Abstract
To investigate the prognostic value of the geriatric nutritional risk index (GNRI) in patients with upper tract urothelial cell carcinoma (UTUC) receiving radical nephroureterectomy (RNU). Between January 2001 and December 2015, we enrolled 488 patients with UTUC underwent RNU in Taichung Veterans General Hospital. GNRI before radical surgery was calculated based on serum albumin level and body mass index. The malnutritional status was defined as GNRI < 92.0. Using Kaplan–Meier analyses and Cox proportional hazards models to analyze the risk factors on disease-free survival (DFS), cancer-specific survival (CSS) and overall survival (OS). 386 patients were categorized as normal nutritional status (GNRI ≥ 92) and 102 patients as malnutritional status (GNRI < 92). We used the receiver operating characteristic (ROC) curve for determined the association between GNRI and OS, with area under the curve (AUC) being 0.69. The 5-year survival rate of DFS, CSS and OS were 48.6%, 80.5% and 80.5% in the normal nutritional group and 28.0%, 53.2% and 40% in the malnutritional group. Using the multivariate analysis, malnutritional status was found as an independent risk factor for OS (hazard ratio [HR] = 3.94, 95% confidence interval [CI] 2.70–5.74), together with age (HR = 1.04, 95% CI 1.02–1.06), surgical margin positive (HR = 1.78, 95% CI 1.13–2.82), pathological T3 (HR = 2.54, 95% CI 1.53–4.21), pathological T4 (HR = 6.75, 95% CI 3.17–14.37) and lymphovascular invasion (HR = 1.81, 95% CI 1.16–2.81). We also found GNRI index as independent risk factor in DFS (HR = 1.90, 95% CI 1.42–2.54) and CSS (HR = 5.42, 95% CI 3.24–9.06). Preoperative malnutritional status with low GNRI is an independent marker in predicting DFS, CSS and OS in UTUC patients underwent RNU.
Similar content being viewed by others
Introduction
Urothelial carcinoma (UC) is a urothelial originated malignant disease, involving mostly the low urinary tract (bladder and urethral) while upper tract urothelial carcinoma (UTUC) is relative uncommon, accounting for only 5–10% of UCs1. Compared with western countries, the incidence of UTUC in Taiwan is much higher due to arsenic water contamination, herb consumption and prevalence hemodialysis, constituting 40.2% of all UCs2,3.
Radical nephroureterectomy (RNU) with bladder cuff excision is the standard treatment for clinical localized UTUCs4. Despite staging and surgical refinements, oncology outcome after RNU remain unchanged over the past decades5. Tumor stage and grade are the main prognostic factors. Analyses of the SEER database on 5-year cancer specific survival (CSS) showed 86% for T1N0, 77% for T2N0, 63% for T3N0 and 39% for locally advanced6. Other tumor-related factors that affect oncology outcomes include variant histology, lymph node involvement, lymphovascular invasion, surgical margins, extensive tumor necrosis and hydronephrosis7.
Patients’ factors such as comorbidity, American Society of Anesthesiologists (ASA), performance status (PS) and Charlson Comorbidity Index are also associated with survival outcome on top of the disease stage8,9.
Malnutrition is a common problem in cancer patients that may progress to cachexia, leading to poor response to therapy, relative poor prognosis and lower quality of life10. The geriatric nutritional risk index (GNRI), which consists of serum albumin level and the ratio of actual and ideal body weights, is a simple and accurate screening method initially designed to predict outcomes in hospitalized elderly patients11. Low GNRI is associated with poor prognosis in many human malignancies regarding less treatment response and shorter survival time12. The index could also be used in predicting perioperative and oncological outcomes for patients with esophageal cancer, gastric cancer, colorectal cancer, bladder cancer and kidney cancer who have received definitive radical surgery13,14,15,16,17. In metastatic urothelial carcinoma, GNRI index is known to be a useful predictive biomarker for chemotherapy and immune checkpoint inhibitors and poor nutrition status associated with less treatment response and survival18,19,20.
No study has yet been published regarding the association between the GNRI and localized UTUC. Here, we aim to investigate the impact of GNRI on survival outcomes of UTUCs receiving RNU.
Patients and methods
Patient selection
This study was retrospective chart reviewed analysis and it was approved by the Institutional Review Broad of Taichung Veterans General Hospital (IRB No. CE13240A-3) and informed consents were obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations. From 2001 to 2015, 728 patients with pathological confirmed UTUC underwent RNU with bladder cuff excision at Taichung Veterans General Hospital. Initially, 520 patients with primary UTUC and available medical record were included in the study. 13 patients were excluded due to loss of follow-up within the first year after operation and 2 patients were excluded due to died related to surgery. 15 patients were excluded due to concurrent radical cystectomy and 2 patients were excluded due to no albumin report. Finally, 488 patients were enrolled in the analysis.
RNU approached included traditional open nephroureterectomy through thoracoabdominal incision (n = 67), laparoscopic transperitoneal nephroureterectomy (n = 403) and retroperitoneoscopy nephroureterectomy (n = 18). We performed hilar lymph node dissection only in patients clinically suspicious lymph node metastasis before 2007. Since 2008, hilar lymph node dissection with or without regional lymph node dissection was routinely performed during RNU. The templates of regional lymph node dissection included para-aortic and peri-caval lymph node for renal pelvis and proximal ureter tumor and pelvic lymph node for distal ureter tumor. Adjuvant chemotherapies with cisplatin-based regimens were performed for those with advanced tumor feature (T3/4 or lymph node positive) but not routinely practiced according to clinicians preference21.
Tumor staging followed the American Joint Committee on Cancer and the International Union for Cancer Control updated tumor-node-metastasis (TNM) cancer staging system22. Tumor grade was determined in accordance with the 2004 World Health Organization/International Society of Urologic Pathology consensus classification23,24.
Surveillance protocol
All patients were under periodic monitoring protocol: every 3 months during the first two years after operation, every 3 or 6 months during the third year in the case of no evidence of recurrence or progression. The follow-up protocol included laboratory studies, urine cytology, computed tomography (or magnetic resonance imaging) and cystoscope evaluation.
The geriatric nutritional risk index
The nutritional status with GNRI values was calculated as follows: GNRI = 1.489 × serum albumin level (g/L) + 41.7 × (actual body weight [kg]/ideal body weight [kg])25. The ideal body weight was identified as [height (m)]2 × 22 (kg/m2). The value of the actual body weight/ideal body weight was set to 1 when the actual body weight exceeded the ideal body weight. Malnutritional status was defined as a GNRI < 92.0, according to previous literature15,20. Patients were divided into either the normal nutrition group (GNRI ≥ 92.0) and malnutrition group (GNRI < 92.0).
Patient characteristics
Patient characteristics included the following: gender, age at operation, Eastern Cooperative Oncology Group (ECOG) performance status, albumin, hypertension, diabetes mellitus, coronary artery disease, Body Mass Index (BMI, kg/m2), smoking status, renal function, surgical modality, tumor ___location, surgical margin status, pathological TNM stage, tumor grade, concomitant carcinoma in situ (CIS), lymphovascular invasion and adjuvant chemotherapy.
Outcome assessment and statistical analysis
End point assessment included: Disease Free Survival (DFS), Cancer Specific Survival (CSS) and Overall Survival (OS), as counted from the date of the RNU. Receiver operating characteristic (ROC) curve was used to determine the cut-off value for overall survival using the Youden index. Mann–Whitney U test was used for continuous variables, and Pearson’s chi-squared test was used for categorical variables. The Kaplain − Meier survival curve and log-rank test was used to determine survival outcomes. For the association between the variables, we used univariate and multivariate Cox hazard regression models to analyze the hazard ratio (HR) and 95% Confidence Interval (CI). Analyses were conducted using the Statistical Package for Social Sciences (SPSS), version 22.0.
Ethics statement
The studies involving human participants were reviewed and approved by certification at Taichung Veteran General Hospital, Taiwan, with Certification of approval with IRB: CE13240A-3. The patients/participants provided their written informed consent to participate in this study.
Results
Patient and characteristics
A total of 488 patients were enrolled in the study.102 patients were in the malnutrition group (GNRI < 92) and 386 patients were in the normal nutrition group (GNRI ≥ 92) (Table 1). The median age was 70.0 years (range 63.8–76.0) in the malnutrition group, and 67.0 years (58.0–76.0) in the normal nutrition group (p = 0.023). The median GNRI was 86.8 (range 83.4–89.3) in the malnutrition group, and 101.3 (range 96.9–104.3) in the normal nutrition group (p < 0.001). There was no statistical difference between the two groups in terms of comorbidity, smoking status, preoperative renal function, history of uremia, surgical modality and tumor ___location. The median follow-up period was 23.2 months (range 11.3–36.1) in the malnutrition group and 41.2 months (range 27.0–65.0) in the normal nutrition group (p < 0.001).
We hypothesized poor nutrition with low GNRI not only result from the patient himself but also the sequela of the more advanced malignant disease. In Table 1, we found that more advanced tumor in malnutrition group than in normal nutrition group such as pathological T stage (p = 0.067), pathological N stage (p < 0.001), positive surgical margin (16.7% vs. 8.5%, p = 0.026) and lymphovascular invasion (34.3% vs. 18.7%, p = 0.001). Additionally in supplementary Fig. 1 to 4, we found that high pathological T stage, high pathological N stage, lymphovascular invasion and surgical margin positive were associated with lower GNRI index score.
GNRI cut-off value
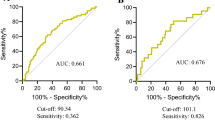
The ROC curve was plotted for GNRI as a predictive factor for OS and revealed the area under curve (AUC) was 0.69 with a cut-off value 93.58 months (Fig. 1a). When using the cut-off value as 93.58 months, the sensitivity was 48.33% and the specificity was 83.15% (AUC 0.657). When using the cut-off value as 92, the sensitivity was 43.33% and the specificity was 86.41% (AUC 0.649), respectively. We used Delong test to examine the two cut-off and showed no significant difference in predicting overall survival (p = 0.430) (Fig. 1b; Table 2). Thus, we think GNRI cut-off value 92 and 93.58 were both efficacy for patients with UTUC receiving RNU. Additionally, we used the cut-off value of 93.58 to categorize patient characteristics and their demographic as shown in Supplementary Table 1 and to exam the predict value in Fig. 3.
Overall survival
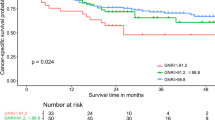
We compared OS between the malnutrition and normal nutrition groups. Using GNRI 92 as cut-off value, the median OS was 30.16 months in the malnutrition group and the median OS could not be calculated due to half of patients were survive in the normal nutrition group (p < 0.001) (Fig. 2a). Using GNRI 93.58 as the cut-off value, the median OS was 34.83 months in patients with GNRI < 93.58 and half of patients were survived in GNRI ≥ 93.58 (p < 0.001) (Fig. 3a). Univariate and multivariate analyses with COX regression revealed GNRI < 92 being an independent risk factor for OS (HR = 3.94, 95% CI 2.70–5.74, p < 0.001) (Table 3). We also found other independent risk factors for OS including the following: age (HR = 1.04, 95% CI 1.02–1.06, p < 0.001), surgical margin positivity (HR = 1.78, 95% CI 1.13–2.82, p = 0.013), pathological T3 (HR = 2.54, 95% CI 1.53–4.21, p < 0.001), pathological T4 (HR = 6.75, 95% CI 3.17–14.37, p < 0.001) and lymphovascular invasion (HR = 1.81, 95% CI 1.16–2.81, p = 0.008).
Kaplan–Meier curve for UTUC patients comparing normal nutrition group and malnutrition group according to GNRI = 92. (a) Overall survival, median 30.16 months in malnutrition group and half of patients were survive in normal nutrition group, p < 0.001. (b) Disease free survival, median 10.97 months in malnutrition group and 52.93 months in normal nutrition group, p < 0.001. (c) Cancer specific survival, median time were not calculated due to half of patients were survive in both group, p < 0.001.
Kaplan–Meier curve for UTUC patients according to cut-off value of GNRI by 93.58. (a) Overall survival, median 34.83 months in GNRI < 93.58 and half of patients were survive in GNRI ≥ 93.58, p < 0.001. (b) Disease free survival, median 12.81 months in GNRI < 93.58 and 52.93 months in GNRI ≥ 93.58, p < 0.001. (c) Cancer specific survival, median time were not calculated due to half of patients were survive in both group, p < 0.001.
Disease free survival
Using GNRI 92 as the cut-off value, the median DFS was 10.97 months in the malnutrition group, and 52.93 months in the normal nutrition group, (p < 0.001) (Fig. 2b). Using GNRI 93.58 as the cut-off value, the median DFS was 12.81 months in patients with GNRI < 93.58 and was 52.93 months in patients with GNRI ≥ 93.58 (p < 0.001) (Fig. 3b). Univariate and multivariate analyses with COX regression revealed GNRI < 92 as an independent risk factor of DFS (HR = 1.90, 95% CI 1.42–2.54, p < 0.001) (Table 4). We also found other independent risk factors for DFS including the following: surgical margin positivity (HR = 1.68, 95% CI 1.13–2.49, p = 0.010), pathological T4 (HR = 1.89, 95% CI 1.06–3.37, p = 0.031) and lymphovascular invasion (HR = 1.67, 95% CI 1.19–2.35, p = 0.003).
Cancer specific survival
Using GNRI 92 as the cut-off value, the median CSS could not be calculated due to half of patients were survive in both group and showed significant difference (p < 0.001) (Fig. 2c). Similar result was found when using GNRI 93.58 as the cut-off value (p < 0.001) (Fig. 3c). Univariate and multivariate analyses with COX regression revealed GNRI < 92 as an independent risk factor of CSS (HR = 5.42, 95% CI 3.24–9.06, p < 0.001) (Table 5). We also found other independent risk factors for CSS including the following: male gender (HR = 1.93, 95% CI 1.15–3.23, p = 0.012), age (HR = 1.04, 95% CI 1.01–1.07, p = 0.005), surgical margin positivity (HR = 2.16, 95% CI 1.26–3.71, p = 0.005), pathological T3 (HR = 6.30, 95% CI 2.57–15.43, p < 0.001) and pathological T4 (HR = 24.79, 95% CI 8.21–74.87, p < 0.001).
Patients age > 70 years old
Because GNRI index was initially designed for elderly patients, survival analysis was performed in patients elder than 70 years old11. In Fig. 4, GNRI index < 92 was associated with shorter OS (p < 0.001, Fig. 4a), DFS (p < 0.001, Fig. 4b) and CSS (p < 0.001, Fig. 4c) in patients age > 70 years old.
Kaplan–Meier curve for overall survival in UTUC patients elder than 70 years old comparing normal nutrition group and malnutrition group according to GNRI = 92. (a) Overall survival, median 25.36 months in GNRI < 92 group and it couldn’t be calculated due to more than half of them were survive in GNRI ≥ 92 group, p < 0.001** (b) Disease free survival, median 9.79 months in GNRI < 92 group and 63.44 months in GNRI ≥ 92 group, p < 0.001** (c) Cancer specific survival, median 37.09 months in GNRI < 92 group and it couldn’t be calculated due to more than half of them were survive in GNRI ≥ 92 group, p < 0.001**.
Perioperative complications
Perioperative complications were no significant differences among the two groups. A total 11 complications (13.7%) were found in malnutrition group, and 33 complications (8.5%) in the normal nutrition group (p = 0.868) (Supplementary table 2). Among these complications, 3 vascular injuries (2.9%) were in the malnutrition group, and 7 (1.8%) in the normal nutrition group (p = 0.506). There were 3 wound infections (2.9%) in the malnutrition group, and 8 (2.1%) in the normal nutrition group (p = 0.162) and 8 cases of ileus (7.8%) in the malnutrition group, and 18 (4.7%) in the normal nutrition group (p = 1.000).
Discussion
Our principal finding is that preoperative GNRI, as a nutritional status evaluation tool, is an independent prognostic factor for UTUC patients receiving RNU. Age, surgical margin positive, pathological T stage and lymphovascular invasion are also independently affect the overall survival under a long-term follow-up. Literature suggests that GNRI index < 92 as clinical trigger for nutritional support in institutionalised elderly26. We also used GNRI < 92 to verify the prognostic value. Additionally, based on the ROC curve analysis in our study, the cut-off value 93.58 may be more sensitive and specific in predicting survival outcome, although cut-off value 92 may be more convenient to use in clinical practice.
In our analysis, we found that low GNRI index was not only associated with individual physical condition, but also due to advanced tumor behavior. In the supplementary Fig. 1 to 4, we found that advanced pathological features including higher pathological T stage, pathological N stage, surgical margin positive and lymphovascular invasion had significant lower GNRI index value. As the result, this may explain why it was associated with poorer survival outcome.
Malnutrition is a common problem in hospitalized elderly patients, and it is associated with their functional decline and higher mortality rate27. Malnutrition may be characterized by loss of muscle or fat mass causing body weight loss, which is common for cancer patients as the result of cachexia and is responsible for 22% of deaths28. Pretreatment serum albumin levels provide useful nutritional assessment and prognostic significance for cancer patients. Serum albumin levels may drop due to tumor progression, immune response to tumor and anticancer therapy29. The nutritional risk index is first used to evaluate nutritional status and postoperative outcome which is calculated by albumin content, in terms of present body weight and usual body weight30. However, this index is not widely used because most elderly patients do not remember their usual body weight, and their weight loss may require correcting multiple contributing factors31. GNRI was proposed by Bouillanne et al., and with the usual body weight replaced by ideal body weight. It became a simplified and more convenient predictive tool11. Although albumin is a well-known index of nutrition status affecting wound healing and postoperative complication, it will be altered by digestive function and systemic inflammation32,33. In contrast, GNRI calculated by albumin, actual body weight and ideal body weight is more objective and easily determined and it was associated with risk of deaths in many human diseases such as diabetes mellitus, cardiovascular disease, end stage renal disease and cancers34.
Several studies reported the value of GNRI in predicting oncologic outcome and comorbidity in cancer patients receiving curative surgical treatment. Kubo et al., found that low GNRI are associated with high incidences of preoperative dysphagia, postoperative lung complications and 5-year overall survival in esophagus cancer13. Similar to our finding, they found that incidence of nodal metastasis and pathological stage were significantly higher in the GNRI-low group than in the GNRI-high group which contributed to the inferior survival outcomes after esophagectomy. Most of reported literatures focused on the gastrointestinal tract malignancies. Hirahara et al. and Sasaki et al. also reported that GNRI is an independent prognostic factor for OS in gastric patients underwent laparoscopic gastrectomy and in colorectal cancer patients after curative surgery14,15. Significantly higher incidence of postoperative complications was found in low-GNRI group, including surgical site infection, ileus, anastomotic leakage, intra-abdominal abscess, colitis, pneumonia, and urinary infection. The difference of post operative complications was not seen in our population. The possible reason is that no intestinal reconstruction during RNU and anastomotic leakage may cause subsequent complications.
Malnutrition is relative less common in genitourinary tract malignancy, and it may present as a sequela of advanced disease or paraneoplastic syndrome35. In a large-scale retrospective study, Kang et al. found that low values of GNRI are associated with aggressive pathologic characteristics and poor survival in patients with renal clear cell carcinoma who have nephrectomy17. Riveros et al. found that in bladder cancer patients receiving radical cystectomy, GNRI independently predicts mortality, blood transfusion, pneumonia, extended length of stay and non-home discharge16. Additional to survival and perioperative complications outcome, they also suggest that low GNRI was associated with extended length of hospital stay and nonhome discharge. Moreover, GNRI may being part of Enhanced Recovery after Surgery (ERAS) protocols for nutritional risk screening before radical cystectomy36.
Our present study is the first to investigate the relationship between GNRI and survival outcomes in UTUC patients receiving RNU, not only elderly but also young age populations. It could be the sequela of advanced tumor stage causing cancer cachexia or paraneoplastic syndrome and advanced malignant features were associated with low GNRI score. Additionally in multivariate analysis, we confirmed GNRI is the independent risk factor for OS, CSS and DFS.
Treatment protocol for UTUC changed over time. For example, whether performing template lymph node dissection during RNU was inconsistent in our populations. Meta-analysis for retrospective articles suggested templated-based lymph node dissection improve cancer specific survival in high-stage UTUC and reduces the risk of local recurrence37. Additionally, population-based cohort studies found that lymph node dissection improved survival outcomes not only in clinical lymph node negative but also pathological lymph node negative UTUC patients38,39. Nevertheless, template lymph node dissection was only routinely performed in our populations since 2008 and it may influence the survival outcome. Similarly, the POUT trial suggests that adjuvant gemcitabine-platinum combination chemotherapy significantly improved disease free survival40. However, only 23.9% patients in our population received adjuvant chemotherapy and this may influence the conclusion of our analysis.
Below are some limitations of our study. First, retrospective design had selection and information bias that had restricted the power of the prognostic role. Prospective cohort study is needed to overcome the limitations of the potential bias. Second, the surgical method is a possible confounder that impacts oncologic outcomes. The difference of surgical approach and template lymph node dissection may influence the outcome. Third, reports in the literature suggested that the nutrition status is associated with physical performance and the quality of life41. However, the research approach using questionnaires was not feasible due to the retrospective nature of our study. Finally, we did not assess the impact of neoadjuvant or adjuvant therapy which may have systemic impacts leading to malnutrition and influence the survival result. In the present era of using immune check-point inhibitors, further large scaled prospective cohort studies are needed to verify the association between GNRI and malignance.
Conclusions
Preoperative malnutritional status with low GNRI is an independent marker in predicting DFS, CSS and OS in UTUC patients underwent RNU. Age, surgical margin positivity, advanced tumor stage and lymphovascular invasion are also independent prognostic factors.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
06 December 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-81780-6
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. https://doi.org/10.3322/caac.21654 (2021).
Yang, M. H. et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 59, 681–687. https://doi.org/10.1016/s0090-4295(02)01529-7 (2002).
Ozsahin, M. et al. Prognostic factors in urothelial renal pelvis and ureter tumours: A multicentre Rare Cancer Network study. Eur. J. Cancer 35, 738–743. https://doi.org/10.1016/s0959-8049(99)00012-x (1999).
Margulis, V. et al. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer 115, 1224–1233. https://doi.org/10.1002/cncr.24135 (2009).
Adibi, M. et al. Oncological outcomes after radical nephroureterectomy for upper tract urothelial carcinoma: Comparison over the three decades. Int. J. Urol. 19, 1060–1066. https://doi.org/10.1111/j.1442-2042.2012.03110.x (2012).
Rosiello, G. et al. Contemporary conditional cancer-specific survival after radical nephroureterectomy in patients with nonmetastatic urothelial carcinoma of upper urinary tract. J. Surg. Oncol. 121, 1154–1161. https://doi.org/10.1002/jso.25877 (2020).
Rouprêt, M. et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2020 Update. Eur. Urol. 79, 62–79. https://doi.org/10.1016/j.eururo.2020.05.042 (2021).
Aziz, A. et al. Comparative analysis of comorbidity and performance indices for prediction of oncological outcomes in patients with upper tract urothelial carcinoma who were treated with radical nephroureterectomy. Urol. Oncol. 32, 1141–1150. https://doi.org/10.1016/j.urolonc.2014.04.008 (2014).
Chromecki, T. F. et al. Chronological age is not an independent predictor of clinical outcomes after radical nephroureterectomy. World J. Urol. 29, 473–480. https://doi.org/10.1007/s00345-011-0677-0 (2011).
Argilés, J. M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs 9(Suppl 2), S39-50. https://doi.org/10.1016/j.ejon.2005.09.006 (2005).
Bouillanne, O. et al. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 82, 777–783. https://doi.org/10.1093/ajcn/82.4.777 (2005).
Lv, G. Y., An, L. & Sun, D. W. Geriatric nutritional risk index predicts adverse outcomes in human malignancy: A meta-analysis. Dis. Markers 2019, 4796598. https://doi.org/10.1155/2019/4796598 (2019).
Kubo, N. et al. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus 16, 147–154. https://doi.org/10.1007/s10388-018-0644-6 (2019).
Hirahara, N. et al. Preoperative geriatric nutritional risk index is a useful prognostic indicator in elderly patients with gastric cancer. Oncotarget 11, 2345–2356. https://doi.org/10.18632/oncotarget.27635 (2020).
Sasaki, M. et al. The geriatric nutritional risk index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci. Rep. 10, 10744. https://doi.org/10.1038/s41598-020-67285-y (2020).
Riveros, C. et al. The geriatric nutritional risk index predicts postoperative outcomes in bladder cancer: A propensity score-matched analysis. J. Urol. 207, 797–804. https://doi.org/10.1097/ju.0000000000002342 (2022).
Kang, H. W. et al. A low geriatric nutritional risk index is associated with aggressive pathologic characteristics and poor survival after nephrectomy in clear renal cell carcinoma: A multicenter retrospective study. Nutr. Cancer 72, 88–97. https://doi.org/10.1080/01635581.2019.1621357 (2020).
Etani, T. et al. Low geriatric nutritional risk index as a poor prognostic marker for second-line pembrolizumab treatment in patients with metastatic urothelial carcinoma: A retrospective multicenter analysis. Oncology 98, 876–883. https://doi.org/10.1159/000508923 (2020).
Naiki, T. et al. First report of oncological outcome and prognostic analysis in a first-line setting of short hydration gemcitabine and cisplatin chemotherapy for patients with metastatic urothelial carcinoma. Oncology 99, 622–631. https://doi.org/10.1159/000517326 (2021).
Isobe, T. et al. Chronological transition in outcome of second-line treatment in patients with metastatic urothelial cancer after pembrolizumab approval: a multicenter retrospective analysis. Int. J. Clin. Oncol. 27, 165–174. https://doi.org/10.1007/s10147-021-02046-z (2022).
Hung, S. C. et al. Comparison of efficacy of adjuvant MEC (methotrexate, epirubicin and cisplatin) and GC (gemcitabine and cisplatin) in advanced upper tract urothelial carcinoma. Anticancer Res. 37, 1875–1883. https://doi.org/10.21873/anticanres.11525 (2017).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474. https://doi.org/10.1245/s10434-010-0985-4 (2010).
Mostofi, F. K., Sobin, L. H., Torloni, H. & World Health, O. International Histological Classification of Tumours, no. 10 (World Health Organization, 1973).
Soukup, V. et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: A European Association of urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur. Urol. 72, 801–813. https://doi.org/10.1016/j.eururo.2017.04.015 (2017).
Yamada, K. et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 87, 106–113. https://doi.org/10.1093/ajcn/87.1.106 (2008).
Cereda, E., Zagami, A., Vanotti, A., Piffer, S. & Pedrolli, C. Geriatric nutritional risk index and overall-cause mortality prediction in institutionalised elderly: a 3-year survival analysis. Clin. Nutr. 27, 717–723. https://doi.org/10.1016/j.clnu.2008.07.005 (2008).
Dent, E., Chapman, I., Piantadosi, C. & Visvanathan, R. Nutritional screening tools and anthropometric measures associate with hospital discharge outcomes in older people. Aust. J. Ageing 34, E1-6. https://doi.org/10.1111/ajag.12130 (2015).
Argilés, J. M., Olivan, M., Busquets, S. & López-Soriano, F. J. Optimal management of cancer anorexia-cachexia syndrome. Cancer Manag. Res. 2, 27–38. https://doi.org/10.2147/cmar.s7101 (2010).
Gupta, D. & Lis, C. G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 9, 69. https://doi.org/10.1186/1475-2891-9-69 (2010).
Buzby, G. P. et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: The rationale and impact of previous clinical trials and pilot study on protocol design. Am. J. Clin. Nutr. 47, 357–365. https://doi.org/10.1093/ajcn/47.2.357 (1988).
Robbins, L. J. Evaluation of weight loss in the elderly. Geriatrics 44(31–34), 37 (1989).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15, e493-503. https://doi.org/10.1016/s1470-2045(14)70263-3 (2014).
Lesourd, B. & Mazari, L. Nutrition and immunity in the elderly. Proc. Nutr. Soc. 58, 685–695. https://doi.org/10.1017/s0029665199000907 (1999).
Hao, X., Li, D. & Zhang, N. Geriatric nutritional risk index as a predictor for mortality: A meta-analysis of observational studies. Nutr. Res. 71, 8–20. https://doi.org/10.1016/j.nutres.2019.07.005 (2019).
Palapattu, G. S., Kristo, B. & Rajfer, J. Paraneoplastic syndromes in urologic malignancy: The many faces of renal cell carcinoma. Rev. Urol. 4, 163–170 (2002).
Burden, S., Billson, H. A., Lal, S., Owen, K. A. & Muneer, A. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst. Rev. 5, Cd010127. https://doi.org/10.1002/14651858.CD010127.pub2 (2019).
Dominguez-Escrig, J. L. et al. Potential benefit of lymph node dissection during radical nephroureterectomy for upper tract urothelial carcinoma: A systematic review by the European Association of Urology Guidelines Panel on non-muscle-invasive bladder cancer. Eur. Urol. Focus 5, 224–241. https://doi.org/10.1016/j.euf.2017.09.015 (2019).
Dong, F. et al. Lymph node dissection could bring survival benefits to patients diagnosed with clinically node-negative upper urinary tract urothelial cancer: A population-based, propensity score-matched study. Int. J. Clin. Oncol. 24, 296–305. https://doi.org/10.1007/s10147-018-1356-6 (2019).
Lenis, A. T. et al. Role of surgical approach on lymph node dissection yield and survival in patients with upper tract urothelial carcinoma. Urol. Oncol. 36(9), e1-9.e9. https://doi.org/10.1016/j.urolonc.2017.09.001 (2018).
Birtle, A. et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 395, 1268–1277. https://doi.org/10.1016/s0140-6736(20)30415-3 (2020).
Beberashvili, I. et al. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin. Nutr. 35, 1522–1529. https://doi.org/10.1016/j.clnu.2016.04.010 (2016).
Author information
Authors and Affiliations
Contributions
Study design and protocol development: L.W.C., S.C.H. and S.F.Y. Manuscript writing and editing: L.W.C. Statistical analysis: L.W.C. and S.C.H. Data collection and patient management: L.W.C., S.C.H., C.S.C., J.R.L., K.Y.C., S.S.W., C.K.Y., K.L., C.C.C., S.C.W., C.Y.L., C.L.C., Y.C.O. and S.F.Y. Supervision and revision: S.C.H. and S.F.Y.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, an Affiliation for Shun-Fa Yang was omitted. Full information regarding the correction made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, LW., Hung, SC., Chen, CS. et al. Geriatric nutritional risk index as a prognostic marker for patients with upper tract urothelial carcinoma receiving radical nephroureterectomy. Sci Rep 13, 4554 (2023). https://doi.org/10.1038/s41598-023-31814-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31814-2
This article is cited by
-
Preoperative geriatric nutritional risk index is useful factor for predicting postoperative delirium among elderly patients with degenerative lumbar diseases
European Spine Journal (2024)
-
Impact of a novel immune and nutritional score on prognosis in patients with upper urinary tract urothelial carcinoma following radical nephroureterectomy
Journal of Cancer Research and Clinical Oncology (2023)