Abstract
Metformin has shown outstanding anti-inflammatory and osteogenic abilities. Mesenchymal stem cell-derived extracellular vesicles (EVs) reveal promising therapeutic potency by carrying various biomolecules. This study explored the effects of metformin on the therapeutic potential of EVs derived from human periodontal ligament stem cells (PDLSCs) for periodontitis. PDLSCs were cultured in osteogenic medium with or without metformin, and the supernatant was then collected separately to extract EVs and metformin-treated EVs (M-EVs). After identifying the characteristics, we evaluated the anti-inflammatory and osteogenic effects of EVs and M-EVs in vivo and in vitro. Osteogenic differentiation of PDLSCs was markedly enhanced after metformin treatment, and the effect was dramatically inhibited by GW4896, an inhibitor of EVs’ secretion. Metformin significantly increased EVs’ yields and improved their effects on cell proliferation, migration, and osteogenic differentiation. Moreover, metformin significantly enhanced the osteogenic ability of EVs on inflammatory PDLSCs. Animal experiments revealed that alveolar bone resorption was dramatically reduced in the EVs and M-EVs groups when compared to the periodontitis group, while the M-EVs group showed the lowest levels of alveolar bone loss. Metformin promoted the osteogenic differentiation of PDLSCs partly through EVs pathway and significantly enhanced the secretion of PDLSCs-EVs with superior pro-osteogenic and anti-inflammatory potential, thus improving EVs’ therapeutic potential on periodontitis.
Similar content being viewed by others
Introduction
Periodontitis is a common chronic inflammatory diseases that occurs in periodontal ligament tissues in response to dental plaque1, and inflammation leads to progressive destruction of periodontal tissue and results in alveolar bone resorption, loose teeth, and ultimately tooth loss. Beyond its negative impact on patients’ oral function and quality of life, periodontitis also correlates with some systemic diseases, such as diabetes and cardiovascular diseases2. Therefore, a therapeutic strategy that can attenuate inflammation, reduce alveolar bone resorption, and promote the repair and regeneration of periodontal tissues, particularly alveolar bone, is pivotal for the management of periodontitis. Traditional treatments, including scaling and root planning and periodontal surgery, have proven to be limited to regenerating periodontal tissue3,4. Despite current advances in treatment methods, achieving effective regeneration of periodontal tissue still poses challenges.
Stem cell-based tissue engineering provides a promising strategy for periodontal regeneration5. Periodontal ligament stem cells (PDLSCs), an easily accessible source of mesenchymal stem cells (MSCs), have potent self-renewal and multi-differentiation capabilities, making them a suitable “seed cell” for periodontal tissue regeneration6,7. However, the capabilities of seed cells to contribute to tissue regeneration are notably hindered under inflammatory conditions. Extracellular vesicles (EVs), being nanoparticles derived from almost all cells, exhibit excellent advantages, such as less ethical concerns and improved safety, providing enormous potential and wide applications in regenerative medicine when compared to its parent cells8,9,10. MSCs-EVs exhibit extensive therapeutic potential for periodontitis by enhancing the biological potential of stem cells, restoring the impaired capacity of inflammatory stem cells, and modulating the inflammatory immune response to mitigate destruction of periodontal tissue and promoting the regeneration of periodontal tissue11. Despite the considerable therapeutic potential of MSCs-EVs for bone regeneration, there exist certain limitations that impede their clinical application, such as limited bioactivity, low yield, poor bone targeting properties, and limited efficacy12,13. The biogenesis, cargo, and function of EVs are highly related to the cultural environment of their parent cells, thereby more studies have focused on the optimized methods of MSCs that can improve the therapeutic effects of EVs14,15,16,17.
Researchers are delving into the mechanism dehind MSCs' response to periodontal inflammation18,19,20, aiming to exploring some drugs or active factors that protect their activity and pluripotency in inflammatory environments, thereby promoting periodontal tissue regeneration21,22,23,24,25. Metformin (Met), an anti-hyperglycemic biguanide compound, is the most widely used oral hypoglycemic agent for the treatment of type 2 diabetes mellitus26. Recently, some other functions of Met have been gradually excavated, such as anti-aging, anti-oxidative stress, anti-tumor, anti-inflammatory, immunoregulatory and anti-cardiovascular diseases27,28,29. Met has been reported that has excellent therapeutic effects for periodontitis by promoting osteoblast proliferation and inhibiting osteoclast activity30. Met enhanced the proliferation, migration, and osteogenic differentiation of PDLSCs31, and promoted bone formation through synergistic regulation of osteogenesis and angiogenesis32. In addition, Met significantly alleviated bone resorption by regulating osteoblast and osteoclast differentiation33, attenuating oxidative and cytotoxic action of hypoxia34, protecting PDLSCs against oxidative stress-induced damage25, and reducing inflammatory immune response in experimental periodontitis rat models.
Recent studies found that Met exerts therapeutic effects by regulating the biogenesis and secretion of EVs derived from human glioblastoma cells35 and stimulated the release of MSCs-EVs and enhanced its therapeutic efficacy in intervertebral disc degeneration36. Therefore, this study was designed to study whether the anti-inflammatory and osteogenic effects of Met for the treatment of periodontitis can be explained by its effects on EVs, and to evaluate the effects of Met on PDLSCs-EVs’ secretion and therapeutic efficacy for periodontitis. The findings indicated that the osteogenic differentiation of PDLSCs was prominently enhanced by Met and dramatically inhibited by GW4869. Moreover, Met significantly increased the secretion of EVs derived from PDLSCs with enhanced pro-osteogenic and anti-inflammatory capabilities, thereby improving their therapeutic efficacy for ameliorating alveolar bone loss in periodontitis. These results provide a theoretical foundation for the application of Met in the treatment of periodontitis and for the optimization of PDLSCs-EVs.
Materials and methods cell culture
This study was approved by the ethics committee of Zunyi Medical University (Lunsheng (2020)1–110) and all guardians of patients signed the informed consent. All experiments were performed in accordance with relevant guidelines and regulations. The isolation procedure of PDLSCs were same as the previously described37. Periodontal ligament tissues were extracted from the harvested premolars due to orthodontic purpose, and these tissues were digested in 3 mg/ml type I collagenase (Sigma-Aldrich, USA) at 37 °C for 30 min. After centrifugation, the precipitate was resuspended and then subsequently seeded in culture dishes cultured with complete medium at 37 ℃ with 5% CO2. The medium was prepared with a-Minimum Essential Medium (a-MEM; HyClone, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 1% Penicillin–Streptomycin Solution (100X; Solarbio, Beijing, China). Cells between passage 3–5 were used for further treatment.
Identification of PDLSCs
PDLSCs were cultured separately in osteogenic medium (50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone in normal growth media), adipogenic medium (0.5 µM dexamethasone, 0.5 mM isobutyl methylxanthine, 60 µM indomethacin, and 10 µg/ml insulin in normal growth media) or chondrogenic medium (OriCell, GuangZhou, China) to verify these multilineage differentiation capabilities. After induction for a predetermined time, cells were washed three times with PBS, fixed with 4% paraformaldehyde and respectively stained with Alizarin Red solution (Solarbio, Beijing, China) to detect the formation of mineralized matrix, stained with the Oil Red O (Solarbio, Beijing, China) to detect the formation of lipid droplets or stained with the Alcian Blue solution (Solarbio, Beijing, China) to detect the formation of chondrocyte. A flow cytometry assay was used to identify the cell immunophenotypes (CD44, CD45, CD73, CD90 and CD105) using the Human MSC Analysis Kit (BD, San Diego, CA, USA) according to the manufacturer’s instructions.
Isolation and identification of EVs
PDLSCs (P5) were seeded to 10 cm plates (5 × 106 cells/plate) and cultured in 9 ml complete medium. The medium was refreshed to osteogenic medium prepared with EVs-free FBS when cells reached 80% confluency. EVs-free FBS was achieved by centrifuged at 120000g 4℃ for 18 h. EVs and M-EVs were respectively isolated from supernatant treated with or without Met for 48 h using sequential ultracentrifugation. Briefly, the supernatant was centrifuged at 300 g for 10 min, 3000 g for 10 min, 10,000 g for 30 min, and finally 110,000 g for 70 min. And the pellet was washed with PBS, then centrifuged at 110,000 g for 70 min, and finally resuspended with PBS before packaging and storing at -80 ℃. Transmission electron microscopy (TEM) (Hitachi, HT-7700) was used to inspect the shape of EVs. Nanoparticle tracking analysis (NTA) was used to examine the particle size distribution of EVs using an NS300 nanoparticle analyzer (Nanosight, Malvern, Worcestershire, UK). BCA Protein Assay Kit (Solarbio, Beijing, China) was used to quantify the total protein concentrations of EVs. Western blotting was performed to assess the expression of CD63 (1:2000; Abcam; ab134045), CD81 (1:2000; Abcam; ab109201), and Calnexin (1:2000; Abcam; ab133615).
Cellular uptake assay
PDLSCs were seeded in glass-bottom cell culture dishes and incubated with PKH26-labeled EVs or M-EVs (10 μg/ml) for 6 h at 37 °C. PKH26 (red) dye (Solarbio, Beijing, China) was used to label EVs and M-EVs according to the manufacturer’s protocol. After co-culture with EVs or M-EVs, cells were washed three times with PBS, fixed with 4% paraformaldehyde, and these nuclei were stained with DAPI (Boster, Wuhan, China). The results were obtained using laser scanning confocal microscopy (Zeiss LSM 900 Airyscan, Germany).
CCK-8 assay
PDLSCs were seeded onto the 96-well plate (4 × 103 cells per well) and incubated overnight. After culture with EVs or M-EVs (10 μg/ml) for 1, 3, 5, and 7 d, the proliferation of PDLSCs was detected using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). As per the manufacturer’s instructions, CCK-8 solution was added to each sample at 10% volume and incubated for 2 h, after which the absorbance was measured at 450 nm using a microplate reader.
Cell Migration Assay
Cell migration was evaluated using a non-contact transwell chamber with 8 μm pore filters (Corning, Kennebunk, ME, USA). Briefly, 2.5 × 103 PDLSCs were seeded onto the top well inserts, and 800 μl of medium containing EVs or M-EVs were added to the lower compartment. After 24 h of culture, the chamber was washed three times with PBS. To eliminate non-migrated cells, the upper side of the top well inserts was gently wiped using cotton swabs. The top well inserts were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Images were captured using a microscope (Leica), and the number of migrated cells was counted in five randomly selected fields per well.
Scratch assay
For the scratch assay, 5 × 104 PDLSCs per well were cultured in 6-well plates for 24 h to allow cell adhesion and reach 80% confluency. After the medium was changed to a low-serum (0.5% FBS) culture medium to reduce the rate of proliferation, the cell monolayer was mechanically “wounded” by scraping with a 200 μl sterile pipette tip. Cell monolayers were immediately washed with PBS, and images were captured. Cell monolayers were then treated with the low-serum culture medium containing EVs or M-EVs. Images were captured at 0, 12, and 24 h after the scratch with the microscope. Cell migration rate has been estimated as percent scratch closure using the ImageJ software (National Institutes of Health, Bethesda, MD, USA; https://imagej.net/ij/). All scratch assays were performed in triplicates, and three fields per well were analyzed.
ALP staining and ALP activity
After 7 days of osteogenic induction, ALP staining and ALP activity detection were used to analyze the osteogenic differentiation level of cells. ALP staining was performed using the BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, Shanghai, China), and the Alkaline Phosphatase Assay kit (Beyotime, Shanghai, China) was used to quantify the ALP activity.
ARS staining and quantification
ARS staining was used to assess the bone matrix mineral synthesis according to the manufacturer’s protocol. PDLSCs were washed with PBS and fixed in 4% paraformaldehyde for 30 min after 21 d of co-culture. After three washes with PBS, PDLSCs were incubated in Alizarin Red S Solution (1%, pH 4.2, Solarbio, Beijing, China) for 60 min at room temperature, followed by three washes with PBS to terminate the reaction. After taking photos with an inverted microscope, the mineralized matrix was dissolved in 10% cetylpyridinium chloride (Solarbio, Beijing, China) and quantitatively analyzed for the absorbance value at 562 nm using a microplate reader.
Quantitative real time-polymerase chain reaction (qRT-PCR)
Total cellular RNA was extracted from cells using TRIzol Reagent (InvivoGen), cDNA was synthesized using the Reverse Transcription Kit (Takara, Kusatsu, Japan), and quantitative polymerase chain reaction was performed using the Real-time SYBR reagent (Takara) and Bio-Rad CFX Real-time PCR system. We assessed the expression levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), alkaline phosphatase (ALP), runt-related transcription factor-2 (Runx2) and osteocalcin (OCN). Relative expressions were calculated using the 2− ΔΔCt method and normalized by the Ct value of the housekeeping gene GAPDH. The primer sequences are listed in Table S1.
Animal experiments
The animal experiments were approved by the ethics committee of Zunyi Medical University (Lunsheng (2020)2–217). Animal experiment was reported in accordance with the ARRIVE guidelines 2.0. 40 Male Sprague Dawley (SD) rats (age: 8 weeks, weight: 200–250 g) were purchased from and housed in the Animal Experiment Center of Zunyi Medical University (Guizhou, China). Ligated stimulated periodontitis rats (PD rats) model were used to evaluate the treatment effect of M-EVs on periodontitis38. In brief, rats were anaesthetized with an intraperitoneal injection of 2% pentobarbital sodium (0.3 ml/100 g body weight); then, surgical nylon thread (3–0) was ligated in gingival sulcus of the right maxillary second molar to induce experimental periodontitis. After induction for 4 weeks, all animals were randomly divided into 5 groups (n = 8): normal group (no ligation treatment), PD group (PD rats without treatment), PBS group (PD rats treated with PBS), EVs group (PD rats treated with 200 ug EVs), M-EVs group (PD rats treated with 200 ug M-EVs). PBS, EVs and M-EVs were injected into the palatal gingiva of the experimental rats over a period of 4 weeks (once every 1 week). These rats were euthanized by cervical dislocation after 4 weeks of treatment, and maxilla samples were collected and evaluated by two researchers who blinded the group allocation and intervention.
Histological staining and histopathological evaluation
After fixation with 4% paraformaldehyde, six samples in each group were scanned and reconstructed by micro-CT (VivaCT40, SCANCO Medical AG, Switzerland). Alveolar bone loss was quantified by the distance between the cementoenamel junction and the alveolar bone crest (CEJ-ABC). The samples were decalcified with 10% ethylenediaminetetraacetic acid disodium and were embedded in paraffin after being dehydrated in a gradient series of alcohol to slice into 5 µm-thick slices. Then, for histological analysis, sections were subjected to hematoxylin and eosin (HE) staining, TRAP staining, and immunohistochemical staining (IHC) to visualize alveolar bone alveolar bone. And the quantitation of IHC was calculated using Average Opitical Density (AOD) of fluorescence expression in the periodontal areas.
Statistical analysis
All experiments were repeated at least three times to confirm the reliability of the study. All data were presented as the mean value ± standard deviation (SD) and analyzed by Student’s t-test (α = 0.05) or one-way analysis of variance (ANOVA) with a Tukey’s post hoc test using GraphPad Prism version 8.3.0 (GraphPad Software, USA; https://www.graphpad.com). P < 0.05 was considered statistically significant.
Results
Isolation and characterization of PDLSCs
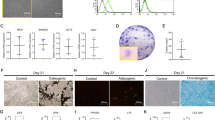
PDLSCs were isolated from human premolar periodontal tissue, and the spindle-like shape of the cultured cells was monitored using an inverted microscope (Fig. 1A). The multi-differentiation capacity of isolated PDLSCs was confirmed by inducing osteogenesis, adipogenesis, and chondrogenesis (Fig. 1B-D). Flow cytometry analysis verified that the majority of PDLSCs were positive for CD44 (99.9%), CD90 (99.94%), CD73 (99.71%) and CD105 (99.16%) markers, and for CD45 (0.84%) markers were relatively negative (Fig. 1E).
Characteristics of human PDLSCs. (A) Primary PDLSCs (Scale bar: 200 μm). (B) Alizarin Red S staining for osteogenic differentiation (scale bar, 100 μm). (C) Alcian blue staining for chondrogenic differentiation (scale bar, 100 μm). (D) Oil Red O staining for adipogenic differentiation (scale bar, 50 μm). (E) Flow cytometric analysis demonstrating that PDLSCs were positive for the mesenchymal stem cell (MSC) markers CD44, CD90, CD73 and CD105 and negative for the hematopoietic stem cell (HSC) marker CD45.
GW4869 impedes the osteogenic effects of Met on PDLSCs
To explore the effect of Met on osteogenic differentiation of PDLSCs, cells were co-cultured with the osteogenic medium added with 0, 10, 50 or 100 µM Met for the planned days. The Met groups showed the improved osteogenic potential when compared to the control group and 50 µM group displayed the most significant osteogenic potential as evidenced by ALP staining, ALP activity, Alizarin Red Staining, and the expression of the osteogenic genes ALP, Runx 2, and OCN (Fig. 2A-C). Taken together, these results showed that Met promoted the osteogenic differentiation of PDLSCs, and 50 µM was chosen as the optimal concentration used in the subsequent experiments.
Effects of Met on osteogenic differentiation of PDLSCs. (A) The ALP staining and ALP activity of PDLSCs treated with Met for 7 days (scale bar, 100 μm; n = 3). (B) Photographs and quantitative analysis of alizarin red S staining (scale bar, 100 μm; n = 3). (C) The osteogenic genes (ALP, Runx2 and OCN) in PDLSCs were analyzed by qRT-PCR analysis (n = 3). (D) The ALP staining and ALP activity of PDLSCs (scale bar, 100 μm; n = 3). (E) Photographs and quantitative analysis of alizarin red S staining (scale bar, 100 μm; n = 3). (F) The osteogenic genes (ALP, Runx2 and OCN) were analyzed by qRT-PCR analysis (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To explore the role of EVs on osteogenic potential of Met, GW4869, an inhibitor of EVs’ secretion, was used to inhibit the release of EVs. PDLSCs were incubated in the osteogenic medium supplemented with Met and/or GW4869 for projected days. The results shown that GW4869 significantly inhibited the effects of Met on osteogenic differentiation of PDLSCs as shown in Fig. 2D-F.
Characterization of EVs and M-EVs
EVs and M-EVs were successfully isolated from the supernatant of PDLSCs treated with or without Met using sequential ultracentrifugation approach. TEM showed that both EVs and M-EVs were characterized by double-membrane structures and cup-shaped morphology (Fig. 3A). WB results demonstrated that these particles positively expressed CD63 and CD81 and negatively expressed Calnexin (Fig. 3B). NTA analysis found that EV and M-EV had a similar size, with diameters ranging from 50 to 200 nm (Fig. 3C). Taken together, the vesicles were proved to have the predominantly characteristic of EVs according to the results of morphology, size, and protein markers.
Identification and internalization of EVs and M-EVs. (A) Morphology of EVs under transmission electron microscopy (scale bar, 100 nm). (B) EVs’ surface markers (CD63 and CD81) and negative marker (Calnexin) were detected by western blotting. All PVDF bands are transferred and tested in the same Wb experiment. (C) The size distribution profile of EVs and M-EVs was identified by nanoparticle analysis. (D) Quantitative analysis of the total protein concentration of EVs and M-EVs by a BCA protein assay (n = 3). (E) Fluorescence microscopy analysis of PKH26-labeled EVs (red) internalized by PDLSCs. Nuclei were stained with DAPI (blue) for counterstaining (scale bar, 20 μm). *P < 0.05, **P < 0.01, ***P < 0.001.
To study the effect of Met on the yields of EVs, EVs and M-EVs were isolated from an equivalent amount of supernatant obtained from the same number of PDLSCs cocultured in the presence or absence of Met using the same procedure. The total protein of EVs was obviously increased in the Met group when compared to the control group (Fig. 3D). To confirm the cellular uptake of EVs, PDLSCs were cultured with PKH67-labeled EVs or M-EVs for 12 h and scanned with a laser scanning confocal microscope. The image showed that PKH26-labelled EVs and M-EVs (red dots) were internalized by cells and distributed in cytoplasm (Fig. 3E).
Met enhanced the osteogenic and anti-inflammatory potential of EVs
To investigate the role of Met on the effect of EVs on cell proliferation and migration, PDLSCs was cultured in medium with EVs (10 μg/ml) or M-EVs (10 μg/ml). The results of CCK-8 indicated that EVs significantly promoted PDLSC proliferation compared to control group and Met significantly enhanced the promotion effect of EVs on cell proliferation (Fig. 4A). The transwell assay found that both EVs and M-EVs demonstrated an effect of increasing cell migration when compared to the control group and Met apparently augmented the effect of EVs on cell migration (Fig. 4B,C). And scratch assay identified the same results (Fig. 4D,E). To investigate the effect of Met on the osteogenic effect of EVs, PDLSCs was cultured in osteogenic induction medium with EVs (10 μg/ml) or M-EVs (10 μg/ml). Figure 4F-J demonstrated that M-EVs have an enhanced osteogenic effect with increased the expression of osteogenic gene (ALP, RUNX2 and OCN), ALP activity and calcium nodules when conpared to EVs.
Met enhanced EVs’ effects on the proliferation, migration, and osteogenic differentiation of PDLSCs. (A) The proliferation of PDLSCs measured by CCK8 assay (n = 3). (B, C) Quantitative analysis and photographs of transwell assay (scale bar, 100 μm; n = 3). (D, E) Quantitative analysis and photographs of scratch assay (scale bar, 200 μm; n = 3). (F) The osteogenic genes (ALP, Runx2 and OCN) in PDLSCs were analyzed by qRT-PCR analysis (n = 3). (G, H) The ALP staining and ALP activity of PDLSCs treated with EVs or M-EVs for 7 days (scale bar, 100 μm; n = 3). (I, J) Photographs and quantitative analysis of alizarin red S staining in EVs or M-EVs treated groups on day 21 (scale bar, 100 μm; n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Considering that PDLSCs in the inflammatory microenvironment are different from those in the normal microenvironment, the effect of PDLSCs-EVs on inflammatory PDLSCs needs to be further explored. Therefore, we further evaluated the effect of Met on the osteogenic and anti-inflammatory potential of EVs. Pg-LPS was used to induce inflammation of PDLSCs, and then the inflammatory PDLSCs (iPDLSCs) were treated with EVs or M-EVs. Figure 5A-B shows that LPS significantly increased the expression of inflammatory factors (IL-6 and IL-8) and dramatically inhibited the expression of osteogenic genes (ALP, Runx2 and OCN) when compared to normal PDLSCs, which identified that Pg-LPS inhibited the the osteogenic differentiation of PDLSCs by inducing inflammation. However, both EVs and M-EVs had the ability to promote the osteogenic differentiation and inhibit the inflammatory cytokines of iPDLSCs, and M-EVs had an enhanced potential for the osteogenic differentiation of iPDLSCs when compared to EVs. ALP activity and ARS staining shows the same results (Fig. 5C-F).
Effects of Met on osteogenic differentiation of inflammatory PDLSCs induced by LPS. (A) The expression of inflammatory factors (IL-6 and IL-8) in PDLSCs were analyzed by qRT-PCR analysis (n = 3). (B) The osteogenic genes (ALP, Runx2 and OCN) in PDLSCs were analyzed by qRT-PCR analysis (n = 3). (CD) ALP staining and ALP activity (scale bar, 100 μm; n = 3). (EF) Photographs and quantitative analysis of alizarin red S staining (scale bar, 100 μm; n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
M-EVs exerted enhanced effects in ameliorating alveolar bone resorption in periodontitis
The ligature-induced experimental periodontitis Sprague–Dawley rat model was utilized to evaluate the therapeutic effect of M-EVs on periodontitis. Micro-CT showed a significant increase in the ABC-CEJ distance in the PD group compared to the normal group; Compared with the PD group, there was no significant change in the ABC-CEJ distance in the PBS group; Compared to the PD group, the ABC-CEJ distance between the Exo group and the M-Exo group was significantly reduced; The difference in ABC-CEJ distance between the Exo group and the M-Exo group is statistically significant (Fig. 6A,B). In addition, H&E staining revealed that M-EVs group had greater new bone regeneration and fewer inflammatory cells compared to the PBS or EVs groups (Fig. 6C). In the progression of periodontitis, TRAP+ osteoclast cells were involved in alveolar bone resorption. TRAP staining indicated that compared to the PBS or EVs groups, the M-EVs group exhibited lesser number of osteoclasts in the periodontium (Fig. 6D,E). To further confirm the osteogenic effect of M-EVs on experimental periodontitis, we performed immunohistochemical staining with osteogenic markers (OCN) (Fig. 6F). Semi-quantitative analysis showed that the M-EVs group had significantly higher OCN expression than other groups (Fig. 6G). These results suggested that EVs can reduce alveolar bone loss in periodontitis rats, and Met can increase the treatment effect of EVs on PD rats. This study provides a foundation to offer an applicable approach to the application of Met in periodontitis and to optimize the therapeutic effects of MSCs-EVs.
Met enhances the effects of EVs to ameliorate alveolar bone loss in SD rats with periodontitis. (A) Micro-CT images of alveolar bone loss (Scale bar: 1 mm). (B) Bone resorption was calculated as the distance from the buccal cementoenamel junction (CEJ) to the alveolar bone crest (ABC) (n = 6). (C) HE staining (Scale bar: 200 μm and 100 μm). (D) TRAP staining: Osteoclasts formation in different groups (Scale bar: 20 μm). (E) Quantitative analysis of TRAP-positive cells (n = 4). (F) Immunofluorescence staining for OCN (Scale bar: 20 μm). (G) Quantitative analysis of immunofluorescence staining for OCN (n = 6).). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In this study, we explored the impact of Met on the therapeutic potential of PDLSCs-EVs for periodontitis (Fig. 7). While previous studies have demonstrated Met's positive influence on PDLSCs proliferation, migration, and osteogenic differentiation25,31,32, the role of EVs in the osteogenic effect of Met remains unclear. We found that Met markedly enhanced the osteogenic differentiation of PDLSCs, while this effect was significantly impeded by inhibiting the release of EVs using GW4869. In order to investigate the effect of Met on the secretion and function of PDLSCs-EVs, we isolated PDLSCs-EVs and Met treated PDLSCs-EVs, respectively, and evaluated the yield and the pro-osteogenic and anti-inflammatory effects of EVs and M-EVs in vitro and in vivo. These results indicated that Met increased EVs yields and enhanced the therapeutic function of EVs for periodontitis. Compared with EVs, M-EVs had an enhanced ability to promote proliferation, migration, and osteogenic differentiation of PDLSCs, moreover, promote osteogenic differentiation and reduce the expression of inflammatory factor in iPDLSCs. Furthermore, Met could significantly enhance the therapeutic efficacy of EVs to reduce the alveolar bone resorption by decreasing inflammatory bone loss and increasing bone regeneration in PDs rats. In general, this study provides a foundation to offer an applicable approach to the application of Met and MSCs-EVs in the treatment of periodontitis and suggests a potential strategy for optimizing the therapeutic effects of MSCs-EVs.
Met is well known as a commonly used oral drug for diabetes, but recent studies has shown that it has some exciting properties, including anti-oxidative capabilities, anticancer, genomic stability, anti-inflammation, and anti-fibrosis39, which have potential for some other disorders other than diabetes mellitus40. Recent evidence indicated that long-term use of Met is associated with a significantly reduced risk of periodontal diseases41. Met significantly reduced the probing depth, increase clinical attachment level, and improve intra-bony defects depth reduction in periodontitis42. Met affects bone cells, including osteocytes, osteoblasts, and osteoclasts, suggesting that Met is a promising drug to promote bone formation by regulating the balance of osteogenesis and osteoclasis to bone formation43. Consistent with Zhang et al.’ results, we found that Met promoted the proliferation, migration, and osteogenic differentiation of PDLSCs. In addition, we evaluated the role of EVs in Met's effect on osteogenic differentiation of PDLSCs and discovered that Met's osteogenic effect was significantly reduced after inhibiting EVs release by GW4869. These findings suggested that EVs could potentially serve as a pivotal pathway for the osteogenic function of Met on PDLSCs. Other studies have also shown that EVs play an important role in regulating the regenerative ability of cells14,44,45.
PDLSCs have now been considered as a potential source for periodontal regeneration with strong proliferative, multi-differentiation, immune regulation, and rapid proliferation capabilities46. Meanwhile, with the in-depth research of MSCs-EVs on regenerative medicine, the research related to PDLSCs-EVs has also attracted the attention of many scholars. Previous studies have confirmed that PDLSCs-EVs have the abilities to enhance proliferation47, promote osteogenic differentiation48, rescue the osteogenic ability of PDLSCs inhibited by inflammation49,50,51, repress macrophage pyroptosis52, induce M1 polarization of macrophages53, alleviate inflammation by regulate the balance of Th17/Treg54, and promote angiogenesis55, thus to effectively attenuate bone loss and promote periodontal regeneration in periodontitis. Our data indicated that PDLSCs-EVs enhance the osteogenic differentiation of PDLSCs in normal and inflammatory microenvironment, and thereby have potential to reduce the alveolar bone resorption and promote new bone regeneration in periodontitis.
However, the limited yield and efficacy of EVs remains the major obstacles to their application in medicine56. Some optimize methods of donor cells, such drug, gene modification, force stimulation, etc., have the effect on the biological activities of cells and thereby affect the EVs’ biogenesis, secretion and function57,58,59,60. Therefore, we further investigated whether Met affect the secretion of PDLSCs-EVs and alters their biological effects. These studies revealed that PDLSCs treated with Met released more EVs compared to control group, which was consistent with Soraya et al.'s research35, and M-EVs had an obvious enhanced osteogenic effect compared to Met or EVs. Therefore, M-EVs could be used as a new strategy for the application of Met in the treatment of periodontitis.
Systemic delivery can unintentionally burden other organs, leading to unwanted reactions and potentially compromising the intended treatment's usefulness. Conversely, local application delivers the needed impact at the targeted ___location using lesser doses for better efficiency61. Therefore, local administration is more optimal for the application of Met on periodontitis. We found that compared to Met, PDLSCs-EVs have stronger effects on promoting cell proliferation, migration, and osteogenic differentiation, reducing the expression of inflammatory factors and alleviating their osteogenic differentiation ability in inflammatory PDLSCs, while Met treatment would significantly augment the therapeutic effects of EVs in periodontitis. Therefore, the application of Met to enhance the treatment efficiency of PDLSCs-EVs, rather than using Met directly, has the relevant advantages of EVs based cell-free therapy and may be a potential method to enhance the therapeutic effect on PDs.
However, there are still some limitations that need to be addressed. Firstly, fow cytometry and NTA are both reliable and accurate methods for measuring EVs’ concentrations62,63. However, we applied NAT instead of flow cytometry to analyze EVs, liking some related high-quality research58,64. And it is necessary to use fow cytometry for EVs analyses in our future research. Notably, the effect of Met on the functions of EVs may be illustrated by the changing cargo (miRNAs and/or proteins). Thus, further RNA-seq and proteomic analyses should be performed to fully understand the mechanism behind the improved therapeutic properties of M-EVs. Subsequently, exploring the appropriate sustained-release scaffold for EVs to simplify the treatment process and precisely regulate host cell function during repair and regeneration to enhance the therapeutic effect is an important research direction in the future. Ultimately, EVs in clinical trials had to comply with good manufacturing practice, which includes cell culture microenvironment, isolation, and purification method of EVs, and the quality control of EVs. The cargos of EVs varies from cell type to culture conditions and batch, which causes differences in biological functions. Therefore, it is necessary to explore more convenient and efficient technologies for the isolation, purification, and storage of EVs to ensure their homogeneity, purity, and repeatability.
Conclusions
Osteogenic differentiation of PDLSCs was significantly enhanced after Met treatment while dramatically inhibited by GW4896. Met significantly increased EVs’ yields and improved their effects on cell proliferation, migration, osteogenic differentiation, and ameliorating alveolar bone resorption in periodontitis. Therefore, Met promoted the osteogenic differentiation of PDLSCs partly through EVs pathway and significantly enhanced the secretion of PDLSCs-EVs with superior pro-osteogenic and anti-inflammatory potential, thus improving EVs’ therapeutic potential on periodontitis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdulkareem, A. A. et al. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral. Microbiol. 15, 2197779. https://doi.org/10.1080/20002297.2023.2197779 (2023).
Sedghi, L. M., Bacino, M. & Kapila, Y. L. Periodontal disease: The good, the bad, and the unknown. Front. Cell Infect. Microbiol. 11, 766944. https://doi.org/10.3389/fcimb.2021.766944 (2021).
Graziani, F., Karapetsa, D., Alonso, B. & Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease?. Periodontol 2000(75), 152–188. https://doi.org/10.1111/prd.12201 (2017).
Deas, D. E., Moritz, A. J., Sagun, R. S. Jr., Gruwell, S. F. & Powell, C. A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol 2000(71), 128–139. https://doi.org/10.1111/prd.12114 (2016).
Ouchi, T. & Nakagawa, T. Mesenchymal stem cell-based tissue regeneration therapies for periodontitis. Regen. Ther. 14, 72–78. https://doi.org/10.1016/j.reth.2019.12.011 (2020).
Tassi, S. A., Sergio, N. Z., Misawa, M. Y. O. & Villar, C. C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 52, 793–812. https://doi.org/10.1111/jre.12455 (2017).
Trubiani, O. et al. Periodontal ligament stem cells: Current knowledge and future perspectives. Stem Cells Dev. 28, 995–1003. https://doi.org/10.1089/scd.2019.0025 (2019).
Adelipour, M., Lubman, D. M. & Kim, J. Potential applications of mesenchymal stem cells and their derived exosomes in regenerative medicine. Expert Opin. Biol. Ther. 23, 491–507. https://doi.org/10.1080/14712598.2023.2211203 (2023).
Marconi, G. D., Diomede, F., Pizzicannella, J. & Trubiani, O. Emerging role of oral mesenchymal stem/stromal cells and their derivates. Int. J. Mol. Sci. https://doi.org/10.3390/ijms241512003 (2023).
Trubiani, O. et al. Human oral stem cells, biomaterials and extracellular vesicles: A promising tool in bone tissue repair. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20204987 (2019).
Yue, C. et al. Human bone marrow stromal cell exosomes ameliorate periodontitis. J. Dent. Res. https://doi.org/10.1177/00220345221084975 (2022).
Ma, S. et al. The emerging biological functions of exosomes from dental tissue-derived mesenchymal stem cells. Cell Reprogram 25, 53–64. https://doi.org/10.1089/cell.2022.0147 (2023).
Lin, H. et al. Advances of exosomes in periodontitis treatment. J. Transl. Med. 20, 279. https://doi.org/10.1186/s12967-022-03487-4 (2022).
Zhang, T. et al. Extracellular vesicles derived from human dental mesenchymal stem cells stimulated with low-intensity pulsed ultrasound alleviate inflammation-induced bone loss in a mouse model of periodontitis. Genes Dis. 10, 1613–1625. https://doi.org/10.1016/j.gendis.2022.06.009 (2023).
Wang, Y. et al. Inflammatory periodontal ligament stem cells drive M1 macrophage polarization via exosomal miR-143–3p-mediated regulation of PI3K/AKT/NF-κB signaling. Stem Cells 41, 184–199. https://doi.org/10.1093/stmcls/sxac087 (2023).
Lin, C. et al. Periodontal ligament fibroblasts-derived exosomes induced by PGE(2) inhibit human periodontal ligament stem cells osteogenic differentiation via activating miR-34c-5p/SATB2/ERK. Exp. Cell Res. 419, 113318. https://doi.org/10.1016/j.yexcr.2022.113318 (2022).
Huang, H. M. et al. Mechanical force-promoted osteoclastic differentiation via periodontal ligament stem cell exosomal protein ANXA3. Stem Cell Rep. 17, 1842–1858. https://doi.org/10.1016/j.stemcr.2022.06.006 (2022).
Zhou, L. L. et al. Oral mesenchymal stem/progenitor cells: The immunomodulatory masters. Stem Cells Int. 2020, 1327405. https://doi.org/10.1155/2020/1327405 (2020).
Zhang, Z., Deng, M., Hao, M. & Tang, J. Periodontal ligament stem cells in the periodontitis niche: Inseparable interactions and mechanisms. J. Leukoc. Biol. 110, 565–576. https://doi.org/10.1002/jlb.4mr0421-750r (2021).
Chen, Q. et al. Periodontal inflammation-triggered by periodontal ligament stem cell pyroptosis exacerbates periodontitis. Front. Cell Dev. Biol. 9, 663037. https://doi.org/10.3389/fcell.2021.663037 (2021).
Marconi, G. D. et al. Ascorbic acid: A new player of epigenetic regulation in LPS-gingivalis treated human periodontal ligament stem cells. Oxid. Med. Cell Longev. 2021, 6679708. https://doi.org/10.1155/2021/6679708 (2021).
Wu, W. et al. Bomidin attenuates inflammation of periodontal ligament stem cells and periodontitis in mice via inhibiting ferroptosis. Int. Immunopharmacol. 127, 111423. https://doi.org/10.1016/j.intimp.2023.111423 (2024).
Tang, M. et al. Flavonoid extract from propolis alleviates periodontitis by boosting periodontium regeneration and inflammation resolution via regulating TLR4/MyD88/NF-κB and RANK/NF-κB pathway. J. Ethnopharmacol. 319, 117324. https://doi.org/10.1016/j.jep.2023.117324 (2024).
Cao, J. et al. Epigenetic regulation of osteogenic differentiation of periodontal ligament stem cells in periodontitis. Oral Dis. 29, 2529–2537. https://doi.org/10.1111/odi.14491 (2023).
Jia, L., Xiong, Y., Zhang, W., Ma, X. & Xu, X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp. Cell Res. 386, 111717. https://doi.org/10.1016/j.yexcr.2019.111717 (2020).
Torunoglu, S. T., Zajda, A., Tampio, J., Markowicz-Piasecka, M. & Huttunen, K. M. Metformin derivatives-Researchers’ friends or foes?. Biochem. Pharmacol. 215, 115743. https://doi.org/10.1016/j.bcp.2023.115743 (2023).
Nojima, I. & Wada, J. Metformin and its immune-mediated effects in various diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24010755 (2023).
Naseri, A. et al. Metformin: New applications for an old drug. J. Basic Clin. Physiol. Pharmacol. 34, 151–160. https://doi.org/10.1515/jbcpp-2022-0252 (2023).
Foretz, M., Guigas, B. & Viollet, B. Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19, 460–476. https://doi.org/10.1038/s41574-023-00833-4 (2023).
Hammad, U. M. K. et al. Applications of metformin in dentistry-a review. J. Taibah Univ. Med. Sci. 18, 1299–1310. https://doi.org/10.1016/j.jtumed.2023.03.014 (2023).
Zhang, R., Liang, Q., Kang, W. & Ge, S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol. Int. 44, 70–79. https://doi.org/10.1002/cbin.11202 (2020).
Sun, Y. et al. Injectable periodontal ligament stem cell-metformin-calcium phosphate scaffold for bone regeneration and vascularization in rats. Dent Mater. https://doi.org/10.1016/j.dental.2023.07.008 (2023).
Hong, C. Y. et al. Metformin reduces bone resorption in apical periodontitis through regulation of osteoblast and osteoclast differentiation. J. Endod. 49, 1129–1137. https://doi.org/10.1016/j.joen.2023.07.005 (2023).
Lai, E. H. et al. Metformin ameliorates periapical lesions through suppression of hypoxia-induced apoptosis of osteoblasts. J. Endod. 44, 1817–1825. https://doi.org/10.1016/j.joen.2018.08.002 (2018).
Soraya, H., Sani, N. A., Jabbari, N. & Rezaie, J. Metformin increases exosome biogenesis and secretion in U87 MG human glioblastoma cells: A possible mechanism of therapeutic resistance. Arch. Med. Res. 52, 151–162. https://doi.org/10.1016/j.arcmed.2020.10.007 (2021).
Liao, Z. et al. Metformin facilitates mesenchymal stem cell-derived extracellular nanovesicles release and optimizes therapeutic efficacy in intervertebral disc degeneration. Biomaterials 274, 120850. https://doi.org/10.1016/j.biomaterials.2021.120850 (2021).
Kuang, Y. et al. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 21, 13–27. https://doi.org/10.1007/s10522-019-09838-x (2020).
Zhang, B., Yang, Y., Yi, J., Zhao, Z. & Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 56, 991–1005. https://doi.org/10.1111/jre.12912 (2021).
Malaekeh-Nikouei, A. et al. Metformin beyond an anti-diabetic agent: A comprehensive and mechanistic review on its effects against natural and chemical toxins. Biomed. Pharmacother. 165, 115263. https://doi.org/10.1016/j.biopha.2023.115263 (2023).
Dong, Y. et al. The development and benefits of metformin in various diseases. Front. Med. 17, 388–431. https://doi.org/10.1007/s11684-023-0998-6 (2023).
Tseng, C. H. Metformin and risk of gingival/periodontal diseases in diabetes patients: A retrospective cohort study. Front. Endocrinol. (Lausanne) 13, 1036885. https://doi.org/10.3389/fendo.2022.1036885 (2022).
Patil, K. S. et al. Efficacy of 1.5% metformin gel as an adjuvant to scaling, root planing, and curettage for the treatment of infrabony defects in chronic periodontitis patients. Contemp. Clin. Dent. 13, 18–23. https://doi.org/10.4103/ccd.ccd_271_20 (2022).
de Vries, T. J., Kleemann, A. S., Jin, J. & Schoenmaker, T. The differential effect of metformin on osteocytes, osteoblasts, and osteoclasts. Curr. Osteoporos. Rep. https://doi.org/10.1007/s11914-023-00828-0 (2023).
Fei, D. et al. Exosomes regulate interclonal communication on osteogenic differentiation among heterogeneous osteogenic single-cell clones through PINK1/Parkin-mediated mitophagy. Front. Cell Dev. Biol. 9, 687258. https://doi.org/10.3389/fcell.2021.687258 (2021).
Wang, Z. et al. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 10, 1310. https://doi.org/10.3389/fimmu.2019.01310 (2019).
Tomokiyo, A., Wada, N. & Maeda, H. Periodontal ligament stem cells: Regenerative potency in periodontium. Stem Cells Dev. 28, 974–985. https://doi.org/10.1089/scd.2019.0031 (2019).
Zhao, B. et al. Periodontal ligament stem cell-derived small extracellular vesicles embedded in matrigel enhance bone repair through the adenosine receptor signaling pathway. Int. J. Nanomed. 17, 519–536. https://doi.org/10.2147/ijn.S346755 (2022).
Zhao, Y. et al. The experimental study of periodontal ligament stem cells derived exosomes with hydrogel accelerating bone regeneration on alveolar bone defect. Pharmaceutics https://doi.org/10.3390/pharmaceutics14102189 (2022).
Lei, F. et al. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 141, 333–343. https://doi.org/10.1016/j.actbio.2021.12.035 (2022).
Xu, X. Y. et al. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl. Med. 9, 1414–1430. https://doi.org/10.1002/sctm.19-0418 (2020).
Čebatariūnienė, A., Kriaučiūnaitė, K., Prunskaitė, J., Tunaitis, V. & Pivoriūnas, A. Extracellular vesicles suppress basal and lipopolysaccharide-induced NFκB activity in human periodontal ligament stem cells. Stem Cells Dev. 28, 1037–1049. https://doi.org/10.1089/scd.2019.0021 (2019).
Han, X. D., Chen, H. M. & Li, C. Effect of human periodontal ligament stem cell-derived extracellular vesicles on macrophage pyroptosis and periodontal inflammatory injury in periodontitis. Cells Tissues Organs https://doi.org/10.1159/000519569 (2021).
Kang, H., Lee, M. J., Park, S. J. & Lee, M. S. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int. J. Mol. Sci. 19, 3843. https://doi.org/10.3390/ijms19123843 (2018).
Zheng, Y. et al. Exosomal microRNA-155–5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J. Cell Physiol. 234, 20662–20674. https://doi.org/10.1002/jcp.28671 (2019).
Zhang, Z. et al. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int. J. Med. Sci. 17, 558–567. https://doi.org/10.7150/ijms.40918 (2020).
Piffoux, M. et al. Extracellular vesicles for personalized medicine: The input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 138, 247–258. https://doi.org/10.1016/j.addr.2018.12.009 (2019).
Li, X. et al. Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles. Cell Mol. Biol. Lett. 28, 9. https://doi.org/10.1186/s11658-023-00422-3 (2023).
Zhuang, Y. et al. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210–3p/EFNA3/PI3K pathway. Acta Biomater. 150, 413–426. https://doi.org/10.1016/j.actbio.2022.07.015 (2022).
Yu, W. et al. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 133, 112646. https://doi.org/10.1016/j.msec.2022.112646 (2022).
Claes, L. & Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 93, 384–398. https://doi.org/10.1016/j.pbiomolbio.2006.07.021 (2007).
Hau, H., Rohanizadeh, R., Ghadiri, M. & Chrzanowski, W. A mini-review on novel intraperiodontal pocket drug delivery materials for the treatment of periodontal diseases. Drug Deliv. Transl. Res. 4, 295–301. https://doi.org/10.1007/s13346-013-0171-x (2014).
Simeone, P. et al. Diameters and fluorescence calibration for extracellular vesicle analyses by flow cytometry. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21217885 (2020).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell Ves. 13, e12404. https://doi.org/10.1002/jev2.12404 (2024).
Ma, S. et al. Skeletal muscle-derived extracellular vesicles transport glycolytic enzymes to mediate muscle-to-bone crosstalk. Cell Metab 35, 2028–20432027. https://doi.org/10.1016/j.cmet.2023.10.013 (2023).
Acknowledgements
We thank Prof. Song (Stomatological Hospital of Chongqing Medical University) for his assistance in the design of LIPUS. We thank Prof. Yu (Zunyi Medical University) for sharing her BD Stemflow™ Human MSC Analysis Kit with us and make the flow cytometry assay for us.
Author information
Authors and Affiliations
Contributions
M.L.X. (Mingli Xiang) contributed to concept, design, formal analysis, investigation, methodology and original draft. Y.L.L. and Q.S.G. contributed to formal analysis, investigation, methodology. C.C.L., L.L.X. and M.L.X. (Meiling Xiang) contributed to statistical analysis, data curation and validation. X.Y.G. and J.G.L. contributed to conceptualization, project administration, resources and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiang, M., Liu, Y., Guo, Q. et al. Metformin enhances the therapeutic effects of extracellular vesicles derived from human periodontal ligament stem cells on periodontitis. Sci Rep 14, 19940 (2024). https://doi.org/10.1038/s41598-024-70688-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70688-w
Keywords
This article is cited by
-
Isolation methods of exosomes derived from dental stem cells
International Journal of Oral Science (2025)