. Capturing images with non-zero OPLDs while reconstructing them under the assumption of a zero OPLD creates a discrepancy that manifests as axial ringing artifacts in the reconstructed images. To address these challenges, we extended a previously reported post-processing strategy28 to enable the estimation of and compensation for OPLD, even in complicated biological samples.
The principle of OPLD estimation for 4Pi-SIM is illustrated in Supplementary Fig. 6, in which a and b denote the unknown phase values due to OPLD for excitation and detection, respectively. It is based on the observation that the phases of the two detection interference fringes exiting the two ports of the NPBS are in theory π shifted or complementary28. This means that, after performing the Fourier transform (FT) on the detected fluorescence interference signals from both exits, the phases of the axially extended sidebands obtained from one exit should be π different from the phases obtained from the other exit. In other words, adding those two FT spectra would remove the sidebands, whereas subtracting one from the other would remove the center band. This relation between the two detection interference fringes holds in 4Pi-SIM, and if the two phase-complementary image stacks are registered and summed, all sidebands derived from those of the I2M OTF would be removed, thus greatly simplifying the FT spectrum (Supplementary Fig. 6i). In this simplified FT spectrum, the overlap between the order-0 and order-1 SIM bands, above region 3 and below region 6, should contain the information related to the OPLD (that is, phase a). However, this information cannot be extracted, because it is mixed with the sample’s phase (that is, phase s) and the initial phase (that is, phase φ0) of the illumination pattern (Supplementary Fig. 6j). Fortunately, the overlap shares the same sample phase once the band is shifted to the correct position. And the residual initial phase can be derived simply by removing φ0 estimated from the overlap 4 and 5. All phases are estimated by a linear regression method. Furthermore, the fluorescence phase b can be simply inferred from the wavelength relationship b = a × λex / λem, where λex and λem represent the excitation and emission wavelengths, respectively.
4Pi-SIM OTF library generation
To compensate for the estimated OPLD and optimize image reconstruction, it was necessary to synthesize a 4Pi-SIM OTF matching the OPLD present in the data, which is possible because a 4Pi-SIM OTF can be obtained by convolving an I2M detection OTF with a 4Pi-SIM illumination OTF28, provided that the I2M detection OTF can be measured experimentally.
For this purpose, we designed a new I2M illumination scheme that utilizes a slightly different excitation wavelength, as opposed to a previously reported scheme using a 20:80 (transmission:reflectance) mirror28. In this design (Supplementary Fig. 7a), a dual-laser setup was used for 4Pi-SIM and I2M. The 473-nm laser, which was aligned with the central optical path of the 488-nm laser and separated by a dichroic mirror, provided single-sided WF illumination. The fluorescence signals then interfered at the NPBS and were subsequently captured by the camera to yield the I2M PSF.
Using this I2M illumination module, we acquired a 4Pi-SIM PSF and the companion I2M PSF, both of which were needed for generating the synthetic OTF library. The only difference from the normal 4Pi-SIM PSF acquisition procedure is that, at the end of each SIM pattern cycle for a focal plane, an additional exposure was taken using the 473-nm laser under WF illumination. The PSF stacks underwent the Fourier transformation to obtain their respective OTFs. To extract the 4Pi-SIM excitation OTF, we separated the bands of the 4Pi-SIM stack and deconvolved each band from the I2M OTF. Finally, we synthesized the desired OTF library by first separately modifying the phases of the excitation and I2M OTFs, which is easy because all components are well separated spatially, and then convolving these phase-modulated OTFs together under a series of OPLDs with 10-nm increments.
4Pi-SIM and 3D-SIM image reconstruction
Parameter estimation was carried out before the final reconstruction was performed. Two phase-complementary images were registered using an affine matrix and then summed to generate a stack without fluorescence interference. This stack underwent band separation and was used for all subsequent estimation steps. The integer-pixel coordinates of the illumination wave vector (k0) were estimated by cross-correlating the shifted order-2 and the order-0 SIM bands. Subpixel precision in locating k0 was achieved by utilizing gradient descent methodology15. The modulation depth and initial phases of the lateral SIM patterns (that is, phase φ0) were estimated from the overlap of the order-0 band and the shifted higher-order bands (regions 4 and 5, Supplementary Fig. 6j). The OPLD was then estimated following the procedure described in the ‘Optical path length difference estimation’ section.
Before reconstruction, the original images underwent background removal and edge-softening with a window function. The resulting stack was then unmixed and adjusted for the estimated modulation depth and the initial phase for each higher-order SIM band. Depending on the estimated OPLD, the closest matched OTF from the preconstructed OTF library was selected to create a generalized Wiener filter used in the final image reconstruction as previously described15.
For single-objective 3D-SIM reconstruction used for comparison throughout this work, a joint Wiener filter was used to integrate images acquired from both exit ports of the NPBS. The images were registered, unmixed and jointly reconstructed using one Wiener filter to improve the quality of the 3D-SIM reconstruction. 4Pi-SIM employs two objectives to collect two-fold fluorescence compared to 3D-SIM but only uses half of it (raw data A, the image with constructive interference) for reconstruction. In contrast, 3D-SIM collects fluorescence from a single objective, which is then split into raw data A and B. Consequently, we performed a joint reconstruction in 3D-SIM but not in 4Pi-SIM, which ensures that the fluorescence signal used in reconstruction is about equal for both 3D-SIM and 4Pi-SIM, allowing for a fair comparison. The Wiener parameters that balance the resolution-noise trade-off were set identically during reconstruction in both modalities.
For two-color imaging, the reconstruction of each channel was carried out separately, following the procedure described above. Because the focal planes of the green and red channels differ by approximately 2 mm, only the image with constructive interference was retained for each color channel. Consequently, there were no complementary image pairs available to estimate OPLDs. In this scenario, we reconstructed the images using a range of OTFs with varying OPLDs. The final result was determined by selecting the reconstructed image with the least artifacts. Subsequently, registration between the two channels was performed in two steps. The initial step involved an affine transformation using a transformation matrix obtained by imaging a fluorescent bead sample. Next, the residual shift was corrected by using a few common fluorescent beads present in both images through Gaussian fitting to accurately determine their positions.
Data analysis and visualization
Data analysis was performed using custom code written in MATLAB R2023a (MathWorks). The reconstructed images generated by 4Pi-SIM have a pixel size of 32 nm in the x–y plane and 40 nm in the z direction, whereas the pixel size of images generated by 3D-SIM is 32 nm in the x–y plane and 120 nm in the z direction. For visualization purposes, we used bilinear interpolation in Fiji (NIH) to interpolate the images from both 4Pi-SIM and 3D-SIM to achieve an isotropic voxel size of 30 nm. The Fiji plugin ‘Bleach Correction’, based on histogram matching, was used to process all time-lapse data and videos except for those shown in Figs. 4–6, Extended Data Figs. 7i,j and 8, Supplementary Figs. 6d and 10g–j, and Supplementary Videos 9–16. Adjacent time points in live-cell videos were registered by the Fiji plugin ‘Correct 3D Drift’, except for those shown in Figs. 4–6 and Supplementary Videos 9–16. FluoRender (University of Utah) and Imaris (Oxford Instruments) were used for 3D rendering and visualization.
Plasmids
To label ER in fixed cells, we coexpressed mEmerald-Sec61β (a gift from J. Bewersdorf, Department of Cell Biology, Yale School of Medicine) and mEmerald-KDEL (a gift from D. Li, Institute of Biophysics, Chinese Academy of Sciences). To label ER in live cells, we coexpressed oxStayGold-Sec61β and oxStayGold-KDEL. The plasmid encoding oxStayGold-KDEL (pcDNA3/er-(n2)oxStayGold(c4)) was obtained from Addgene (plasmid no. 185822)32. To generate oxStayGold-Sec61β, we PCR-amplified the oxStayGold gene from pcDNA3/er-(n2)oxStayGold(c4) and replaced the mEmerald gene in mEmerald-Sec61β using Gibson assembly.
To label mitochondria, we expressed eGFP-OMP25 (a gift from J. Bewersdorf, Department of Cell Biology, Yale School of Medicine) in fixed cells and coexpressed oxStayGold-OMP25 and TOM20-oxStayGold in live cells. To generate oxStayGold-OMP25 and TOM20-oxStayGold, we amplified the oxStayGold gene using PCR and replaced the eGFP and mCherry genes in eGFP-OMP25 and TOM20-mCherry, respectively.
To label microtubules in live cells, we expressed oxStayGold-Ensconsin, which was constructed by replacing the mEmerald gene with the oxStayGold gene in mEmerald-Ensconsin (a gift from D. Li, Institute of Biophysics, Chinese Academy of Sciences).
To label actin in live cells, we constructed Lifeact-mStayGold, Lifeact-mEGFP and Lifeact-mScarlet-H. The mStayGold36, mEGFP and mScarlet-H genes were amplified by PCR from the plasmids pAAV-CAG-(n1) QC2-6 FIQ(C4)-P2A-FusionRed (Wekwikgene no. 0000267), mEGFP-tublin (a gift from P.Xu, Institute of Biophysics, Chinese Academy of Sciences) and pRset-A-mScarlet-H (a gift from M. Zhang, Biomedical Engineering Department, Peking University), respectively. Lifeact genes were amplified by PCR from the plasmids Lifeact-RFP. The mStayGold, mEGFP and mScarlet-H genes were fused to the carboxy terminus of Lifeact and cloned into the vector pcDNA3/er-(n2)oxStayGold(c4), digested with HindIII and EcoRI.
For two-color ER-microtubules samples in fixed COS-7 cells, we expressed mEmerald-Sec61β. For two-color mitochondria-microtubules samples in fixed U-2 OS cells, we expressed eGFP-OMP25.
For two-color ER-mitochondria samples in live cells, we co-expressed oxSayGold-OMP25 and dCyOFP2s-KDEL. The plasmid encoding dCyOFP2s-KDEL was generated by PCR amplification of the dCyOFP2s gene from pMito-dCyOFP2s (a gift from K. Piatkevich, School of Life Sciences, Westlake University) and replacement of the mEmerald gene in mEmerald-KDEL.
Cell culture and transfection
HeLa (CCL-2), COS-7 (CRL-1651) and U-2 OS (HTB-96) cell lines were purchased from the American Type Culture Collection (ATCC). HeLa and COS-7 cells were cultured in DMEM (Gibco, catalog no. C11965500CP), and U-2 OS cells were cultured in McCoy’s 5A medium (Gibco, catalog no. 16600-082), and supplemented with 10% fetal bovine serum (Sigma-Aldrich, catalog no. F8318) and 1% penicillin–streptomycin (Gibco, catalog no. 15140122). The cells were maintained at 37 °C with 5% CO2 and routinely tested for mycoplasma contamination.
Before cell plating, high-precision coverslips (Marienfeld, catalog no. 0117650) were cleaned as follows: the coverslips were immersed in 1 M KOH (Sigma-Aldrich, catalog no. 484016), sonicated for 15 minutes and then subjected to three cycles of sonication in double-distilled H2O for 10 minutes each. The coverslips were subsequently sterilized with 100% ethanol (J&K Scientific, catalog no. 258449) for 1 minute and incubated with poly-l-lysine (Sigma-Aldrich, catalog no. P4707).
On the day before transfection, approximately 1 × 105 cells were seeded on poly-l-lysine-coated coverslips in a six-well plate. The cells were transiently transfected with the aforementioned plasmids using Lipofectamine 3000 (Thermo Fisher Scientific, catalog no. L3000015), following the manufacturer’s instructions. The transfection mixture contained 1 μg of DNA per well in a six-well plate. For co-transfections, 0.75 μg of each DNA sample was used, for a total of 1.5 μg.
Sample labeling
Microtubule labeling
To label microtubules in fixed cells, immunofluorescence labeling was performed in COS-7 cells using antibodies. Approximately 5 × 105 cells were seeded on poly-l-lysine-coated coverslips in a six-well plate. After overnight incubation, the cells were pre-fixed with pre-warmed 0.3% glutaraldehyde (GA, Electron Microscopy Sciences, catalog no. 16020) and 0.25% Triton X-100 (Sigma-Aldrich, catalog no. T8787) in cytoskeletal buffer (CBS buffer: 10 mM MES, 150 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5 mM glucose, pH 6.1; all reagents were purchased from Sigma-Aldrich) for 2 minutes, followed by fixation with pre-warmed 2% GA in CBS buffer for 10 minutes. The cells were rinsed three times with PBS (1× PBS, Gibco, catalog no. 10010023). Next, the cells were blocked and permeabilized with blocking buffer (3% BSA (Jackson ImmunoResearch, catalog no. 001-000-162) and 0.2% Triton X-100 in PBS) for 1 h at room temperature. Subsequently, the cells were incubated overnight at 4 °C with mouse anti-α-tubulin (Sigma-Aldrich, catalog no. T5168, 1:500) and mouse anti-β-tubulin (Sigma-Aldrich, catalog no. T5293, 1:500) in dilution buffer (1% BSA and 0.2% Triton X-100 in PBS). Afterward, the cells were washed three times for 5 minutes each with wash buffer (0.1% Triton X-100 in PBS) and then incubated with goat anti-mouse Alexa Fluor 488 (AF488) (Thermo Fisher Scientific, catalog no. A11029, 1:500) in dilution buffer for 1 h at room temperature. Following another three washes with wash buffer, the samples were post-fixed with 3% paraformaldehyde (PFA, Electron Microscopy Sciences, catalog no.15710) and 0.1% GA in PBS for 10 minutes. The samples were then rinsed three times with PBS and stored in PBS at 4 °C until imaging.
For live-cell samples, COS-7 cells expressing oxStayGold-Ensconsin were cultured on poly-l-lysine-coated coverslips for 24 h. Before imaging, the culture medium was replaced with 1 ml of live-cell imaging medium (FluoroBrite DMEM (Gibco, catalog no. A1896701) supplemented with additional glucose to achieve a final concentration of 15 mM).
ER labeling
To label ER in fixed cells, we transfected COS-7 cells with the mEmerald-Sec61β and mEmerald-KDEL plasmids. The cells were grown at 37 °C with 5% CO2 for 24 h before being fixed in 3% PFA and 0.1% GA in PBS for 15 minutes, followed by three rinses with PBS. Subsequently, the cells were permeabilized and blocked in a blocking buffer for 1 h at room temperature. Rabbit anti-GFP primary antibody (Thermo Fisher Scientific, catalog no. A11122, 1:500) in dilution buffer was applied to the cells, which were then incubated overnight at 4 °C. The cells were then washed three times for 5 minutes each with wash buffer and incubated with donkey anti-rabbit AF488 (Yeason, catalog no. 34206ES60, 1:500) for 1 h at room temperature. Next, the cells were washed three times with wash buffer. Finally, the samples were post-fixed in 3% PFA and 0.1% GA in PBS for 10 minutes, rinsed three times with PBS and stored in PBS at 4 °C until imaging.
For live-cell samples, COS-7 cells were transfected with HaloTag-Sec61β and subsequently incubated with Janelia Fluor JFX554 HaloTag Ligand (Promega, catalog no. HT1030) at a final concentration of 1 μM for 1.5 hours. For two-color imaging, COS-7 cells were transfected with oxStayGold-KDEL and oxStayGold-sec61β. These cells were then cultured at 37 °C with 5% CO2 for 24 h. Before imaging, the culture medium was replaced with live-cell imaging medium.
Mitochondrial labeling
To label mitochondria in fixed cells, HeLa cells were transfected with eGFP-OMP25 and incubated at 37 °C with 5% CO2 for 24 h. Next, the cells were fixed with 3% PFA and 0.1% GA in PBS for 15 minutes and then rinsed three times with PBS. Subsequently, the cells were permeabilized and blocked in blocking buffer for 1 h at room temperature. The cells were then incubated overnight at 4 °C with rabbit anti-GFP (1:1,000) and rabbit anti-TOMM20 (Abcam, catalog no. ab78547, 1:1,000) primary antibodies in dilution buffer. Following three washes for 5 minutes each with wash buffer, the cells were incubated with donkey anti-rabbit AF488 (1:500) for 1 h at room temperature. The cells were then washed three times with wash buffer. Finally, the samples were post-fixed in 3% PFA and 0.1% GA in PBS for 10 minutes, rinsed three times with PBS and stored in PBS at 4 °C until imaged.
For live-cell samples, HeLa cells were transfected with oxStayGold-OMP25 and TOM20-oxStayGold and then cultured at 37 °C with 5% CO2 for 24 hours. Before imaging, the culture medium was replaced with live-cell imaging medium.
Actin labeling
To label actin in live cells, COS-7 cells were transfected with Lifeact-mStayGold, Lifeact-mEGFP and Lifeact-mScarlet-H and then cultured at 37 °C with 5% CO2 for 24 h. Before imaging, the culture medium was replaced with live-cell imaging medium.
PM labeling
To label the PM in fixed cells, approximately 5 × 105 HeLa cells were seeded onto cleaned, poly-l-lysine-coated coverslips and cultured for 24 h. Subsequently, the cells were fixed with 4% PFA in Hank’s Balanced Salt Solution (HBSS) (WISENT, catalog no. 311-512-CL) for 15 minutes at room temperature. Following fixation, the samples were washed three times with HBSS and incubated with WGA-AF488 (Thermo Fisher Scientific, catalog no. W11261) diluted at a concentration of 1:200 in HBSS for 20 minutes. After incubation, the samples were washed twice with HBSS and stored in PBS at 4 °C until imaging.
Golgi labeling
Mouse anti-GM130 (BD Biosciences, catalog no. 610823), rabbit anti-GOLGB1 (Sigma-Aldrich, catalog no. HPA011555) and rabbit anti-golgin97 (Sigma-Aldrich, catalog no. HPA044329) were used as the primary antibodies for the cis-, medial- and trans-Golgi, respectively. The primary antibodies were used at a dilution of 1:500. HeLa cells were plated on poly-l-lysine-coated coverslips at a density of approximately 5 × 105 cells and were cultured for 24 h before being fixed in 4% PFA for 15 minutes. Following fixation, the cells were permeabilized and blocked with blocking buffer for 1 h. The primary antibodies were then incubated with the samples overnight at 4 °C, followed by three washes with wash buffer for 5 minutes each. After washing, the samples were labeled with a secondary antibody, goat anti-mouse AF488 or donkey anti-rabbit AF488, at a dilution of 1:500 for 1 h at room temperature. The samples were then rinsed three times with wash buffer. Finally, the samples were stored in PBS at 4 °C until they were ready for imaging.
Vimentin labeling
To label vimentin in fixed cells, immunofluorescence labeling was conducted using antibodies. COS-7 cells were grown on poly-l-lysine-coated coverslips for 24 h and then pre-fixed with a pre-warmed solution of 0.3% GA and 0.25% Triton X-100 in CBS buffer for 2 minutes. Subsequently, the cells were fixed with 2% pre-warmed GA in CBS buffer for 10 min. Afterward, the cells were rinsed three times with PBS. Next, the COS-7 cells were permeabilized and blocked with blocking buffer for 1 h at room temperature. Next, the cells were incubated overnight at 4 °C with a mouse anti-vimentin primary antibody (BioLegend, catalog no. 677802) diluted in dilution (1:500). The cells were then washed three times for 5 minutes each with wash buffer and incubated for 1 h at room temperature with goat anti-mouse AF488, which was also diluted at 1:500. After another three washes with wash buffer, the samples were post-fixed with a solution of 3% PFA and 0.1% GA in PBS for 10 minutes. The samples were then rinsed three times with PBS and stored in PBS at 4 °C until imaging.
Synaptonemal complex samples
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Westlake University. The testicles were removed from 3-week-old C57 mice. First, the tunica albuginea was removed from the testis. Then, the seminiferous tubules were incubated in buffer I (30 mM Tris-HCl, pH 8.2, 17 mM sodium citrate, 5 mM EDTA, 0.5 mM dithiothreitol and 0.1 mM phenylmethylsulfonyl fluoride; all reagents were purchased from Sigma-Aldrich) or PBS for 15 minutes at room temperature. Spermatocytes were then released into buffer I using forceps and mixed with an equal volume of buffer II (100 mM sucrose, Sigma-Aldrich). This mixture was then filtered through a 70-μm cell strainer. Finally, the filtered cells were spread onto buffer-III-presoaked glass slides containing 1% PFA and 0.15% Triton X-100, pH 9.2, and dried overnight in humidified chambers. Next, the samples were rinsed three times with 0.1% Triton X-100 in PBS. Image-iT (Invitrogen, catalog no. I36933) was applied to enhance the signal for 30 minutes, followed by rinsing the samples three times with 0.1% Triton X-100 in PBS. Subsequently, the samples were blocked with 3% BSA in PBS for 30 minutes at room temperature and incubated with mouse anti-SCP-3 (D-1) primary antibody (Santa Cruz Biotechnology, catalog no. sc-74569, 1:1,000) in 1% BSA in PBS at 4 °C overnight. The cells were then washed three times with 0.05% CA-630 (Sigma-Aldrich, catalog no. I8896), 0.05% Triton X-100 and 0.2% BSA in PBS and subsequently incubated with a goat anti-mouse AF488 secondary antibody (1:500 dilution) for 1 hour at room temperature. Finally, the cells were washed three times for 5 minutes with PBS.
Hippocampal neuron samples
Primary hippocampal cultures were prepared from neonatal C57BL/6J mice within 12 hours of birth, with both sexes mixed. Newborn pups were anesthetized and dissected on a 4 °C water-cooled platform. The hippocampi were isolated and washed twice with 10 ml HBSS. Subsequently, they were digested with papain (Worthington Biochemical Corporation, catalog no. LS003127) at 37 °C for 20 minutes and rinsed twice with 5 ml of preheated plating medium. This medium was composed of MEM (Gibco, catalog no. 51200038) supplemented with 2.5% glucose, 0.4% NaHCO3, 0.1 mg ml–1 transferrin (Sigma-Aldrich, catalog no. T8158), 10% FBS, 2 mM l-glutamine and 25 μg ml–1 insulin (Solarbio, catalog no. I8830). After aspirating the supernatant, the hippocampi were pipetted up and down with plating medium until the tissues were mostly dissolved. The resulting cell suspension was then passed through a cell strainer (Greiner, catalog no. 542040) to remove any remaining undissociated tissue and collected in a 50-ml tube. Neurons were cultured on 4% Matrigel-treated (Corning, catalog no. 354234) 25 mm diameter high-precision coverslips. The cultures were maintained in a 37 °C culture incubator, and the plating medium was replaced with culture medium after 24 h. The culture medium had the same composition as the plating medium, except for the addition of 2% B-27 supplement (Gibco, catalog no. 17504044). To inhibit the growth of glial cells, 2 μM cytarabine (Tocris Bioscience, catalog no. 4520/50) was added to the culture medium 3 d after plating. Fresh culture medium was added during the maturation period of the neurons, which were used for imaging after 2 weeks of culture. Mouse anti-beta spectrin II (BD Biosciences, catalog no. 612563, used at a 1:200 dilution) and goat anti-mouse AF488 (1:500 dilution) were used as primary and secondary antibodies, respectively. For fixation, the cultured neuron samples were treated with 4% PFA in PBS for 15 minutes at room temperature and then washed three times for 5 minutes each with wash buffer. These fixed samples were subsequently blocked in blocking buffer for 1 h and stained with the primary antibody in dilution buffer overnight at 4 °C. After three washes for 5 minutes each using washing buffer, the samples were stained with the secondary antibody in wash for 1 h at room temperature. After another three washes for 5 minutes each using wash buffer, the samples were immediately mounted for imaging.
Two-color samples
To label two-color ER-microtubules in fixed cells, we transfected COS-7 cells with mEmerald-Sec61β. The transfected cells were cultured at 37 °C with 5% CO2 for 24 h before fixation with 3% PFA and 0.1% GA in PBS for 15 minutes, followed by three rinses with PBS. Subsequently, the cells were permeabilized and blocked in a blocking buffer for 1 h at room temperature. Rabbit anti-GFP (1:500 dilution) and mouse anti-α-tubulin (Sigma-Aldrich, catalog no. T5168, 1:500) primary antibodies were applied in dilution buffer, and cells were incubated overnight at 4 °C. After three 5-minute washes with wash buffer, cells were incubated overnight at 4 °C with donkey anti-rabbit AF488 (1:500 dilution) and goat anti-mouse Alexa Fluor 568 (Thermo Fisher Scientific, catalog no. A11031, 1:500) secondary antibodies. The following day, the cells underwent three washes with wash buffer. The samples were then post-fixed in 3% PFA and 0.1% GA in PBS for 10 minutes, subjected to three PBS rinses and stored in PBS at 4 °C until imaging.
U-2 OS cells were used to label mitochondria and microtubules for two-color imaging in fixed cells. Approximately 1 × 105 cells were plated on cleaned and poly-l-lysine-coated glass coverslips before being transfected with eGFP-OMP25. The cells were cultured at 37 °C with 5% CO2 for 24 h before fixation with 3% PFA and 0.1% GA for 15 minutes, followed by three rinses with PBS. Subsequently, the cells were permeabilized and blocked in blocking buffer for 1 hour at room temperature. Rabbit anti-GFP (1:500 dilution) and mouse anti-α-tubulin (1:500 dilution) primary antibodies were applied in dilution buffer, and cells were then incubated overnight at 4 °C. The following day, the cells were washed three times for 5 minutes each with wash buffer and incubated with Alexa Fluor 488-conjugated donkey anti-rabbit (1:500 dilution) and Alexa Fluor 568-conjugated goat anti-mouse (1:500 dilution) secondary antibodies overnight at 4 °C. After being washed three times with wash buffer, the samples were post-fixed in 3% PFA and 0.1% GA in PBS for 10 minutes, rinsed thrice with PBS. Finally, the samples were stored in PBS at 4 °C until imaging.
For live-cell samples, HeLa cells were used to label mitochondria and the ER for two-color imaging. Before transfection with oxStayGold-OMP25 and dCyOFP2s-KDEL, approximately 1 × 105 cells were plated on cleaned and poly-l-lysine-coated glass. COS-7 cells were used to label microtubules and the ER. These cells were transfected with oxStayGold-Ensconsin and dCyOFP2s-KDEL. Following transfection, the cells were cultured for 24 h, and the culture medium was replaced with 1 ml of live-cell imaging medium before imaging.
Sample mounting
The samples were mounted in a manner similar to what has previously been described26,27. First, 100 nm green–yellow fluorescent beads (Thermo Fisher Scientific, catalog no. F8803) were diluted in imaging buffer (PBS for fixed samples and live-cell imaging media for live cells) at a ratio of approximately 1:1 × 105 to 1:1 × 107. Next, the sample coverslip was immersed in 1 ml of bead solution in a dish for 5–10 minutes. The coverslip was removed from the dish and placed on a custom sample holder. Then, 100 μl imaging buffer was evenly distributed on the sample coverslip, and another clean coverslip was placed on top. Excess solution was gently removed with Kimwipes. Finally, the samples were sealed with two-component silicone glue (Picodent Twinsil, Picodent) and were transferred to the microscope for imaging after the glue had hardened (typically in 10–20 minutes).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
An example dataset is available at https://doi.org/10.6084/m9.figshare.25714068 (ref. 63). Owing to the extensive size of the image data (>5 TB), it is currently not feasible to upload it to an online repository. However, the datasets generated and/or analyzed during the current study are available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The code for 4Pi-SIM reconstruction is available at https://github.com/zhanglab-srm/4Pi-SIM.
References
Sahl, S. J., Hell, S. W. & Jakobs, S. Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol. 18, 685–701 (2017).
Sigal, Y. M., Zhou, R. & Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887 (2018).
Schermelleh, L. et al. Super-resolution microscopy demystified. Nat. Cell Biol. 21, 72–84 (2019).
Rainer Heintzmann, C. C.& Cremer, C. G. In Optical Biopsies and Microscopic Techniques III 185–196 (SPIE, 1999).
Gustafsson, M. G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Shao, L., Kner, P., Rego, E. H. & Gustafsson, M. G. Super-resolution 3D microscopy of live whole cells using structured illumination. Nat. Methods 8, 1044–1046 (2011).
Fiolka, R., Shao, L., Rego, E. H., Davidson, M. W. & Gustafsson, M. G. Time-lapse two-color 3D imaging of live cells with doubled resolution using structured illumination. Proc. Natl Acad. Sci. USA 109, 5311–5315 (2012).
Li, D. et al. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 349, aab3500 (2015).
Guo, Y. et al. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell 175, 1430–1442 (2018).
Huang, X. et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat. Biotechnol. 36, 451–459 (2018).
Wu, Y. & Shroff, H. Faster, sharper, and deeper: structured illumination microscopy for biological imaging. Nat. Methods 15, 1011–1019 (2018).
Kner, P., Chhun, B. B., Griffis, E. R., Winoto, L. & Gustafsson, M. G. Super-resolution video microscopy of live cells by structured illumination. Nat. Methods 6, 339–342 (2009).
Heintzmann, R. & Huser, T. Super-resolution structured illumination microscopy. Chem. Rev. 117, 13890–13908 (2017).
Frohn, J. T., Knapp, H. F. & Stemmer, A. True optical resolution beyond the Rayleigh limit achieved by standing wave illumination. Proc. Natl Acad. Sci. USA 97, 7232–7236 (2000).
Gustafsson, M. G. et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94, 4957–4970 (2008).
Schermelleh, L. et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–1336 (2008).
Frohn, J. T., Knapp, H. F. & Stemmer, A. Three-dimensional resolution enhancement in fluorescence microscopy by harmonic excitation. Opt. Lett. 26, 828–830 (2001).
Manton, J. D., Strohl, F., Fiolka, R., Kaminski, C. F. & Rees, E. J. Concepts for structured illumination microscopy with extended axial resolution through mirrored illumination. Biomed. Opt. Express 11, 2098–2108 (2020).
Li, X. et al. Three-dimensional structured illumination microscopy with enhanced axial resolution. Nat. Biotechnol. 41, 1307–1319 (2023).
Hell, S. & Stelzer, E. H. K. Properties of a 4Pi confocal fluorescence microscope. J. Opt. Soc. Am. A 9, 2159–2166 (1992).
Hell, S. W., Schrader, M. & van der Voort, H. T. Far-field fluorescence microscopy with three-dimensional resolution in the 100-nm range. J. Microsc. 187, 1–7 (1997).
Gustafsson, M. G. L., Agard, D. A. & Sedat, J. W. in Three-Dimensional Microscopy: Image Acquisition and Processing II (eds Wilson, T. & Cogswell, C. J.) 147–156 (SPIE, 1995).
Gustafsson, M. G., Agard, D. A. & Sedat, J. W. I5M: 3D widefield light microscopy with better than 100 nm axial resolution. J. Microsc. 195, 10–16 (1999).
Shao, L. et al. I5S: wide-field light microscopy with 100-nm-scale resolution in three dimensions. Biophys. J. 94, 4971–4983 (2008).
Huang, F. et al. Ultra-high resolution 3D imaging of whole cells. Cell 166, 1028–1040 (2016).
Zhang, Y. et al. Nanoscale subcellular architecture revealed by multicolor three-dimensional salvaged fluorescence imaging. Nat. Methods 17, 225–231 (2020).
Wang, J. et al. Implementation of a 4Pi-SMS super-resolution microscope. Nat. Protoc. 16, 677–727 (2021).
Shao, L., Winoto, L., Agard, D. A., Gustafsson, M. G. & Sedat, J. W. Interferometer-based structured-illumination microscopy utilizing complementary phase relationship through constructive and destructive image detection by two cameras. J. Microsc. 246, 229–236 (2012).
Xu, K., Zhong, G. & Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Phillips, M. J. & Voeltz, G. K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82 (2016).
Schroeder, L. K. et al. Dynamic nanoscale morphology of the ER surveyed by STED microscopy. J. Cell Biol. 218, 83–96 (2019).
Hirano, M. et al. A highly photostable and bright green fluorescent protein. Nat. Biotechnol. 40, 1132–1142 (2022).
Wang, C. et al. Dynamic tubulation of mitochondria drives mitochondrial network formation. Cell Res. 25, 1108–1120 (2015).
Vincent, A. E., Turnbull, D. M., Eisner, V., Hajnoczky, G. & Picard, M. Mitochondrial nanotunnels. Trends Cell Biol. 27, 787–799 (2017).
Qin, J. et al. ER-mitochondria contacts promote mtDNA nucleoids active transportation via mitochondrial dynamic tubulation. Nat. Commun. 11, 4471 (2020).
Ando, R. et al. StayGold variants for molecular fusion and membrane-targeting applications. Nat. Methods 21, 648–656 (2024).
Jin, M. et al. Branched actin networks are organized for asymmetric force production during clathrin-mediated endocytosis in mammalian cells. Nat. Commun. 13, 3578 (2022).
Nixon-Abell, J. et al. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354, aaf3928 (2016).
Takakura, H. et al. Long time-lapse nanoscopy with spontaneously blinking membrane probes. Nat. Biotechnol. 35, 773–780 (2017).
Ball, G. et al. SIMcheck: a toolbox for successful super-resolution structured illumination microscopy. Sci. Rep. 5, 15915 (2015).
Subach, O. M. et al. LSSmScarlet, dCyRFP2s, dCyOFP2s and CRISPRed2s, genetically encoded red fluorescent proteins with a large stokes shift. Int. J. Mol. Sci. 22, 12887 (2021).
Bindels, D. S. et al. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56 (2017).
Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Grimm, J. B. et al. A general method to improve fluorophores using deuterated auxochromes. JACS Au 1, 690–696 (2021).
Efstathiou, C. & Draviam, V. M. Electrically tunable lenses—eliminating mechanical axial movements during high-speed 3D live imaging. J. Cell Sci. 134, jcs258650 (2021).
Chen, B. et al. Resolution doubling in light-sheet microscopy via oblique plane structured illumination. Nat. Methods 19, 1419–1426 (2022).
Temma, K. et al. Selective-plane-activation structured illumination microscopy. Nat. Methods 21, 889–896 (2024).
Richardson, D. S. et al. Tissue clearing. Nat. Rev. Methods Primers 1, 84 (2021).
Wassie, A. T., Zhao, Y. X. & Boyden, E. S. Expansion microscopy: principles and uses in biological research. Nat. Methods 16, 33–41 (2019).
Hampson, K. M. et al. Adaptive optics for high-resolution imaging. Nat. Rev. Methods Prim. 1, 68 (2021).
Zhao, W. et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nat. Biotechnol. 40, 606–617 (2022).
Jin, L. et al. Deep learning enables structured illumination microscopy with low light levels and enhanced speed. Nat. Commun. 11, 1934 (2020).
Qiao, C. et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy. Nat. Methods 18, 194–202 (2021).
Qiao, C. et al. Rationalized deep learning super-resolution microscopy for sustained live imaging of rapid subcellular processes. Nat. Biotechnol. 41, 367–377 (2023).
Fan, J. et al. High-fidelity reconstruction of structured illumination microscopy by an amplitude-phase channel attention network with multitemporal information. IEEE Trans. Instrum. Meas. 72, 1–13 (2023).
Shtengel, G. et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl Acad. Sci. USA 106, 3125–3130 (2009).
Aquino, D. et al. Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores. Nat. Methods 8, 353–359 (2011).
Bates, M. et al. Optimal precision and accuracy in 4Pi-STORM using dynamic spline PSF models. Nat. Methods 19, 603–612 (2022).
Schmidt, R. et al. Spherical nanosized focal spot unravels the interior of cells. Nat. Methods 5, 539–544 (2008).
Hao, X. et al. Three-dimensional adaptive optical nanoscopy for thick specimen imaging at sub-50-nm resolution. Nat. Methods 18, 688–693 (2021).
Siegmund, R., Werner, F., Jakobs, S., Geisler, C. & Egner, A. isoSTED microscopy with water-immersion lenses and background reduction. Biophys. J. 120, 3303–3314 (2021).
Bohm, U., Hell, S. W. & Schmidt, R. 4Pi-RESOLFT nanoscopy. Nat. Commun. 7, 10504 (2016).
Ouyang, Z. et al. Example dataset for “Elucidating subcellular architecture and dynamics at isotropic 100 nm resolution with 4Pi-SIM”. figshare https://doi.org/10.6084/m9.figshare.25714068.v3 (2024).
Acknowledgements
We thank D. Li at the Institute of Biophysics, Chinese Academy of Sciences, for sharing the mEmerald-KDEL and mEmerald-Ensconsin plasmids, P. Xu at the Institute of Biophysics, Chinese Academy of Sciences, for sharing the mEGFP-tubulin plasmid, M. Zhang at Peking University for sharing the mScarlet-H plasmid and J. Bewersdorf at Yale University for sharing the mEmerald-Sec61β and eGFP-OMP25 plasmids. Y.Z. acknowledges support from the National Science Foundation of China (32150015). Liangyi Chen acknowledges support from the National Key Research and Development Program of China (2022YFC3400600) and the National Science Foundation of China (81925022, T2288102, 32227802). X.H. acknowledges support from the National Science Foundation of China (32322049, 32350009, 32170691), the National Key Research and Development Program of China (2022YFF0712503, 2021YFA1100201), the Beijing Nova Program (20220484033) and the Clinical Medicine Plus X-Young Scholars Project of Peking University. J.F. acknowledges support from the National Science Foundation of China (62103071) and the National Key Research and Development Program of China (2023YFF1501002). X.Y. acknowledges support from the ‘Pioneer’ and ‘Leading Goose’ R&D Program of Zhejiang (2024SSYS0033). This work was also supported by the Westlake Laboratory of Life Sciences and Biomedicine, the Westlake Education Foundation, the Research Center for Industries of the Future at Westlake University, the New Cornerstone Science Foundation and the High-performance Computing Platform of Peking University.
Author information
Authors and Affiliations
Contributions
Y.Z., X.H., J.F., Liangyi Chen and L.S. supervised the project; Y.Z., X.H. and J.F. initiated and conceived the research; Z.O., Q.W. and X.L. designed the optical layout and built the optical system with guidance from Y.Z., X.H., J.F. and Liangyi Chen; Z.O., Q.W., W.G. and X.L. developed the algorithm and implemented the corresponding software with guidance from J.F., X.B. and B.X.; Z.O., Q.W. and X.L. performed experiments, analyzed the data and prepared the figures; Q.D., Linlin Chen and X.Y. prepared the synaptonemal complex samples; Y.C., C.P. and C.L. prepared the neuron samples; Q.D., M.T., Z.Y. and B.Z. prepared all other samples with guidance from Y.Z.; Y.Z., X.H., J.F., L.S. and Liangyi Chen wrote the manuscript with input from all authors. All authors participated in the discussions and data interpretation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Xuesong Li, Lothar Schermelleh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 OTF comparison of 3D-SIM, four-beam SIM, and 4Pi-SIM.
The first column shows the simulated OTFs for wide-field (WF) microscopy and image interference microscopy (I2M). The second column shows the illumination frequency components of 3D-SIM, four-beam SIM, and 4Pi-SIM, from top to bottom. The magenta dots indicate the additional frequency components introduced by four-beam interference, while the green dots represent frequency components further introduced by six-beam interference. The third column compares the x-z cross-sections of the illumination patterns for 3D-SIM, four-beam SIM, and 4Pi-SIM. The fourth column compares the system OTFs of 3D-SIM, four-beam SIM, and 4Pi-SIM by convolving the illumination frequency components with the detection OTFs. All the simulated OTFs are shown on a logarithmic scale for visualization purposes. The dashed line in the frequency distribution of illumination indicates the WF detection OTF. The dashed lines in the System OTF indicate WF (for 3D-SIM and four-beam SIM) and I2M (for 4Pi-SIM) detection OTF, respectively. Scale bars: 1 μm for illumination pattern in the spatial ___domain; 1/500 nm-1 for all simulated OTFs. Simulation parameters: pixel size, 30 nm; excitation NA = 1.15; emission NA = 1.27; refractive index of the immersion medium = 1.33; excitation wavelength, 488 nm; and emission wavelength, 525 nm.
Extended Data Fig. 2 Optical layout of 4Pi-SIM.
Refer to the Methods section for a detailed description. The excitation laser (blue beam) emerges from the lower right, is reflected by the dichroic mirror (DM1), is then split by the non-polarizing beamsplitter (NPBS), and finally interferes at the sample plane. The emitted fluorescence (green beam) undergoes interference at the NPBS before being captured by the sCMOS camera (CAM1). The focus-lock laser (red beam) emerges from the upper left, is reflected by the dichroic mirror (DM2), is directed toward the objectives (OBJ1, 2), and is finally captured by a compact CMOS camera (CAM2). The excitation laser for I2M illumination (dark blue) emerges from the lower left and is reflected by a dichroic mirror (DM3) to the sample. AOTF: acousto-optic tunable filter; MMF: multimode fiber; SMF: single-mode fiber; PBS: polarization beam splitter; HWP: half-wave plate; SLM: spatial light modulator; HS-LCVR: high-speed liquid crystal variable retarder; DM: dichroic mirror; NPBS: non-polarizing beamsplitter; FTS: fine-tuning translation submodule; CTS: coarse-tuning translation submodule; PM: knife-edge right-angle prism protective silver mirror; QWP: quarter-wave plate; L1-L18: lenses; M1-M19: mirrors; CY1: cylindrical lens; and F1: filter.
Extended Data Fig. 3 SolidWorks design and photograph of 4Pi-SIM.
a-c, Overview (a), side view (b), and front view (c) of the SolidWorks model of 4Pi-SIM. d, Photograph of the 4Pi-SIM microscope.
Extended Data Fig. 4 OPLD adjustment module.
a, Schematic diagram of the 4Pi-cavity. The red dashed box at the bottom indicates the OPLD coarse-tuning translation submodule (CTS) while the one on top indicates the OPLD fine-tuning translation submodule (FTS). PM: knife-edge right-angle prism protective silver mirror. b, SolidWorks model of the CTS, designed to adjust the OPLD with sub-μm resolution. c, SolidWorks model of the FTS, designed to adjust the OPLD with sub-nm resolution. d, Process of coarse adjustment of the OPLD, capturing fluctuations in the intensity of bead images as the CTS stage moves to pinpoint the maximum interference ___location. The blue line indicates the average intensity profile of 20 beads from the same field of view. The magenta dashed line indicates a fitted sinusoidal envelope. e, Process of fine adjustment of the OPLD. Another scan with FTS was performed to accurately locate the maximum interference, that is zero OPLD. The blue line indicates the average intensity profile of the same 20 beads as in d. The magenta dashed line indicates a fitted sinusoidal envelope.
Extended Data Fig. 5 Polarization rotation module.
a, The upper or lower system introduces an additional phase delay, leading to the degradation of the polarization linearity. ①②③ The p-polarized beams from the SLM-based pattern generator are modulated by a polarization rotation system, which includes a HS-LCVR (with its fast axis at 45°) and a QWP (with its fast axis at 0°). This design rotates the linearly polarized beams to s-polarization relative to the back pupil plane of the objective lens. However, the large number of optical components fail to preserve the polarization states with arbitrary incident angles, introducing a static phase shift. ④⑤ Consequently, the beams become elliptically polarized, diminishing interference contrast. b, Compensation for system phase delays with a modified module. ①② The p-polarized beams are first rotated to 45° by a HWP (with its fast axis at 22.5°). ③④⑤ Each arm has two phase delays: a variable one from the HS-LCVR (fast axis: 0°) and a static one from either the upper or lower system. ⑥⑦ Finally, a pair of QWPs (fast axis: 45°) are mounted before the objective lenses to demodulate elliptically polarized beams, converting them to s-polarized beams relative to the objective lens. All directions of the fast axis are marked relative to the propagation direction of the incident light.
Extended Data Fig. 6 Flow chart for 4Pi-SIM reconstruction.
Refer to the Methods section for a detailed description. "4Pi-SIM raw data A" and "4Pi-SIM raw data B" denote the complementary image stacks of 4Pi-SIM.
Extended Data Fig. 7 Reconstruction with estimated OPLD in beads, fixed cells, and live cells.
a, 4Pi-SIM image of 100-nm fluorescent beads. b, The x-z cross-sections along the dashed line in a, comparing the reconstruction results with an OPLD of 0 nm (upper) and an estimated OPLD of -160 nm (lower). c, Intensity profiles for a single bead reconstructed with OPLDs of 0 nm (blue, triangle) and -160 nm (red, circle) along the magenta dashed line in b, showing ringing artifacts when using an OTF with a mismatched OPLD. d, 4Pi-SIM image (maximum intensity projection) of immunolabeled ER in a COS-7 cell. e, The x-z cross-sections along the dashed line in d, comparing the reconstruction results with an OPLD of 0 nm (upper) and an estimated OPLD of -180 nm (lower). f, Intensity profiles of the single-layer ER indicated by the magenta dashed line in e, contrasting reconstruction with OPLDs of 0 nm (blue, triangle) and -180 nm (red, circle). g, 4Pi-SIM image (maximum intensity projection) of actin filaments in a live COS-7 cell. h, The estimated OPLDs varied over time, with green arrows marking the specific moments for i and j, alongside their estimated OPLDs. i, Comparative reconstructed images, with and without estimated OPLD, from the zoomed area within the yellow dashed box in g. The top panels show the x-y time-lapse images. The middle panels show the x-z cross-sections of reconstruction without estimated OPLD, corresponding to the dashed line marking the same actin filament. The bottom panels show x-z cross-sections of reconstruction with estimated OPLDs, demonstrating that our algorithm can effectively remove these axial artifacts. j, Comparative reconstructed images, with and without OPLD adjustment, from the magnified area within the magenta dashed box in g. The images from top to bottom represent time-lapse x-y cross-sections, and x-z cross-sections reconstructed without and with estimated OPLD, emphasizing the importance of OPLD-corrected reconstruction in distinguishing real signals from potential artifacts. All slices are 30 nm thick. Scale bars: 2 µm (a, d, e, g) and 1 µm (b, i, j). a.u., arbitrary units. Representative results are shown from three to six independent experiments.
Extended Data Fig. 8 Time-lapse volumetric imaging with 4Pi-SIM using conventional fluorophores.
a, 4Pi-SIM image (maximum intensity projection) of the actin filaments (Lifeact-mEGFP) in a live COS-7 cell. b, Time-lapse magnified views of the boxed region denoted ‘b’ in a. c, 4Pi-SIM image (maximum intensity projection) of the actin filaments (Lifeact-mScarlet-H) in a live COS-7 cell. d, Time-lapse magnified views of the boxed region denoted ‘d’ in c. e, 4Pi-SIM image (maximum intensity projection) of the ER (HaloTag-sec61β) labeled with the JFX554 dye in a live COS-7 cell. f, Time-lapse magnified views of the boxed region denoted ‘f’ in e. Scale bar: 2 μm (a-f). Representative results are shown from two to three independent experiments.
Extended Data Fig. 9 Two-color imaging of 4Pi-SIM using dyes with different spectra in fixed cells.
a, 4Pi-SIM image (maximum intensity projection) of immunolabeled ER (AF488, green) and tubulin (AF568, magenta) in a fixed COS-7 cell. b, Magnified 3D rendering of the white boxed region denoted ‘b’ in a. c, Magnified x-z cross-section along the yellow dashed line in a. d, Axial intensity profiles along the white dashed line in c. a.u., arbitrary units. e, 4Pi-SIM image (maximum intensity projection) of immunolabeled mitochondria outer membrane (AF488, green) and tubulin (AF568, magenta) in a fixed U2-OS cell. f, Magnified x-z cross-section along the dashed line labeled ‘f’ in e. g, Magnified y-z cross-section along the dashed line labeled ‘g’ in e. Scale bar: 5 μm (a, e); 1 μm (b); 500 nm (c, f, g). Representative results are shown from two to three independent experiments.
Extended Data Fig. 10 Locally ringing artifacts arise from refractive index inhomogeneity.
a, 4Pi-SIM image (maximum intensity projection) of immunolabeled vimentin in a fixed COS-7 cell. Color denoted the depth. b, Magnified x-z cross-section along the white dashed line in a. The result was reconstructed using an OTF with a globally estimated OPLD of -50 nm. c-f, Insets show magnified sub-regions of the corresponding colored dashed boxes in b. The upper panels showed reconstruction results under the globally estimated OPLD of -50 nm with non-uniform artifacts, potentially coming from refractive index mismatch at the nuclear boundary. The lower panels showed the reconstruction results with locally estimated OPLD-matched OTFs, effectively eliminating the axially ringing artifacts. Scale bar: 2 μm (a); 1 μm (b); 500 nm (c-f). Representative results are shown from three independent experiments.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, and Tables 1 and 2.
Supplementary Video 1
The video shows the SolidWorks model of the 4Pi-SIM microscope (see also Extended Data Fig. 3a–c). A detailed description of the optical components can be found in the Methods section.
Supplementary Video 2
The video shows the 3D rendering of the immunolabeled mitochondrial outer membrane in a fixed HeLa cell (see also Fig. 2a,b). It sequentially compares axial cross-sections obtained from WF microscopy, 3D-SIM, and 4Pi-SIM.
Supplementary Video 3
The video shows the 3D rendering of the immunolabeled vimentin in a fixed COS-7 cell (see also Fig. 2c,d). It sequentially compares axial cross-sections obtained from WF microscopy (left), 3D-SIM (middle), and 4Pi-SIM (right).
Supplementary Video 4
The video shows the 3D rendering of the immunolabeled βII-spectrin in a fixed hippocampal neuron (see also Fig. 2f–j). A zoomed-in sub-region compares the axial cross-sections obtained from 4Pi-SIM, 3D-SIM, and WF microscopy.
Supplementary Video 5
The video shows the 3D rendering of the immunolabeled synaptonemal complex (SYCP3) in a fixed mouse spermatocyte (see also Fig. 2k–m). It compares axial cross-sections obtained from 4Pi-SIM, 3D-SIM, and WF microscopy.
Supplementary Video 6
The video shows the 3D rendering of the immunolabeled ER in a fixed COS-7 cell (see also Fig. 3a–c). A zoomed-in sub-region compares the axial cross-sections of 4Pi-SIM, 3D-SIM, and WF microscopy.
Supplementary Video 7
The video shows the 3D rendering of the immunolabeled Golgi apparatus in fixed HeLa cells (see also Fig. 3d–f). It sequentially displays movie segments of the cis-Golgi (Part 1), medial-Golgi (Part 2), and trans-Golgi (Part 3). Each part includes a 3D rendering and a comparison of the axial view among 4Pi-SIM, 3D-SIM, and WF microscopy.
Supplementary Video 8
The video shows the 3D rendering of the immunolabeled plasma membrane (WGA-AF488) in a fixed HeLa cell (see also Fig. 3g,h). It shows axial cross-sections comparing 4Pi-SIM, 3D-SIM, and WF microscopy in sequence.
Supplementary Video 9
The video shows the time-lapse 4Pi-SIM images of the mitochondrial outer membrane (TOM20-oxStayGold and oxStayGold-OMP25) in a live HeLa cell (see also Fig. 4a–d). It displays three independent zoomed-in sub-regions with different mitochondrial dynamics. This dataset includes 32 time points, with a total acquisition time of 25.0 min.
Supplementary Video 10
The video shows the time-lapse 4Pi-SIM images of the microtubules (oxStayGold-Ensconsin) in a live COS-7 cell (see also Fig. 4e,f). This dataset includes 175 time points, with a total acquisition time of 112.6 min.
Supplementary Video 11
The video shows the time-lapse 4Pi-SIM images of the actin filaments (Lifeact-mStayGold) in a live COS-7 cell (see also Fig. 4g–j). This dataset includes 169 time points, with a total acquisition time of 71.3 min.
Supplementary Video 12
The video shows the time-lapse 4Pi-SIM images of ER (oxStayGold-Sec61β and oxStayGold-KDEL) in a live COS-7 cell (see also Fig. 5a–d). This dataset includes 231 time points, with a total acquisition time of 263.7 min.
Supplementary Video 13
The video shows the time-lapse 4Pi-SIM images of ER (oxStayGold-Sec61β and oxStayGold-KDEL) in a live COS-7 cell (see also Fig. 5e–g). This dataset includes 493 time points, with a total acquisition time of 151.7 min.
Supplementary Video 14
The video shows the time-lapse 4Pi-SIM images of ER (oxStayGold-Sec61β and oxStayGold-KDEL) in a live COS-7 cell (see also Fig. 5h–k). This dataset includes 300 time points, with a total acquisition time of 7.0 min.
Supplementary Video 15
The video shows the time-lapse two-color 4Pi-SIM images of ER (dCyOFP2s-KDEL, magenta) and mitochondria (oxStayGold-OMP25, green) in a live HeLa cell (see also Fig. 6a–d). This dataset includes 13 time points, with a total acquisition time of 8.4 min.
Supplementary Video 16
The video shows the time-lapse two-color 4Pi-SIM images of ER (dCyOFP2s-KDEL, magenta) and microtubules (oxStayGold-Ensconsin, green) in a live COS-7 cell (see also Fig. 6e–h). This dataset includes 14 time points, with a total acquisition time of 9.0 min.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1f,g.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ouyang, Z., Wang, Q., Li, X. et al. Elucidating subcellular architecture and dynamics at isotropic 100-nm resolution with 4Pi-SIM. Nat Methods 22, 335–347 (2025). https://doi.org/10.1038/s41592-024-02515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-024-02515-z