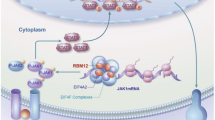
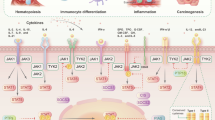
Abstract
Yu-Ping-Feng-San (YPF) is a famous classical Chinese medicine formula known for its ability to boost immunity. YPF has been applied to enhance the immune status of tumor patients in clinical practice. However, there is still a lack of research on its immune regulatory effects and mechanisms in the tumor microenvironment. This study was designed to investigate the effects and mechanism of YPF on improving the immune suppression state of hepatocellular carcinoma (HCC) microenvironment. In an orthotopic mouse model of HCC, YPF improved the immune microenvironment of HCC immunosuppression, enhanced the maturation of dendritic cells (DCs), promoted the release of IL-12, and decreased the presence of JAK2, p-JAK2, STAT3, and p-STAT3 proteins in both tumor tissue and paracancerous tissues. YPF also could promote the maturation and reduce the activation of JAK2, p-JAK2, STAT3, and p-STAT3 proteins of mouse bone marrow-derived DCs induced by culture medium or tumor supernatant in vitro. Transient transfection of siRNA-STAT3 with DCs resulted in an increase in its maturation and its secretion of IL-12. On the whole, these combined effects of YPF served to ameliorate the HCC immune suppression microenvironment, which conducive to immune cells play the role of immune surveillance and killing liver cancer cells. The mechanisms of these combined effects were, at least in part, related to its inhibition of the activated JAK2-STAT3 signaling pathway in DCs within the HCC microenvironment.
Similar content being viewed by others
Introduction
Primary carcinoma of the liver, mainly hepatocellular carcinoma (HCC), despite the availability of surgical and non-surgical treatment options, stays as the third primary contributor to global cancer-related fatalities due to a considerable number of HCC patients being diagnosed in advanced stages. Hence, there is a pressing demand to discover novel therapeutic strategies to enhance the survival and prognosis for HCC patients1,2.
Traditional Chinese medicine (TCM) plays a significant part in treatment of liver cancer by improving clinical symptoms, enhancing quality of life, prolonging survival, and preventing postoperative recurrence3,4. Main advantage of TCM treatment lies in improving the body’s internal environment, particularly reshaping the tumor-related microenvironment, thereby inhibiting tumor growth and preventing metastatic relapse5. Yet, the exact mechanisms responsible for these enhancements remains unclear.
Dendritic cells (DCs), considered the most effective antigen-presenting cells of the human immune system, play a crucial part not just in triggering innate immune responses but also in initiating adaptive immune responses to selectively combat and eliminate tumor cells. The manipulation of DCs holds significant potential for inducing potent anti-tumor immune responses. DCs are crucial for activating naïve T cells (Th0) and promoting their proliferation and differentiation6. While in an immature state, DCs circulate extensively in blood and peripheral tissues, capturing antigens from pathogenic infections or tumor cells. Upon taking up these antigens, DCs undergo phenotypic and functional maturation processes. They then migrate towards secondary lymphoid tissues, where they present processed antigens to cytotoxic T lymphocytes (CTLs), activating them and initiating antigen-specific immune responses aimed at eliminating target cells expressing the antigen7,8. Mature DCs play a crucial part in inducing anti-tumor immune responses, primarily in their capacity to activate tumor-specific T cell responses and provoke T helper 1 (Th1) cell responses9. However, immature DCs can lead to immune tolerance. Therefore, promoting DCs maturation is beneficial for enhancing anti-tumor immunity to effectively eliminate tumors, holding significant therapeutic implications10,11.
The immune status of the tumor microenvironment greatly influences tumor progression, and HCC characterized by immune suppression features is often associated with poor prognosis. Within the tumor microenvironment, tumor cells secrete numerous immune-suppressive factors, such as interleukin-10 (IL-10), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and more, which inhibit the maturation and activation of DCs, resulting in an immune-suppressive phenotype and function. Additionally, liver cancer cells enlist immunosuppressive DCs to inhibit CD8+ T cells, enabling tumor evasion from immune surveillance. Exosomes derived from tumors disrupt the differentiation and maturation of DCs through the IL6/STAT3 signaling pathway. This prevents myeloid progenitor cells from evolving into CD11c+ DCs, promoting apoptosis and, in turn, diminishing T cell activation while mediating immune suppression12,13. These aforementioned immune-suppressive factors are also activators of signal transducer and activator of transcription 3 (STAT3). Recent research focusing on the inflammatory microenvironment of tumor immune suppression indicates that STAT3, as a central regulatory factor, becomes highly activated in tumor tissues, participating in regulating tumor immune evasion. It has emerged as a significant target for tumor immunotherapy. STAT3 shows sustained high expression in human liver cancer tissue, intimately linked to the onset and progression of HCC. Moreover, it correlates positively with clinical staging and pathological grading of HCC14,15. In the HCC microenvironment, activation of STAT3 in DCs suppresses the expression of CD80, CD86, MHC class II molecules, and the release of IL-12, thereby hindering the DCs maturation. Consequently, effective presentation of tumor antigens to CD8+ T cells and NK cells is impaired, preventing their anti-tumor activity. Simultaneously, it promotes the buildup of Treg cells within tumor tissues, thereby inhibiting CD8+ T cell proliferation16,17. Targeting the blockade of STAT3 signaling favors the maturation of DCs, improves the immune status of tumor microenvironment, reverses immune tolerance in HCC, and has become a hot topic in the HCC immunotherapy ___domain18,19.
Yu-Ping-Feng-San (YPF) is a famous classical immune enhancing formula in traditional Chinese medicine. YPF is composed of Astragalus membranaceus (Fisch.) Bunge (Huangqi), Atractylodes macrocephala Koidz. (Baizhu) and Saposhnikovia divaricata (Turcz.) Schischk. (Fangfeng). Recent researches have shown that YPF could boost the immune function of the body by enhancing the activity of phagocyte, promoting the production of IL-2, facilitating the lymphocyte transformation, and improving the immune enhancement such as increasing the activity of NK cells which inhibit tumor development20,21. Our earlier research has validated that YPF enhances the cytotoxicity of spleen lymphocytes to Hepa1-6 murine HCC cells in vitro, increase the Th1 phenotype cytokines levels in tumor tissues and paracancerous tissues of Hepa1-6 implanted mice with HCC, reduce the Th2 phenotype cytokines levels, increase the Th1/Th2 ratio, and improve the immunosuppressive status of Th2 predominance in HCC. Also it could reduce the OX40 ligand (OX40L) expression on DCs induced by thymic stromal lymphopoietin (TSLP) through inhibiting the production of TSLP in Hepa1-6 murine HCC cells, this in turn inhibiting the ability of TSLP-DCs to polarize Th2-type cells and the secretion of Th2-type immune cytokines such as IL-13 and IL-4, thereby inhibiting the progression of HCC22. However, it remains to be clarification whether YPF can inhibit the activation of STAT3 in DCs, promote their maturation, affect the secretion of corresponding cytokines, and ameliorate the suppression of HCC immune microenvironment. This research conducted research on the above. It provided experimental basis for expanding the application of YPF in tumor immunotherapy.
Materials and methods
Reagents and antibodies
Anti-Mouse CD4 FITC(88-8111), Anti-Mouse CD25 APC(88-8111), PE anti-mouse/rat Foxp3 (88-8111), Anti-Mouse CD3e PE (12-0031-82), Anti-Mouse CD8a APC (17-0081-81), Anti-Mouse CD45 FITC (11–0451), Anti-Mouse NK1.1 APC (17-5941), Anti-Mouse CD80 (B7-1) PE (12–0801), Anti-Mouse CD86 (B7-2) PE (12–0862), Anti-Mouse MHC Class II (I-A/I-E) PE (12-5321) were purchased from Thermo Fisher Scientific (MA, USA). Mouse IL-12p70 ELISA Kit were purchased from Solarbio (Beijing, China). Recombinant Murine GM-CSF, Recombinant Murine IL-4 were purchased from PeproTech (Jiangsu, China). Stat3 Rabbit mAb (#4904S), Phospho-Stat3 XP Rabbit mAb (#9134S), Jak2 XP Rabbit mAb (#3230S), Phospho-Jak2 Antibody (#3771S), GAPDH Antibody (#5174S) were purchased from Cell Signaling Technology (MA, USA). Lipofectamine 3000 was purchased from Invitrogen (MA, USA). The standard compounds (purity > 98%) of Prim-O-glucosylcimifugin (#P0094), calycosin-7-O-β-D-glucoside (#P0616), cimifugin (#P0081), 4’-O-β-D-glucopyranosyl-5-O-methylvisamminol (#P0422), psoralen (#P0062), calycosin (#P0148), sec-O-glucosylhamaudol (#P0921), formononetin (#P0205) and atractylon (#P1293) were purchased from ShangHai PureOne Biotechnology (China).
YPF preparation and HPLC analysis of the components
YPF was made up of Astragalus membranaceus (Fisch.) Bunge, Atractylodes macrocephala Koidz. and Saposhnikovia divaricata (Turcz.) Schischk., which were purchased from Chunhui Tang Pharmaceutical Co., Ltd (Jiangsu, China) and were identified by Professor Duorong Shen, a botanist from Pharmacy Department, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine. The extraction procedure and HPLC analysis of YPF were according to our previous study23.
Cell culture
Hepa1-6 cell line (C57BL/6-derived hepatoma), gifted by professor Limin Zheng (School of Life Sciences, Sun Yat-sen University), were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, MA, USA) containing 10% heat-inactivated fetal bovine serum (Gibco, MA, USA) at 37 °C in a humidified atmosphere with 5% CO2.
Animal model
The six-week-old C57/BL6 male mice (20–22 g) provided by the animal experiment center of Matt Albert Technology Co. Ltd (Jiangsu, China), and animal certificate number is SCXK (SU) 2018-0006. Mice were adaptively raised for 3 days for further experiments. According to our previous study, the mice were anesthetized with 300 mg/kg tribromoethanol (ALADDIN, Shanghai, China) and an orthotopic transplanted model of HCC in mice was successfully established. After 10 days, the mice were randomly allocated into five groups. YPF groups were orally administered with 20 g, 30 g, and 40 g herb / kg in 0.2 ml, respectively24. Model control group was treated with saline. Cisplatin (China Qilu Drug Producing Co. Ltd., Shandong, China) served as the positive for drugs once every other day for 2 weeks. Each group consisted of 8 mice. After 14 days of pharmaceutic treatment, the mice were euthanized using carbon dioxide inhalation for at least 5 min, and their livers were collected for subsequent analysis. Tumor inhibition rate (%) = (mean tumor weight of model control group - mean tumor weight of experiment group) /mean tumor weight of model control group × 100%.
The animal experiments was approved by the Ethics Committee of Suzhou Chinese Medicine Hospital (Ethical Number: 2021-LDP-049), and all experimental programs and procedures were in compliance with the Guide for the Care and Use of Laboratory Animals at Nanjing University of Chinese Medicine. The feeding conditions were temperature at 24–25 °C and 50–60% relative humidity, according to ethics guidelines. We verify that all of our studies involving live animals adhere to the ARRIVE guidelines, and we confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Preparation of single-cell suspension and flow cytometry detection
Harvested spleen, tumor tissues and paracancerous tissues, and the preparation method of single-cell suspension was according to our previous study22. The cell suspension were tested by flow cytometry. Among them, CD3+CD45+NK1.1+ represented NK cells, CD4+CD25+Foxp3+ represented Treg cells, and CD3+CD8+ represented CD8+ T cells. CD80+, CD86+ and MHC-II+ represented DC cells.
Preparation of homogenate in tumor tissues and paracancerous tissues
Tumor tissue and paracancerous liver tissue samples of HCC-bearing mice were homogenized on ice with PBS (100 mg tissue and 1mL PBS). The Homogenized liquid underwent centrifugation at 3,000 × g for 10 min in 4˚C. The resulting supernatants (100 µl) were utilized for analysis.
Culture bone marrow-derived DCs
The culture method for mouse DCs was previously reported in our study22. On day 6, when bone marrow cells from femur and tibia specimens of mice differentiated into DCs, YPF / tumor supernatant were introduced into the culture. The cells only incubated with medium contained cytokine for C group. And tumor supernatant was added into the culture contained cytokine for M group. The semi-adherent DCs were collected on day 7 and subjected to flow cytometry for the assessment of the markers CD80, CD86, and MHC-II.
ELISA assays of IL-12
The concentrations of IL-12 (Solarbio Science & Technology Co. Ltd., Beijing, China) in the tissue homogenate / culture supernatants were quantitatively measured using commercially available ELISA kits following the manufacturer’s protocols.
Western blotting
The method used for western blotting was reported by our previous study23. Tumor and paracancerous liver tissue protein loading amount was 100 µg, meanwhile the DCs protein was 50 µg. Dilution ratio of primary antibody was as follow: anti-JAK2 was 1:1000, anti-p-JAK2 was 1:1000, anti-STAT3 was 1:2000, anti-p-STAT3 was 1:1000, and anti-GAPDH was 1:2000. The PVDF membranes were cut before hybridization with a single specific target antibody during blotting for better imaging. The PVDF membranes were detected by a FluoChem HD2 fluorescence visible imaging system (ProteinSimple, USA). The images were quantified using Image J. The quantification was normalized to the comparable value of GAPDH expression.
Plasmid transfection
SiRNA-STAT3 Plasmid construction commissioned by the Public Protein/Plasmid Library (Jiangsu, China). DCs were transfected with 5 µg plasmids using Lipofectamine 3000 (Invitrogen, CA, USA) in 6-well plates and utilized for subsequent experiments after 24 h.
Statistical analysis
The data were presented as mean ± SD. All statistical analyses were performed using SPSS 22.0. Before Student’s t-test, a variance homogeneity test and normality analysis were carried out. The statistical significance of differences was assessed using the Student’s t-test. A significance level of P < 0.05 was considered statistically significant.
Results
The contents of nine components in YPF
Using the quality control methods we previously established23, we employed HPLC to analyze the content of nine components in the YPF extract used in this study (Table 1; Fig. 1A–C).
HPLC analysis of YPF. (A) HPLC chromatograms of YPF. (B) Standard compounds. (5) Prim-O-glucosylcimifugin, (6) calycosin-7-O-β-D-glucoside, (7) cimifugin, (8) 4’-O-β-D-glucopyranosyl-5-O-methylvisamminol, (10) psoralen, (11) calycosin, (12) sec-O-glucosylhamaudol, (13) formononetin, and (15) atractylon. (C) Chemical structures of the compounds.
Effect of YPF on immunity and tumor progression in HCC bearing mice
YPF could lower the Treg cells proportion in the spleen, tumor tissues, and paracancerous tissues, while increasing the NK cells proportion and CD8+ T cells proportion in HCC bearing mice, thereby improving the immune microenvironment (Fig. 2A–C). At the same time, YPF inhibited the tumor growth in HCC bearing mice at a dose-dependent (the tumor inhibition rates of the YPF group 40, 30, and 20 g crude herb/kg were 53.45%, 35.86%, and 23.99%, respectively). A significant difference was observed when compared with the model control group (P < 0.05, P < 0.01, Fig. 2D). Cisplatin exhibits broad-spectrum anti-tumor activity, making it an ideal choice as the positive control drug for HCC treatment. It has cytotoxic effects on tumor cells and immunosuppressive at the same time, as demonstrated in our previously published paper25. Therefore, the experimental design in this study did not take cisplatin’s immunological effects into account. These results indicated that YPF had the potential to enhance anti-tumor immunity, improve the microenvironment of HCC immunosuppression, and so as to exert its anti-HCC effect.
Effect of YPF on the immune microenvironment in HCC bearing mice. (A–C) The proportion of immune cells in the spleen, tumor tissues, and paracancerous tissues. (D) Tumor weight and tumor inhibition rate. (E) The images of the tumors from six mice per treatment group (scale bar: 1 cm). Data are presented as the means ± SD. *P < 0.05 and **P < 0.01 vs. Model group.
The effect of YPF on the maturation of DCs
The maturation of DCs is crucial for initiating anti-tumor immune responses. Therefore, we further analyzed the impact of YPF on the DCs maturation in HCC bearing mice. The results showed that YPF elevated the expression of surface factors CD80, CD86, and MHC-II on DCs in spleen cells, primary myeloid cells, tumor tissues, and paracancerous tissues of HCC bearing mice. Furthermore, YPF elevated the product of IL-12 in tumor tissue, but had no effect in paracancerous tissues (Fig. 3A–E). These findings suggested that YPF had the ability to enhance the maturation of DCs and promote the release of IL-12 in HCC bearing mice.
In addition to in vivo experiments, we also observed the effects of YPF on the maturation and IL-12 secretion of cultured DCs in vitro. Results revealed that YPF promoted the DCs maturation and increased the IL-12 secretion by DCs. Furthermore, when we cultured DCs with tumor supernatant to simulate the growth state of DCs in tumor microenvironment. YPF also promoted the DCs maturation in the tumor microenvironment and increase the IL-12 secretion by DCs (Fig. 3F–H). These results suggested that YPF might enhance anti-tumor immunity and improve immunosuppression of HCC by affecting the maturation of DCs.
YPF demonstrated a positive impact on the maturation of DCs. (A–D) DCs in spleen, primary myeloid cells, tumors tissues, and paracancerous tissues. (E) IL-12 secretion of tumor tissues and paracancerous tissues. (F,G) The maturation of mouse bone marrow-derived DCs induced by normal culture medium or tumor supernatant. (H) IL-12 secretion of mouse bone marrow-derived DCs induced by normal culture medium or tumor supernatant. Data are presented as the means ± SD.*P < 0.05 and **P < 0.01 vs. Model group; #P < 0.05 and ##P < 0.01 vs. Control group.
YPF promoted the maturation of DCs through the JAK2-STAT3 pathway
How did YPF modulate the DCs maturation? To unveil the underlying mechanisms of YPF in promoting the maturation of DCs, we first observed its effects on the presence of JAK2 and STAT3 proteins in tumor tissues and paracancerous tissues of HCC bearing mice. The findings indicated that YPF decreased the overall protein presence of JAK2 and STAT3, at the same time including the levels of p-JAK2 and p-STAT3 proteins (Fig. 4A,B). These findings suggested that the inhibition effects on the JAK2-STAT3 pathway by YPF in tumor tissues and paracancerous tissues might be related to the promotion of DCs maturation.
Furthermore, we investigated the impact of YPF on JAK2 and STAT3 protein expression in DCs cultured in vitro. The results indicated that YPF demonstrated the ability to decrease the expression of JAK2, p-JAK2, STAT3, and p-STAT3 proteins. In tumor microenvironment, YPF decreased the expression of JAK2 and STAT3 total protein, p-JAK2 and p-STAT3 protein (Fig. 4C,D). These results suggested that YPF could facilitate the maturation of DCs by inhibiting the JAK2-STAT3 pathway activation.
YPF promoted the DCs maturation through the JAK2-STAT3 pathway. (A,B) JAK2-STAT3 pathway in tumor and paracancerous tissues. (C,D) JAK2-STAT3 pathway in mouse bone marrow-derived DCs induced by normal culture medium or tumor supernatant. Data are presented as the means ± SD.*P < 0.05 and **P < 0.01 vs. Model group; #P < 0.05 and ##P < 0.01 vs. Control group.
STAT3 as a key target molecule for YPF to regulate the maturation of DCs
To investigate whether STAT3 is a crucial target molecule in the regulation of DCs maturation by YPF, we employed the transient transfection plasmid method to interfere STAT3 expression in DCs and observed the effect of YPF. The results demonstrated that after transient transfection of siRNA-STAT3 plasmid with DCs, the total STAT3 protein expression was downregulated (Fig. 5A), indicating the feasibility of this method. Upon knocking down STAT3 total protein expression in DCs, we found an increase in the expression of DCs surface factors CD80, CD86, MHC-II, and the secretion of IL-12. This indicated that knocking down STAT3 in DCs could enhance DCs maturation and the secretion of IL-12. Interestingly, the addition of YPF to DCs transfected with siRNA-STAT3 plasmid did not further enhance the promoting effect on DCs maturation. These results suggested that blocking the activation of STAT3 was a key component of YPF’s mechanism in promoting DCs maturation (Fig. 5B,C). Similar effects on the maturation and IL-12 secretion of DCs cultured in tumor supernatant were observed (Fig. 5D,E).
Then, we further investigated the effects of STAT3 protein expression on DCs transfected with siRNA-STAT3 plasmid. In comparison to the vector group, the tumor supernatant could promote the expression of STAT3 and p-STAT3 in DCs, while YPF had an inhibitory effect on STAT3 and p-STAT3 expression. Compared with the model (tumor supernatant cultured DCs) group, the YPF group, the transfected siRNA-STAT3 group, and the transfected siRNA-STAT3 with YPF group all reduced the expression of STAT3 and p-STAT3. However, compared with the transfected siRNA-STAT3 group, the transfected siRNA-STAT3 with YPF group did not further reduce the expression of p-STAT3 (Fig. 5F). It indicated that STAT3 was a key target molecule for YPF in regulating the maturation of DCs.
STAT3 was a key target molecule for YPF to regulate the maturation of DCs. (A) DCs transfected with siRNA-STAT3 plasmid. (B) The maturation, (C) IL-12 secretion of mouse bone marrow-derived DCs induced by normal culture medium transfected with siRNA-STAT3 plasmids. (D) The maturation, (E) IL-12 secretion, (F) STAT3 protein expression of mouse bone marrow-derived DCs induced by tumor supernatant transfected with siRNA-STAT3 plasmids. Data are presented as the means ± SD. *P < 0.05 and **P < 0.01 vs. Vector group; #P < 0.05 and ##P < 0.01 vs. Control group; △P < 0.05 and △△P < 0.01 vs. Model group.
Discussion
Immune system plays a pivotal part in safeguarding host from cancer invasion. Some scholars have recognized tumors as microenvironmental immune disease, highlighting the significant role of immune state within tumor immune microenvironment (TIME) in cancer occurrence and development. This classification includes suppressive (S-TIME), equilibrated (E-TIME), and active (A-TIME) states26. Within the tumor microenvironment, tumor cells secrete large amounts of immunosuppressive factors, including VEGF, IL-10, and IL-6, and more, which reduce the quantity and activity of effector NK cells, T cells, DC-cells, and M1-type TAMs involved in anti-tumor effects. Simultaneously, they gather suppressive immune cells such as regulatory myeloid suppressive cells (MDSCs), T cells (Tregs), and M2-type TAMs, placing TIME in an immunosuppressive state27,28, – 29. This immunosuppressive state within TIME promotes tumor growth, which is an important reason for tumor recurrence and treatment resistance30,31. Numerous researches have demonstrated that in HCC patients, immune status within the tumor’s local microenvironment skews toward an inhibitory phenotype. NK and CD8+T immune cells decreased, while immunosuppressive Tregs significantly increased32. The degree of immune suppression was strongly correlated with the poor prognosis of HCC patients33.
Therefore, ameliorating the immunosuppressive state of HCC microenvironment has become an important target for treatment. The research results declared that YPF reduced the Tregs proportion in tumor tissue, paracancerous tissue, and spleen of HCC bearing mice, increased the NK cells proportion and CD8+T cells proportion, and improved the immune microenvironment and overall immunity of HCC. At the same time, it showed a certain inhibitory effect on tumor growth. It indicated that YPF could enhance anti-tumor immunity, improve the microenvironment of HCC immune suppression, and play a part in delaying the growth of HCC.
DCs are recognized as the strongest and exclusive antigen-presenting cells in the body that activates quiescent T cells. They serve as the central link in initiating, regulating, and sustaining immune responses. DCs binding to T cells can induce substantial quantity of cytokines secretion, such as IL-18 and IL-12, and promote T cell proliferation, and induce CTLs generation, and dominate Th1 immune response. However, Tumor cells in TIME secrete a large amount of immunosuppressive factors through autocrine signaling.
These cytokines can cause a decrease in the number of immature DCs in the tumor tissue rendering them functionally deficient and unable to stimulate antigen-specific CD8+T cells, resulting in immune tolerance and placing TIME in an immunosuppressive inflammatory state34,35. We discovered that YPF promoted the DCs maturation and IL-12 secretion in TIME. It indicated that YPF could improve the immunosuppressive state in TIME of HCC bearing mice, which might be related to promoting the maturation of DCs and initiating corresponding immune responses.
JAK-STAT pathway serves as a middle signaling center that able to be activated by an excess of growth factors, cytokines, and hormones. This pathway regulates various cellular course, encompassing cell division, proliferation, and determination of cell destiny. It was reported that 45.5% HCC patients exhibited changes in JAK-STAT pathway, and activated STAT3 was strongly associated with tumor invasiveness. It was worth noting that mice lacking STAT3 expression in liver cells could be immune to HCC invasion36. We found that STAT3 in DCs within HCC microenvironment was activated, which inhibited the expression of MHCII class molecules, CD86, CD80, and IL-12 in DCs, hindered their maturation, and prevented them from effectively presenting antigens. These findings aligned with the notion that the tumor microenvironment hinders the maturation and activation of DCs, resulting in an immunosuppressive phenotype and function11. Importantly, our study revealed that YPF not only promoted the expression of MHCII class molecules, CD86, CD80, and IL-12 in DCs, but also inhibited the expression levels of JAK2-STAT3 signaling pathway activating proteins in DCs. These results implied that YPF could promote the DCs maturation through inhibiting STAT3 activation.
STAT3 has a negative regulatory function in differentiated conventional DCs. Targeted blockade of STAT3 signal is beneficial for DCs maturation37. In our in vitro experiments, we observed that downregulating the expression of STAT3 total protein in DCs could promote the DCs maturation and IL-12 secretion. Interestingly, YPF did not further enhance its promoting effect on the maturation of siRNA-STAT3 DCs, reducing the expression of p-STAT3 proteins. When siRNA-STAT3 DCs were cultured with tumor supernatant to simulate the tumor microenvironment, the results were consistent with the above findings. These observations were suggested that blocking the activation of STAT3 was involved in the regulation of YPF in promoting the maturation of DCs. STAT3 was a key target molecule for YPF to regulate the maturation of DCs. However, we did not research the HCC bearing mouse model that blocked STAT3 signaling in DCs, which would be an avenue for future research. Our findings may help to better understand the targeted inhibitory effect of STAT3 in HCC, providing new clues for YPF to improve immune status of TIME and reverse immune tolerance of HCC.
Our preliminary research (in Supplementary Materials 1) indicated that the main components of YPF, including calycosin, psoralen, formononetin, and sec-O-glucosylhamaudol, effectively inhibited the proliferation of Hepa1-6 murine HCC cells. Furthermore, calycosin, calycosin-7-O-β-D-glucoside, Prim-O-glucosylcimifugin, and psoralen promoted the proliferation of RAW264.7 macrophages. Additionally, formononetin significantly stimulated the proliferation of splenocytes in C57BL/6 mice. It had been reported that calycosin-7-O-β-D-glucoside, cimifugin, 4’-O-β-D-glucopyranosyl-5-O-methylvisamminol, psoralen, and formononetin exerted their antitumor effects primarily by inhibiting proliferation, inducing apoptosis, suppressing migration, and reversing multidrug resistance. These effects had been observed in various cancer cell lines, such as SK-OV-3 human ovarian carcinoma, MKN28 human gastric cancer, and H460 human lung carcinoma38,39,40,41,42. Calycosin and atractylon effectively suppressed the growth of hepatocellular carcinoma cells by modulating proliferation, promoting apoptosis, and inhibiting invasion. These antitumor effects were intricately linked to the ROS-mediated activation of the MAPK, STAT3, and NF-κB signaling pathways43,44. Among these compounds, Prim-O-glucosylcimifugin enhanced the immunosuppressive tumor microenvironment by promoting increased CD8 + T-lymphocyte infiltration in the spleen, bone marrow, and tumors of B16-F10-bearing mice45. Based on these findings, we believed that the specific active components of YPF responsible for promoting DCs maturation still needed to be identified. In the tumor microenvironment, the specific key components and their precise roles remained unclear. Identifying these critical components will likely be a focus of our future research. Our preliminary studies have shown that YPF significantly delays tumor growth in advanced HCC patients, alleviates symptoms, and improves their quality of life. The underlying mechanism may be related to the improvement of the body’s immune status46. In the future, we will explore the use of YPF for the treatment of other types of tumors, thereby broadening its potential for application in clinical. The results of this study showed that YPF not only promoted DCs maturation but also enhanced TIME in HCC. This may highlight the potential of TCM in modulating and improving the immunosuppressive microenvironment. In the future, for the clinical treatment of HCC, we will consider combining YPF with chemotherapy drugs or PD-1 inhibitors, to observe its regulatory effects on immune suppression in the HCC microenvironment. We will further explore the application of YPF in clinical (including but not limited to HCC), both as a monotherapy and in combination with other therapeutic approaches.
Conclusion
In summary, this study displayed that YPF not solely restored the DCs maturation and IL-12 secretion, but also increased the CD8+T cells proportion and NK cells proportion, while concurrently decreasing the Treg cells proportion. These collective effects contribute to improve the immune suppression TIME in HCC and enhance the immune cells cytotoxicity to against HCC. The machines of these combined effects were, at least partially related to its inhibition on the JAK2-STAT3 signaling pathway activation in DCs within HCC microenvironment. It provided experimental evidence for expanding the application of YPF in tumor immunotherapy.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- YPF:
-
Yu-Ping-Feng-San
- DCs:
-
Dendritic cells
- HCC:
-
Hepatocellular carcinoma
- TCM:
-
Traditional Chinese medicine
- CTLs:
-
Cytotoxic T lymphocytes
- Th1:
-
T helper 1
- TGF-β:
-
Transforming growth factor β
- VEGF:
-
Vascular endothelial growth factor
- IL:
-
Interleukin
- STAT3:
-
Signal transducer and activator of transcription 3
- OX40L:
-
OX40 ligand
- TSLP:
-
Thymic stromal lymphopoietin
- PBS:
-
Phosphate-buffered saline
- ELISA:
-
Enzyme-linked immunosorbent assay
- HPLC:
-
High-performance liquid chromatography
- TIME:
-
Tumor immune microenvironment
- Tregs:
-
Regulatory T cells
- MDSCs:
-
Myeloid suppressive cells
References
Yang, C. et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 20, 203–222. https://doi.org/10.1038/s41575-022-00704-9 (2023).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Liu, X. L. et al. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine 62, 152930. https://doi.org/10.1016/j.phymed.2019.152930 (2019).
Wei, L. et al. Frontier progress of the combination of modern medicine and traditional Chinese medicine in the treatment of hepatocellular carcinoma. Chin. Med. 17, 90. https://doi.org/10.1186/s13020-022-00645-0 (2022).
Zhang, L. Y. et al. Targeting tumor immunosuppressive microenvironment for the prevention of hepatic cancer: Applications of traditional Chinese medicines in targeted delivery. Curr. Top. Med. Chem. 20, 2789–2800. https://doi.org/10.2174/1568026620666201019111524 (2020).
He, M. Y. et al. CD5 expression by dendritic cells directs T cell immunity and sustains immunotherapy responses. Science 379, eabg2752. https://doi.org/10.1126/science.abg2752 (2023).
Jeng, L. B., Liao, L. Y., Shih, F. Y. & Teng, C. F. Dendritic-cell-vaccine-based immunotherapy for hepatocellular carcinoma: Clinical trials and recent preclinical studies. Cancers (Basel) 14, 4380. https://doi.org/10.3390/cancers14184380 (2022).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24. https://doi.org/10.1038/s41577-019-0210-z (2020).
Gardner, A. & Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 37, 855–865. https://doi.org/10.1016/j.it.2016.09.006 (2016).
Schlitzer, A., McGovern, N. & Ginhoux, F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell. Dev. Biol. 41, 9–22. https://doi.org/10.1016/j.semcdb.2015.03.011 (2015).
Böttcher, J. P. & Sousa, C. R. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 4, 784–792. https://doi.org/10.1016/j.trecan.2018.09.001 (2018).
Qiao, D. R. et al. The mononuclear phagocyte system in hepatocellular carcinoma. World J. Gastroenterol. 28, 6345–6355. https://doi.org/10.3748/wjg.v28.i45.6345 (2022).
Fasano, R. et al. Immunotherapy for hepatocellular carcinoma: New prospects for the cancer therapy. Life (Basel) 11, 1355. (2021). https://doi.org/10.3390/life11121355
Lee, C. & Cheung, S. T. STAT3: An emerging therapeutic target for hepatocellular carcinoma. Cancers (Basel) 11, 1646. https://doi.org/10.3390/cancers11111646 (2019).
Yang, T. F. et al. WWOX activation by toosendanin suppresses hepatocellular carcinoma metastasis through JAK2/Stat3 and Wnt/β-catenin signaling. Cancer Lett. 513, 50–62. https://doi.org/10.1016/j.canlet.2021.05.010 (2021).
Shang, N. et al. Dendritic cells based immunotherapy. Am. J. Cancer Res. 7, 2091–2102 (2017).
Pang, Y. B. et al. Experimental study on the immune response of fusion tumor vaccine of HepG2 and dendritic cells in vitro. Zhonghua Yi Xue Za Zhi 97, 535–539. https://doi.org/10.3760/cma.j.issn.0376-2491.2017.07.013 (2017).
Li, Y. et al. Targeted inhibition of STAT3 induces immunogenic cell death of hepatocellular carcinoma cells via glycolysis. Mol. Oncol. 16, 2861–2880. https://doi.org/10.1002/1878-0261.13263 (2022).
Xu, J. N. et al. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front. Oncol. 11, 760971. https://doi.org/10.3389/fonc.2021.760971 (2021).
Li, Y. H. et al. Yupingfeng Powder relieves the immune suppression induced by dexamethasone in mice. J. Ethnopharmacol. 200, 117–123. https://doi.org/10.1016/j.jep.2017.01.054 (2017).
Wang, Y. L. et al. Yu-Ping-Feng formula exerts antilung cancer effects by remodeling the tumor microenvironment through regulating myeloid-derived suppressor cells. Evid. Based Complement. Alternat. Med. 6624461. (2021). https://doi.org/10.1155/2021/6624461 (2021).
Yao, F. et al. Yupingfeng granule improves th2-biased immune state in microenvironment of hepatocellular carcinoma through TSLP-DC-OX40L pathway. Evid. Based Complement. Alternat. Med. 1263053. (2020). https://doi.org/10.1155/2020/1263053 (2020).
Yuan, Q. et al. Yu Ping Feng San exert anti-angiogenesis effects through the inhibition of TSLP-STAT3 signaling pathways in hepatocellular carcinoma. Evid. Based Complement. Alternat. Med. 2019 (1947156). https://doi.org/10.1155/2019/1947156 (2019).
Yao, F. et al. Osthole attenuates angiogenesis in an orthotopic mouse model of hepatocellular carcinoma via the downregulation of nuclear factor-κB and vascular endothelial growth factor. Oncol. Lett. 16, 4471–4479. https://doi.org/10.3892/ol.2018.9213 (2018).
Zhang, L. R. et al. Osthole promotes anti-tumor immune responses intumor-bearing mice with hepatocellular carcinoma. Immunopharmacol. Immunotoxicol. 3, 301–307. https://doi.org/10.3109/08923973.2015.1035391 (2015).
Wang, S. H. et al. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: Results of a phase II clinical trial. Clin. Cancer Res. 26, 4921–4932. https://doi.org/10.1158/1078-0432.CCR-20-0113 (2020).
Taube, J. M. et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod. Pathol. 31, 214–234. https://doi.org/10.1038/modpathol.2017.156 (2018).
Pinton, L. et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J. Immunother. Cancer 7, 58. https://doi.org/10.1186/s40425-019-0536-x (2019).
Gao, S. et al. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 9, 126–151. https://doi.org/10.7150/thno.29431 (2019).
Hinshaw, D. C. & Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 79, 4557–4566. https://doi.org/10.1158/0008-5472.CAN-18-3962 (2019).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34. https://doi.org/10.1038/s41571-020-0403-1 (2021).
Fu, Y. J., Liu, S. S., Zeng, S. & Shen, H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38, 396. https://doi.org/10.1186/s13046-019-1396-4 (2019).
Pinato, D. J. et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 39, 3620–3637. https://doi.org/10.1038/s41388-020-1249-9 (2020).
Vesely, M. D. & Schreiber, R. D. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 1284, 1–5. https://doi.org/10.1111/nyas.12105 (2013).
O’Sullivan, T. et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 209, 1869–1882. https://doi.org/10.1084/jem.20112738 (2012).
Lokau, J., Schoeder, V., Haybaeck, J. & Garbers, C. Jak-stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma. Cancers (Basel) 11, 1704. https://doi.org/10.3390/cancers11111704 (2019).
Li, H. S. & Watowich, S. S. Diversification of dendritic cell subsets emerging roles for STAT proteins. JAKSTAT 2, e25112. https://doi.org/10.4161/jkst.25112 (2013).
Huang, J. Z. et al. The effect of calycosin-7-O-β-D-glucoside and its synergistic augmentation of cisplatin-induced apoptosis in SK-OV-3 cells. Curr. Pharm. Des. 28, 2161–2166. https://doi.org/10.2174/1381612828666220610164100 (2022).
Zhu, Z. M. et al. Potential molecular metabolic mechanisms underlying the effects of cimifugin in gastric cancer through single-cell and bulk RNA sequencing combined with network pharmacology. J. Gastrointest. Oncol. 15, 1409–1430. https://doi.org/10.21037/jgo-24-413 (2024).
Ma, S. Y. et al. Two new chromone glycosides from the roots of saposhnikovia divaricate. Chem. Biodivers. 15, e1800253. https://doi.org/10.1002/cbdv.201800253 (2018).
Meng, D. D., Dong, Y. L., Shang, Q. X. & Sun, Z. Y. Anti-tumor effect and hepatotoxicity mechanisms of psoralen. Front. Pharmacol. 15, 1442700. https://doi.org/10.3389/fphar.2024.1442700 (2024).
Ong, S. K. L. et al. Focus on formononetin: Anticancer potential and molecular targets. Cancers (Basel) 11, 611. https://doi.org/10.3390/cancers11050611 (2019).
Ren, F. B. et al. Exploring the multi-targeting phytoestrogen potential of Calycosin for cancer treatment: A review. Medicine (Baltimore) 103, e38023. (2024). https://doi.org/10.1097/MD.0000000000038023
Cheng, Y., Chen, T. Y., Yang, X. L., Xue, J. H. & Chen, J. J. Atractylon induces apoptosis and suppresses metastasis in hepatic cancer cells and inhibits growth in vivo. Cancer Manag. Res. 11, 5883–5894. https://doi.org/10.2147/CMAR.S194795 (2019).
Gao, W. F. et al. Prim-O-glucosylcimifugin enhances the antitumour effect of PD-1 inhibition by targeting myeloid-derived suppressor cells. J. Immunother. Cancer 7, 231. https://doi.org/10.1186/s40425-019-0676-z (2019).
Wang, Z. A. et al. Clinical study on the treatment of advanced liver cancer of Qi deficiency and toxic stasis type by Jiawei Yupingfeng San. J. Nanjing Univ. Tradit. Chin. Med. 40, 413–418 (2024).
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.82474119 & 81303276), “333 Project” Fund of Jiangsu Province (Grant No. BRA2019141), Medical Research Project Fund of Jiangsu Commission of Health (Grant No. M2020004), Project Fund of Suzhou City (Grant No. SZS2024031, SKJY2021132 & SYS2018092).
Author information
Authors and Affiliations
Contributions
Conceptualization: Lurong Zhang; Methodology: Lurong Zhang, Guoqiang Liang; Formal analysis and investigation: Fei Yao, Qin Yuan, Liang Zhou, Heng Xu, Yichao Yan, Shaomei Gao, Ting Zou; Writing—original draft preparation: Fei Yao, Qin Yuan; Writing—review & editing: Lurong Zhang, Yichao Yan; Funding acquisition: Lurong Zhang; Resources: Guoqiang Liang; Supervision: Lurong Zhang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Animal experiments was agreed by the Ethics Committee of Suzhou Chinese Medicine Hospital (Ethical Number: 2021-LDP-049).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, F., Yuan, Q., Yan, Y. et al. Yu-Ping-Feng-San improve the immunosuppression of microenvironment in hepatocellular carcinoma by promoting the maturation of DCs through the JAK2-STAT3 pathway. Sci Rep 14, 31522 (2024). https://doi.org/10.1038/s41598-024-83197-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83197-7