Abstract
Prenatal ethanol exposure (PE) causes Fetal Alcohol Spectrum Disorders (FASD), characterized by cognitive, behavioral, and emotional deficits, including anxiety and depression. PE-induced alteration in the function of dorsal raphe nucleus (DRN) serotonin (5-HT) neurons is thought to be major contributing factor for increased anxiety. However, the precise neuronal circuits involved are unknown. Using electrophysiology, optogenetics, chemogenetics, and behavioral approaches, we find that PE preferentially potentiates medial prefrontal cortex (mPFC) glutamatergic inputs, but not lateral habenula (LHb), to DRN 5-HT neurons projecting to mPFC. Additionally, PE also increases the strength of LHb but not mPFC excitatory inputs to DRN 5-HT neurons projecting to central amygdala (Ce). This input and target selective effect of PE was mediated by a circuit-specific increase in nitric oxide (NO) signaling. Importantly, chemogenetic inhibition of mPFC-DRN neuronal circuit blunted anxiety-like behaviors in PE rats. As such, our results unraveled the DRN neuronal circuitries affected by PE, which gate FASD-induced anxiety-like behaviors.
Similar content being viewed by others
Introduction
Prenatal ethanol (PE) exposure causes a broad range of persistent behavioral and cognitive deficits commonly known as fetal alcohol spectrum disorders (FASD). Despite growing awareness of the detrimental effects of PE on brain development, PE remains by far the most common cause of preventable neurodevelopmental disorders1. FASD affects an estimated 7.7 per 1000 children and youth population worldwide2. In addition to cognitive deficits, additional and prominent comorbidities associated with FASD are abnormal stress responses3 and increased susceptibility to psychiatric disorders, including anxiety and depression4,5.
Serotonin (5-HT) neurons of the dorsal raphe nucleus (DRN)6,7 send widespread projections to major forebrain areas8,9 and control diverse physiological functions, including stress homeostasis and emotional processes10,11,12. The dysfunction of central DRN 5-HT system has long been associated with anxiety and depression-related disorders13,14. Importantly, during embryogenesis, DRN 5-HT neurons are vulnerable to ethanol exposure. Indeed, previous studies in rats and mice have established that PE impairs the normal development of 5-HT neurons15,16,17, alters 5-HT synthesis18,19,20, and affects the expression of 5-HT transporter (5-HTT) and receptors21. In addition, results from a recent functional study have revealed that PE persistently enhances the activity of DRN 5-HT neurons by potentiating their glutamate synaptic synapses22. These effects on 5-HT system are thought to mediate at least in part, the anxiety phenotype reported in rat and mice models of FASD22,23.
Dorsal raphe nucleus 5-HT neurons are a heterogeneous group of neurons that exhibit considerable molecular and cellular heterogeneity24,25,26,27. Thus, based on their molecular and cellular profile, DRN 5-HT neurons are grouped in different neuronal clusters with distinct anatomical connectivity27,28 and behavior functions29,30. Although results from previous studies have shown that the PE-induced anxiety-like behaviors in rats are associated with enhanced activity of DRN 5-HT neurons and potentiation of their glutamatergic inputs22, the precise serotoninergic circuits altered by PE remain to be determined. In the present study, we examined the effects of PE on the medial prefrontal cortex (mPFC) and lateral habenula (LHb) glutamatergic inputs to a specific subset of DRN 5-HT neurons projecting to mPFC and central amygdala (CeA), two serotoninergic pathways critically involved in controlling stress-related disorders31,32,33. In addition, we assessed whether chemogenetic inhibition of mPFC-DRN circuit reverses the anxiety-like behaviors reported in in PE rats.
Results
Prenatal ethanol induces input-specific potentiation of glutamate synapses of DRNmPFC 5-HT neurons
Dorsal raphe 5-HT neurons send widespread projections that innervate most forebrain areas, including the mPFC34,35,36, and receive direct excitatory inputs from mPFC37,38. The mPFC inputs to DRN exert a top-down control on the function of DRN 5-HT neurons38, which are implicated in the regulation of stress responses39. To examine the impact of PE on glutamatergic inputs to DRN 5-HT neurons projecting to mPFC (DRNmPFC 5-HT neurons), we first retrogradely labeled these neurons by bilateral stereotaxic injections of retrograde AAV-CAG-tdTomato in mPFC (Fig. 1A) and characterize their electrical properties. Consistent with earlier reports36,40, we found that DRNmPFC neurons were mainly clustered in the interfascicular ventromedial subregions of the DRN (vmDRN) (Fig. 1B). Co-immunostaining for Tph2 showed that the vast majority of tdTomato labeled DRNmPFC neurons were also Tph2 positive demonstrating their 5-HT phenotype (Fig. 1B-C). The proportion of Tph2 positive DRNmPFC neurons exhibited a rostro-caudal gradient, with a significant increase in the number of double-labeled DRNmPFC neurons (Fig. 1D) in the caudal region, which was consistent with previous reports34,41. We next assessed the electrophysiological properties of DRNmPFC 5-HT neurons, by examining their voltage responses to 600 ms suprathreshold depolarizing current pulses (50 pA). Based on the evoked firing patterns, DRNmPFC 5-HT neurons can be subdivided into three subgroups (Fig. 1E), delayed firing (23%), regular firing (44%), and adapting firing (33%) neurons, thereby indicating that they exhibit heterogenous firing properties.
Distribution and firing patterns of DRNmPFC 5-HT neurons in the Raphe. (A) Schematic of injection of retrograde pAAV-CAG-tdTomato in the mPFC. (B) Rostro-caudal distribution of tdTomato labeled DRNmPFC neurons for (red), immunostaining for Tph2 (green). Note that most of the DRNmPFC 5-HT neurons are located in ventral DRN (vDRN). (C) High magnification images of DRN neurons expressing Tph2 (green) and tdTomato (RED). (D) Percentage of Tph2 and tdTomato positive DRN neurons. (E) The distinct firing patterns of DRNmPFC 5-HT neurons elicited by suprathreshold depolarizing current pulses.
Using bulk in vitro extracellular electrical stimulation of DRN, we have previously shown that PE increases glutamatergic synaptic transmission onto DRN 5-HT neurons22. To dissect the precise excitatory circuits potentiated by PE, we used ChR2-assisted circuit mapping and examined the impact of PE on the strength of mPFC and LHb glutamatergic inputs to DRNmPFC 5-HT neurons. To that end, we first expressed ChR2 in the mPFC and retrogradely labeled DRNmPFC neurons using a viral approach (Fig. 2A). We found that in the presence of GABAA receptor antagonist, picrotoxin (100 µM), photostimulation of mPFC fibers in DRN induced optical excitatory post-synaptic currents (oEPSCs) in DRNmPFC 5-HT neurons. These oEPSCs were blocked with tetrodotoxin (TTX, 1µM) and rescued with the voltage-dependent potassium channel blocker 4-aminopyridine (4-AP, 0.1mM), indicating that DRNmPFC 5-HT neurons receive direct monosynaptic glutamatergic inputs from mPFC (Fig. 2A, n = 9 neurons). Using the same optical stimulation intensity (3 mW), we found that PE increased the average amplitude of oEPSCs (PC: 47.25 ± 11.25 pA, n = 12; PE: 110.06 ± 24.04 pA, n = 15; p = 0.028; Fig. 2B) and significantly reduces their PPR (PC: 0.79 ± 0.037, n = 14; PE: 0.54 ± 0.038, n = 10, p = 1.74E-4; Fig. 2C-D). Collectively, these results demonstrate that DRNmPFC 5-HT neurons receive monosynaptic glutamatergic inputs from the mPFC, which are potentiated by PE through an enhanced glutamate release.
PE potentiates mPFC glutamate synapses of DRNmPFC 5-HT neurons. (A) Top panel is a schematic of stereotaxic injections of rAAV-CAG-tdTomato and AAV-Syn-ChR2 in the mPFC. Lower panel illustrates oEPSCs induced by photostimulation of mPFC axon fibers and recorded from DRNmPFC neurons under baseline condition (orange), in TTX (black), and TTX + AP-5 (blue). (B) Scatter plots of the amplitude of oEPSCs in PC (47.25 ± 11.25 pA, n = 12) and PE (97.83 ± 22.71 pA, n = 17, p = 0.058 vs. PC) rats. (C) Representative oEPSCs traces evoked by paired optical stimuli in PC and PE rats, scale bars: 50 ms and 25 pA. (D) Summary plot of paired-pulse ratio of oEPSCs recorded in PC (0.79 ± 0.037, n = 14) and PE (0.54 ± 0.038, n = 10, p = 1.49E-4 vs. PC at 50 ms SI) rats. (E) Top panel is a schematic of stereotaxic injections of rAAV-CAG-tdTomato and AAV-Syn-ChR2 in mPFC and LHb, respectively. Bottom panel illustrates oEPSCs induced by photostimulation of LHb fibers and recorded from DRNmPFC neurons under baseline condition (orange), in TTX (black), and TTX + AP-5 (blue). (F) Scatter plots of the average amplitude of oEPSCs recorded from DRNmPFC neurons in PC (46.55 ± 7.42 pA, n = 14) and PE (52.83 ± 6.12 pA, n = 23, p = 0.51 vs. PC) rats. (G) Representative oEPSCs traces evoked by paired optical stimuli in PC and PE rats, scale bars: 50 ms and 20 pA. (H) Summary plot of paired-pulse ratio of oEPSCs recorded in PC (0.80 ± 0.10, n = 10) and PE (0.96 ± 0.07, n = 22, p = 0.22 vs. PC at 50 ms SI) rats. Data are represented as mean ± SEM. *p < 0.05, n.s. not significant.
Next, we expressed ChR2 in the LHb (Fig. 2E) and recorded DRNmPFC 5-HT neurons. In the presence of picrotoxin 0.1 mM, photostimulation of DRN LHb fibers, elicited oEPSCs (Fig. 2G, n = 8 neurons). These oEPSCs were blocked with TTX (1 µM) and rescued with 4-AP (0.1mM) (Fig. 2E, n = 8 neurons), thereby indicating that DRNmPFC 5-HT neurons also receive monosynaptic glutamatergic inputs from LHb. Examination of the effects of PE on LHb glutamate synapses of DRNmPFC 5-HT neurons revealed that PE did not significantly alter the average amplitudes of oEPSCs (PC: 46.55 ± 7.42 pA, n = 14; PE: 52.83 ± 6.12 pA, n = 23, p = 0.51; Fig. 2F) nor their PPR (PC: 0.80 ± 0.10, n = 10; PE: 0.96 ± 0.07, n = 22, p = 0.22; Fig. 2G-H), suggesting that PE did not significantly alter the strength of LHb glutamatergic inputs to DRNmPFC 5-HT neurons. Collectively, these findings indicate that PE exerts an input-specific potentiation of DRNmPFC 5-HT neurons glutamate synapses.
PE enhances tonic NO signaling at glutamate synapses of mPFC-DRNmPFC 5-HT neurons
We have previously shown that NO signaling increases the strength of glutamatergic inputs onto DRN 5-HT neurons42, and that PE increases tonic NO signaling in the DRN22. Therefore, to test whether an enhanced tonic NO signaling mediates the PE-induced potentiation of mPFC glutamatergic inputs onto DRNmPFC 5-HT neurons, we first examined the expression of neuronal nitric oxide synthase (nNOS) in these neurons. Using a retrograde labeling approach of DRNmPFC neurons (Fig. 3A) and co-immunostaining for nNOS, we found that nNOS positive neurons are distributed throughout all rostro-caudal DRN subdivisions, with a high density in ventromedial DRN (vmDRn). Importantly, more than 73% of retrogradely tdTomato labeled DRNmPFC neurons were also nNOS positive (Fig. 3B-C), which is consistent with earlier studies reporting that the majority (84%) of DRNmPFC 5-HT neurons are also nNOS positive43,44.
PE enhances tonic NO signaling at mPFC glutamate synapses of DRNmPFC 5-HT neurons. (A) Schematic of viral injections of rAAV-CAG-tdTomato in mPFC. (B1,B2) Immunostaining of retrogradely labelled DRNmPFC neurons for tdTomato (red) and nNOS (green). (C) Quantification of tdTomato and nNOS positive DRNmPFC neurons. (D) Schematic of the ChR2 assisted analysis of mPFC -DRN-mPFC neuronal circuit. (E1) Summary graph of the effect of NOS activator SNAP on the amplitude of oEPSCs induced by photostimulation of DRN mPFC fibers and recorded from DRNmPFC neurons in PC (Baseline: 102.08 ± 2.44%, n = 5; SNAP: 184.32 ± 34.48%, n = 5, p = 0.026 vs. baseline) and PE (Baseline: 98.23 ± 1.66%, n = 5; SNAP: 103.57 ± 1.66%, n = 5, p = 1 vs. baseline) rats. (E2) Representative sample of oEPSCs traces recorded before (1) and during SNAP application (2) from PC and PE rats, scale bars: 25 ms, 50 pA. (F) Schematic of the ChR2 assisted analysis of LHb-DRNmPFC neuronal circuit. (G1) Summary graph of the effect of nNOS activator SNAP on the amplitude of oEPSCs recorded from DRNmPFC neurons by stimulation of mPFC fibers expressing ChR2, obtained from PC (Baseline: 100 ± 2.85%, n = 7; SNAP: 117.74 ± 3.93%, n = 7, p = 0.062 vs. baseline) and PE (Baseline: 100 ± 2.63%, n = 11; SNAP: 104.28 ± 6.11%, n = 11, p = 1 vs. baseline) rats. (G2) Representative sample oEPSCs traces taken before (1) and during SNAP application (2) in PC and PE (orange) rats, scale bars: 25 ms, 40 pA.
We next assessed the impact of NO signaling on the strength of mPFC glutamatergic inputs onto DRNmPFC 5-HT neurons (Fig. 3D), by examining the effects of NO donor (S)-nitroso-N-acetyl-D, L-penicillamine (SNAP) on the amplitude of oEPSCs evoked by photostimulation of mPFC fibers in DRN. As illustrated in Fig. 3E1-E2, bath application of SNAP (100 µM) potentiated the amplitude of oEPSCs in control rats (PC: 164.49 ± 28.89% of baseline, n = 5, p = 0.042 vs. baseline), thereby indicating that NO regulates mPFC glutamatergic inputs to DRNmPFC 5-HT neurons. Interestingly, the SNAP-induced potentiation of oEPSCs was blunted in PE rats (PE: 98.23 ± 1.66% of baseline, n = 5; PC vs. PE, p = 0.03; Fig. 3E1), suggesting that PE occluded the effect of SNAP. We also examined whether NO signaling regulates the strength of LHb glutamatergic inputs to DRNmPFC 5-HT neurons (Fig. 3F). Our results revealed that SNAP (100 µM) did not affect the amplitude of oEPSCs induced by activation of LHb axon terminals in either control or PE rats (PC: 117.74 ± 3.93% of baseline, n = 7; p = 0.06 vs. baseline; PE: 104.28 ± 6.11, n = 11; PC vs. PE, p = 0.17; Fig. 3G1-2), indicating that NO signaling does not regulate the strength of LHb glutamatergic input to DRNmPFC 5-HT neurons. Taken together, these results indicate that PE induces an input-specific potentiation of excitatory synaptic transmission onto DRNmPFC 5-HT neurons, which is mediated by a synapse-specific increase in NO signaling.
Prenatal ethanol strengthens glutamate synapses of DRNCeA 5-HT neurons
To target DRNCeA 5-HT neurons, we performed retrograde labeling by injecting the rAAV-CAG-tdTomato in CeA (Fig. 4A). As shown in Fig. 4B, the distribution pattern of tdTomato labeled neurons was different according to rostro-caudal axis and subdivisions of DRN. In the rostral region, DRNCeA neurons were homogenously distributed throughout the DRN subdivisions. Whereas in the caudal region, they were mainly located in the midline region of the vmDRN. Co- immunostaining for Tph2 revealed that the vast majority of these neurons were also Tph2 positive (80%, Fig. 4B-D), and were clustered along the dmDRN-vmDRn midline. In contrast, Tph2 negative neurons were mostly localized in the proximity to the cerebral aqueduct. The evoked firing activity of DRNCeA 5-HT neurons exhibited three distinct firing patterns, regular firing (43%), adapting firing (38%), and delayed firing (19%) (Fig. 4E).
Distribution and firing patterns of DRNCeA 5-HT neurons in the raphe. (A) Schematic of viral injections of rAAV-CAG-tdTomato in the CeA. (B) Rostro-caudal distribution of tdTomato labeled DRNCeA neurons (red) expressing Tph2. (C) High magnification images of Tph2 (green) and tdTomato labeled DRNCeA neurons (Red). (D) Percentage of tdTomato and Tph2 positive DRNCeA neurons. (E) The distinct firing patterns of DRNCeA 5-HT neurons elicited by suprathreshold depolarizing current pulses.
We next expressed ChR2 in mPFC or LHb neurons and recorded oEPSCs from tdTomato retrogradely labeled DRNCeA 5-HT neurons (Fig. 5A). As shown in Fig. 5A, photostimulation of mPFC fibers elicited monosynaptic oEPSCs that was blocked by TTX and rescued by 4-AP (n = 7 neurons). The average amplitude of oEPSCs induced by 3mW photostimulation was comparable in PC and PE rats (PC: 49.21 ± 2.76 pA, n = 22; PE: 49.27 pA ± 9.46, n = 31, p = 0.99; Fig. 5B). Examination of the PPR of oEPSCs showed a significant decrease in PE compared to control rats (PC50ms: 0.73 ± 0.03, n = 8; PE50ms: 0.51 ± 0.05, n = 11; p = 0.0061; Fig. 5C-D), suggesting that PE increase the probability of glutamate release from mPFC fibers impinging onto DRNCeA 5-HT neurons. Similar to mPFC inputs, photostimulation of LHb fibers also induced monosynaptic oEPSCs in DRNCeA 5-HT neurons (Fig. 5E). The average amplitude of oEPSCs induced by stimulation of LHb inputs was not different between PE compared to control rats (PC: 50.74 ± 11.24 pA, n = 20; PE: 84.45 ± 15.13 pA, n = 37, p = 0.13; Fig. 5F). Furthermore, we found that PE significantly reduced the PPR of oEPSCs induced by photostimulation of LHb inputs (PC50ms: 0.99 ± 0.13, n = 9; PE50ms: 0.33 ± 0.066, n = 6; p = 0.001; Fig. 5G-H). Combined, these data suggested that PE increases glutamate release at both mPFC and LHb excitatory synapses impinging onto DRNCeA 5-HT neurons, albeit the effect of PE was more profound at LHb than mPFC inputs to DRNCeA 5-HT neurons.
PE potentiates glutamate release at mPFC and LHb inputs onto DRNCeA 5-HT neurons. (A) Top panel is a schematic of viral injections of rAAV-CAG-tdTomato and AAV-Syn-ChR2 injections in the CeA and mPFC, respectively. Bottom panel depicts oEPSC traces induced by photostimulation of mPFC axon fibers in the DRN in the absence of TTX (red), in TTX (black), and in TTX + AP-5 (blue). (B) Scatter plots of the oEPSC amplitude evoked by photostimulation of mPFC fibers and recorded from DRNCeA neurons of PC (49.21 ± 2.76 pA, n = 22) and PE (49.27 ± 9.46 pA, n = 31, p = 0.99 vs. PC) rats. (C) Representative oEPSCs traces evoked by paired optical stimuli (blue bars) in PC and PE rats, scale bars: 50 ms and 25 pA. (D) Summary plot of paired-pulse ratio of oEPSCs recorded in PC (0.73 ± 0.031, n = 8) and PE (0.51 ± 0.056, n = 11, p = 0.006 vs. PC at 50 ms SI) rats. (E) Top panel, schematic of viral injections of rAAV-CAG-tdTomato and AAV-Syn-ChR2 in CeA and LHb, respectively. Bottom panel, representative sample oEPSC traces induced by photostimulation of LHb fibers recorded from DRNCeA neurons under baseline condition (orange), in TTX (black), and in TTX + AP-5 (blue). (F) Scatter plots of the amplitude of oEPSCs recorded from DRNCeA neurons in PC (50.74 ± 11.24 pA, n = 20) and PE (84.45 ± 15.13 pA, n = 37, p = 0.13 vs. PC) rats. (G) Representative oEPSCs traces evoked by a pair of optical stimuli in PC and PE rats, scale bars: 50 ms and 50 pA. (H) Summary plot of paired-pulse ratio of oEPSCs recorded in PC (0.99 ± 0.13, n = 9) and PE (0.33 ± 0.06, n = 6, p = 0.001 vs. PC at 50 ms SI) rats. Data are represented as mean ± SEM. *p < 0.05, n.s. not significant.
Prenatal ethanol exposure enhances tonic NO signaling at LHb-DRNCeA 5-HT neurons glutamate synapses
To test whether PE potentiates mPFC and LHb glutamates synapses of DRNCeA 5-HT neurons via an increased NO signaling, we first assessed the expression of nNOS in these neurons. We found that approximately 40% of DRNCeA neurons (40%) express nNOS (Fig. 6A-C) and are distributed throughout the various DRN subdivisions. Interestingly, administration of the NO donor SNAP (100 µM) increased the amplitude of oEPSCs induced by optical stimulation of mPFC inputs in control but not in PE rats (PC: 129.26 ± 8.03% of baseline, n = 5; p = 0.043 vs. baseline; PE: 121.04 ± 9.86% of baseline, n = 6; p = 0.16 vs. baseline; Fig. 6D-E), suggesting that mPFC glutamatergic inputs to DRNCeA 5-HT neurons are modulated by NO. Such findings also suggest that the PE-induced potentiation of these inputs is mediated by increased NO signaling. Similar to mPFC inputs, we found that SNAP potentiated the amplitude of oEPSCs induced by activation of LHb inputs in control by not PE rats (PC: 127.09 ± 6.79% of baseline, n = 6; p = 0.019 vs. baseline; PE: 94.47 ± 8.50%, n = 7; p = 1 vs. baseline; PE vs. PC, p = 0.003; Fig. 6F-G). Taken together, these results indicate that both LHb and mPFC glutamatergic inputs to DRNCeA 5-HT neurons are modulated by NO and potentiated by PE-induced increase in tonic NO signaling.
PE increases tonic NO signaling at LHb glutamate synapses of DRNCeA 5-HT neurons. (A) Schematic of rAAV-CAG-tdTomato injections in the CeA. (B1,B2) Immunostaining of tdTomato-labeled DRNmPFC neurons (red) and nNOS expression (green). (C) Quantification of tdTomato and nNOS expression in DRNCeA neurons. (D) Schematic of the ChR2 assisted analysis of mPFC -DRN-CeA neuronal circuit. (E1) Summary graph of the effect of nNOS activator SNAP on the amplitude of oEPSCs induced by photostimulation of mPFC fibers and recorded from DRNCeA neurons in PC (Baseline: 100.00 ± 0.23%, n = 5; SNAP: 129.26 ± 8.03%, n = 5, p = 0.043 vs. baseline) and PE (Baseline: 100.00 ± 0.30%, n = 6; SNAP: 121.04 ± 9.86%, n = 6, p = 0.16 vs. baseline) rats. (E2) Representative sample of oEPSCs traces taken before (1) and during SNAP application (2) in PC and PE rats. Scale bars: 25 ms, 25 pA. (F) Schematic of ChR2 assisted analysis of LHb -DRN-CeA neuronal circuit. (G1) Summary of the effect of nNOS activator SNAP (100 µM) on the amplitude of oEPSCs induced by photoactivation of LHb fibers and recorded from DRNCeA neurons in PC (Baseline: 100.00 ± 4.21%, n = 6; SNAP: 127.09 ± 6.79%, n = 6, p = 0.019 vs. baseline) and PE (Baseline: 99.05 ± 0.94%, n = 7; SNAP: 94.47 ± 8.50%, n = 7, p = 1 vs. baseline) rats. (G2) Representative sample oEPSCs traces taken before (1) and during SNAP application (2) in PC and PE rats. Scale bars: 50 ms, 50 pA.
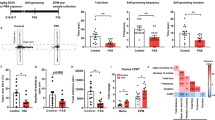
Inhibition of mPFC inputs to DRN blunts PE-induced anxiety-like behaviors
Although numerous studies have shown that PE increases anxiety-like behaviors22,23,45,46, the precise neuronal circuits involved remain unknown. Therefore, using chemogenetic approach we tested whether PE-induced potentiation of mPFC -DRN pathway contributes to increased anxiety-like behaviors in PE rats. To that end, we expressed inhibitory DREADD (hM4Di/mCherry) in mPFC pyramidal neurons projecting to DRN (mPFCDRN) by injection of rAAV-pgk-Cre in the DRN and AAV-hSyn-DIO-hM4D(Gi)-mCherry in the mPFC (Fig. 7A). As expected, injection of these viruses resulted in a strong mCherry labeling in the mPFC and DRN (Fig. 7B). Importantly, in acute mPFC brain slices, administration of a DREADD agonist C21 (10 µM) induced a marked membrane hyperpolarization (11.61 ± 0.5 mV, n = 4, p = 2.28E-4, Fig. 7C) and inhibition of the firing activity of mPFC DRN neurons, thereby confirming that activation of the inhibitory DREADD depresses the activity of mPFC-DRN circuit. Next, we tested whether inhibition of mPFCDRN circuit blunts the increased anxiety-like behaviors in PE. The results of this experiment showed that in PC rats, administration of the DREADD agonist C21 (3 mg/Kg) induced a significant increase in the number of entries (PCVEH: 7.9 ± 1.74, PCC21: 13.7 ± 2.23, n = 10; p = 0.014, Fig. 7D2 and 7D4) and duration (PCVEH: 12.59 ± 3.24, PCC21: 27.73 ± 5.30, n = 10; p = 0.0049, Fig. 7D3) in the center zone with no significant effect on the total distance traveled (PCVEH: 47.13 ± 6.26 m; PCC21: 57.21 ± 4.98, n = 9, p = 0.09, Fig. 7D1). Such results indicate that inhibiting mPFC-DRN circuit reduces basal anxiety-like behaviors in PC rats. In PE rats, activation of DREADD with C21 (3 mg/Kg) increased the total distance travelled (PEVEH: 40.45 ± 6.50, PEC21: 52.54 ± 4.72, n = 9; p = 0.045, Fig. 7E1), the number of entries to the center zone (PEVEH: 4 ± 1.33, PEC21: 10 ± 2.51, n = 9; p = 0.02, Fig. 7E2 and 7E4) and an increase albeit, non-significant in the duration of stay in the center zone (PEVEH: 7.35 ± 2.71, PEC21: 16.03 ± 4.94, n = 9; p = 0.062, Fig. 7E3). Collectively, these results suggest that inhibition of mPFCDRN neuronal circuits reduces basal anxiety in control rats and reduces PE-induced increase in anxiety-like behaviors observed in the OF test.
Inhibition of mPFC inputs to DRN blunts PE-induced anxiety-like behaviors. (A) Schematic of viral injections of rAAV-pgk-Cre in the DRN and AAV-hSyn-DIO-hM4D(Gi)mCherry in the mPFC to express Cre-dependent inhibitory DREADD. (B) Right panel, image of mCherry-labeling indicating DREADD expression in the DRN. Left panel, image of mCherry expression in the mPFC. (C) Voltage response of a layer V mPFC pyramidal neuron to bath application of C21 (10 µM), a DREADD agonist. Note the inhibition of firing activity and hyperpolarization of mCherry-labeled mPFC neuron (11.61 ± 0.5 mV, n = 4). (D) Chemogenetic inhibition of mPFC-DRN circuit inhibits basal anxiety-like behaviors of PC rats in the open field test (OF). (D1) Average total distance traveled of PC rats treated with vehicle (47.13 ± 6.26, n = 10) and C21 (57.21 ± 4.98, n = 10, p = 0.09 vs. VEH) in the OF. (D2) Average number of entries PC rats treated with vehicle (7.9 ± 1.74, n = 10) or C21(: 13.7 ± 2.23, n = 10; p = 0.014 vs. VEH) to the OF inner zone. (D3) Average duration of stay of PC rats treated with vehicle (12.59 ± 3.24, n = 10) or C21(27.73 ± 5.30, n = 10; p = 0.0049 vs. VEH) in the OF inner center. (D4) Movement tracking plots of PC rats treated with vehicle (top) and C21 (bottom) in the OF. (E) Chemogenetic inhibition of mPFC-DRN circuit inhibits basal anxiety-like behaviors of PE rats. (E1) Average total distance traveled of PE rats treated with vehicle (40.45 ± 6.50, n = 9) or C21 (52.54 ± 4.72, n = 9, p = 0.045 vs. VEH) in the open field. (E2) Average number of entries of PE rats injected with vehicle (4 ± 1.33, n = 9) and C21 (10 ± 2.51, n = 9; p = 0.02 vs. VEH) to the OF inner center. (E3) Average duration of stay of PE rats treated with vehicle (7.35 ± 2.71 s, n = 9) or C21 (16.03 ± 4.94 s, n = 9, p = 0.062 vs. VEH) time in the OF inner center. (E4) Movement tracking plots of PE rats treated with vehicle (top) or C21 (bottom). Data are represented as mean ± SEM. *p < 0.05, n.s. not significant.
Discussion
Prenatal ethanol (PE) exposure causes a constellation of cognitive and emotional disorders, including depression47,48,49 and anxiety-like behaviors3,22,23. Emerging evidence suggests that the anxiogenic effects of PE are associated with dysfunction of central 5-HT system22,50,51, and altered excitability of DRN 5-HT neurons22. However, the precise serotoninergic neuronal circuits and the molecular mechanisms underlying PE-induced anxiety-like behaviors remain unknown. Here, using a rodent model of FASD, we found that PE preferentially potentiated mPFC and LHb glutamatergic synaptic transmission onto DRNmPFC and DRNCeA 5-HT neurons, respectively. These inputs and cell-specific effects of PE were mediated by targets and synapses specific increase in tonic NO signaling. Importantly, we also showed that selective chemogenetic inhibition of mPFC inputs to DRN neurons blunted anxiety-like behaviors. As such, the present study unraveled a potential signaling mechanism and the DRN neuronal circuits that could underlie the anxiety phenotype induced by gestational exposure to ethanol.
Serotonin neurons of DRN are clustered in distinct subgroups exhibiting different and unique molecular, physiological features and distinct functional role25,26. Each subpopulation of DRN 5-HT neurons is preferentially interconnected with specific brain areas29,35,52 enabling it to fulfill specific physiological and behavioral functions29,30,53,54. DRN 5-HT neurons projecting to the amygdala and mPFC are part of the stress neuronal circuits55,56,57. Activation of these neurons controls stress homeostasis and gate anxiety-like behaviors30,32,10,58. In the present study, we first characterized the functional organization of mPFC and LHb inputs to DRNmPFC and DRNCeA 5-HT neurons. Although previous studies have reported that DRN 5-HT neurons receive excitatory inputs from mPFC38 and LHb59 the precise cellular target of these inputs remains unknown. Our result showed that DRNmPFC 5-HT neurons receive monosynaptic glutamatergic inputs from mPFC indicating that the DRN and mPFC neurons are engaged in a neuronal loop. In addition, the finding that DRNmPFC 5-HT neurons are also under the control of monosynaptic glutamatergic inputs from LHb, suggests that the DRN-mPFC circuit loop is modulated by LHb inputs. An interesting finding is that PE potentiates mPFC, but not, LHb glutamatergic inputs to DRNmPFC 5-HT neurons. We have previously shown that PE potentiates DRN glutamate synapses via a persistent increase in tonic NO signaling22. Thus, it is conceivable that this input-specific effect could be attributed to a difference in NO-mediated gating of glutamatergic inputs onto DRN mPFC 5-HT neurons. Consistent with this notion, our results show that although the vast majority of DRNmPFC 5-HT neurons express nNOS, the enzyme that synthesizes NO, tonic NO signaling selectively potentiates mPFC, but not, LHb glutamate synapses impinging onto DRNmPFC 5-HT neurons.
Similar to DRNmPFC 5-HT neurons, DRNCeA 5-HT neurons also receive monosynaptic glutamatergic inputs from both mPFC and LHb. These glutamatergic inputs are potentiated in PE rats as shown by the increase in the probability of glutamate release. Examination of the mechanisms underlying this effect shows that although a significant number of DRNCeA 5-HT neurons express NOS, LHb, but not, mPFC glutamate synapses of DRNCeA 5-HT are potentiated by an increase in NO signaling. Importantly, the finding that the potentiation of LHb glutamatergic inputs onto DRNCeA 5-HT neurons by the NO donor SNAP is occluded in PE rats supports the conclusion that an increase in NO signaling mediates the PE-induced potentiation of LHb glutamatergic inputs to DRNCeA 5-HT neurons. It is interesting to note that in addition to enhancing NO signaling22,60, PE impairs eCB-mediated depression of central synapses22,61. Thus, it is conceivable that impaired eCB signaling mediates the PE-induced potentiation of mPFC glutamate synapses of DRNCeA 5-HT neurons. Thought, additional studies are required to thoroughly test this notion.
The top-down control exerted by the mPFC over the activity of DRN 5-HT neurons is involved in regulating stress homeostasis and emotional behaviors39,62,63. In rodents, stimulation of this pathway has been shown to exert an antidepressant-like effect in the forced swim test (FST)39,64 and enhances resilience to social defeat stress-induced depression-like behaviors62,65. In addition, activation of the mPFC has been reported to either increase anxiety-like behaviors66 or exert an anxiolytic-like effect64. These divergent behavioral effects could be attributed to the recruitment of different stress-related circuits downstream from the mPFC. Here, we show that in control rats selective chemogenetic inhibition of mPFC-DRN neuronal circuit reduces anxiety-like behaviors assessed in the OF test. Importantly, inhibition of this pathway also blunts the anxiety phenotype observed in PE rats. Thus, the present study reveals that mPFC inputs onto DRN 5-HT neurons gate anxiety-like behaviors in the OF test and that persistent activation of this input mediates, at least in part, the anxiety phenotype of PE rats. However, the present study does not exclude the possibility that mPFC inputs impinging onto DRN 5-HT neurons may also innervate other brain regions, which could in theory contribute to the anxiolytic effects of chemogenetic inhibition of the mPFC. Furthermore, we have previously shown that mPFC inputs establish direct monosynaptic connections with DRN GABA neurons, which provide a feedforward inhibition to DRN 5-HT neurons38. Consequently, it is conceivable that alterations of GABA signaling in the DRN may also mediate the anxiolytic effect induced by chemogenetic inhibition of the mPFC-DRN inputs in PE rats.
Results from a previous study have shown that early postnatal exposure to selective serotonin uptake inhibitors (SSRIs) enhances mPFC-DRN synaptic connectivity and increases depression-like behaviors in mice67. Thus, it is tempting to speculate that the PE-induced potentiation of mPFC inputs to the DRN may also contribute to depression phenotype of PE rats. In addition, our results also reveal that PE enhances the strength of LHb excitatory inputs to DRN 5-HT neurons, which has been shown to control stress-induced depression-like behaviors68,69. Indeed, inhibition of this pathway exerts an antidepressant-like effect in various animal models of depression70,71,72,73. Consequently, it is possible that the PE-induced potentiation of both mPFC-DRN and LHb-DRN neuronal circuits could mediate the increased depression-like behaviors reported in PE rats74,75. However, future studies are required to directly assess the precise role of these circuits in mediating the depression phenotype of PE rats.
In summary, the present study deciphered the effects of PE on two majors glutamatergic (i.e. mPFC and LHb) inputs impinging onto two distinct subpopulations of DRN 5-HT neurons known to be involved in stress-mediated behaviors. It also revealed the differential role exerted by tonic NO signaling in gating these glutamatergic circuits and their contribution to anxiety-like behaviors in PE rats. However, further studies are required to explore the functional interactions between these neuronal circuits in mediating PE-induced anxiety-like behaviors.
Materials and methods
Animal breeding
All experiments and procedures used in this study were approved by the University at Buffalo Animal Care and Use Committee and the experiment was performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals, and reported in complaince with the ARRIVE guidelines. Breeding and prenatal ethanol treatment were performed as previously described76. During gestational day (GD) 8 to 20, pregnant Sprague Dawley (Envigo, Indianapolis, IN, USA) rats were administered twice (5–6 h apart) with 3 g/kg/treatment ethanol (15% w/v in 0.9% saline) or vehicle (22.5% w/v sucrose in 0.9% saline, isocaloric to ethanol) via intragastric gavage every weekday. A single daily treatment with 4 g/kg solutions was given on weekends. The blood ethanol concentration was 116.3 ± 10.4 mg/dL, measured 1 h after the 2nd gavage on weekdays. Both groups received thiamine (8 mg/kg, i.m; twice/week) to avoid vitamin B1 deficiency induced by PE or pair-feeding. On postnatal day (PD) 1, litters were randomly culled to 10 pups/litter (50–75% males), PE offspring were fostered to untreated dams which gave birth two days earlier to avoid the impact of ethanol withdrawal on maternal behavior, and PC were cross-fostered among themselves. On PD 21, PE and PC male pups were weaned and housed in pairs in standard cages until the completion of the experiments.
Acute brain slice preparation
Brain slices containing the DRN were obtained from PC and PE male rats (8–10 weeks old) as previously described22. Briefly, adult male rats (8 to 10 weeks old) were decapitated under isoflurane anesthesia and the brainstem area containing the DRN was isolated. Coronal slices (350 μm) of the DRN were cut using a Leica VT1200S vibratome (Leica Biosystem, St Louis, MO, USA) in ice-cold modified Ringer’s solution of the following composition (in mM): 110 choline-Cl; 2.5 KCl; 0.5 CaCl2; 7 MgSO4; 1.25 NaH2PO4; 26.2 NaHCO3; 11.6 sodium L-ascorbate; 3.1 sodium pyruvate, 25 glucose and equilibrated with 95% O2/5% CO2. Slices were first incubated for 15 min in a holding chamber containing modified Ringer’s solution at 35 °C, then transferred to regular Ringer’s solution of the following composition (in mM): 119 NaCl; 2.5 KCl; 2.5 CaCl2; 1.3 MgSO4; 1 NaH2PO4; 26.2 NaHCO3; 11 glucose and continuously bubbled with a mixture of 95% O2/5% CO2. After recovery at room temperature for at least 1 h, slices were transferred to a recording chamber (Warner Instruments, Hamden, CT, USA) mounted on an upright microscope and continuously perfused (2 to 3 ml/min) with Ringer’s solution saturated with 95% O2/5% CO2 and heated to 30 ± 1 °C using a solution heater (Warner Instruments, Hamden, CT, USA).
Ex-vivo electrophysiology and optogenetic
Dorsal raphe neurons were visualized using an Olympus BX51 microscope equipped with a 40X water-immersion objective, differential interference contrast (DIC), and fluorescence systems. tdTomato retrogradely labeled DRN 5-HT neurons were visualized using Texas red fluorescence filters (λ = 540–580 nm) and targeted for whole-cell patch clamp recordings. Somatic whole-cell recordings were performed using patch electrodes with a resistance of 3–5 when mΩ filled with an internal solution containing (in mM): 120 potassium gluconate; 10 KCl; 10 Na2-phosphocreatine; 10 HEPES; 1 MgCl2; 1 EGTA; 2 Na2-ATP; 0.25 Na-GTP (pH adjusted to 7.3–7.4 with KOH, osmolality 280–290 mOsmol/l). Membrane current and voltage were amplified with an Axoclamp 2B or Multiclamp 700B amplifier (Molecular Devices, Union City, CA, USA), filtered at 3 kHz, digitized at 10 kHz with Digidata 1440, and acquired using pClamp 10.7 software (Molecular Devices, San Jose, CA, USA). Optically induced postsynaptic currents (oEPSCs) were triggered at a frequency of 0.05 Hz with light pulses (duration: 5 ms, intensity: 2–5 mW) using Prizmatix LED module (465 nm). The light was delivered through patch optical cable (200 μm tip diameter and 1 cm bare exposed glass). The oEPSCs were recorded from neurons voltage clamped at -70 mV in the presence of GABAA receptor antagonist picrotoxin (100 µM). To determine the paired-pulse ratio (PPR), a pair of oEPSCs were evoked with an inter-stimulus interval of 50 to 150 ms. The cell input resistance and access resistance were monitored throughout the experiments using 5 mV hyperpolarizing voltage steps (500 ms duration). Recordings were discarded when the input and series resistance changed by more than 10–20%. Membrane voltage and current were acquired using the pClamp 11 software (Molecular Devices, Union City, CA, USA).
In vivo stereotaxic viral injections
Males Sprague Dawley rats from PE and PC groups (4–5 weeks old) were intraperitoneally anesthetized with a mixture of ketamine (60 mg/kg of body weight) and xylazine (5 mg/kg of body weight) and used for stereotaxic surgeries. To express channelrhodopsin 2 (ChR2) in the medial prefrontal cortex (mPFC) or lateral habenula (LHb) neurons, AAV-hSyn-hChR2(H134R)-EYPF (Addgene # 26973) was bilaterally injected either into mPFC (coordinates relative to bregma: anteroposterior; +3, mediolateral; ± 0.6, dorsoventral; -4 to brain surface: 500 nl/side, 100 nl/min), or into LHb (coordinates relative to bregma: anteroposterior; -3.6, mediolateral; ± 0.6, dorsoventral; -4.6 to brain surface: 300 nl/side, 100 nl/min). To retrogradely label DRn 5-HT neurons projecting to mPFC or CeA, rAAV-CAG-tdTomato virus (Addgene, 59462) was bilaterally injected into mPFC or central amygdala (CeA) (coordinates relative to bregma: anteroposterior; -2, mediolateral; ± 4, dorsoventral; -8 to brain surface: 300 nl/side, 100 nl/min). To specifically express the Cre-dependent DREADDS in mPFC neurons projecting to the DRN, retrograde rAAV-pgk-Cre (Addgene # 24593) was injected into DRN (coordinates relative to bregma: anteroposterior; -8, mediolateral; 2.5 with 20°ML angle, dorsoventral; -6 to brain surface: 500 nl, 100 nl/min) and AAV-hSyn-DIO-hM4D(Gi)-mCherry (Addgene # 44362) was bilaterally injected into mPFC. The injections were performed using a 33-gauge needle attached to a 5 µl syringe (Hamilton, Reno, NV) and driven by a Stoelting pump (Stoelting Co, Wood Dale, IL, USA). After surgery, rats were individually housed and observed two days post-surgery and allowed to fully recover at least three weeks before the start of electrophysiological and behavioral experiments.
Drug administration
DREADD agonist 21 (C21) (11-(1-piperazinyl)-5 H-dibenzo[b, e][1,4]diazepine); Tocris Bioscience) was used to activate inhibitory (hM4Di) DREADDs. C21 was dissolved in 3% dimethyl sulfoxide (DMSO) (Sigma) and 10% cremophor (Sigma) in 0.9% sterile saline. C21 at a dose of 3 mg/Kg or vehicle were administrated intraperitoneally 30 min before the open field test.
Open field test
Rats were habituated in an open field (OF) room for 15 min before being introduced to the center of an OF apparatus (100 cm*100 cm *46 cm) with a black rubber floor and light intensity of 65 lx in the center and 30 to 45 lx in the corners. Rats were allowed to spend 18 min in the OF and their activity was recorded with a digital camera and analyzed with ANY-maze version 6 software (Stoelting Co., Illinois, United States). Anxiety-like behavior was measured by the total distance traveled in the entire OF, the number of entries, and time spent in the center zone.
Immunohistochemistry
Prenatal ethanol and PC rats were deeply anesthetized with pentobarbital and transcardially perfused with 100 ml phosphate-buffered saline (PBS) (0.9%), followed by 250 ml ice-cold 4% paraformaldehyde (PF) in 1X phosphate buffer (pH 7.4). Their brains were removed and post-fixed in 4% PF for an hour, and sucrose solution (10%) at 4 °C until they sank. Coronal brain Sect. (40 μm) containing the DRN were cut using American Optical 860 sliding microtome. The brain sections were first incubated overnight at 4 °C in a phosphate buffer saline (PBS) solution containing 10% normal goat serum (NGS) and 0.5% Triton X-10 to block unspecific binding. Sections were then incubated with the following primary antibodies: anti-tdTomato (Biorbyt, Cambridge, UK) at 1/500 dilution, anti-neuronal nitric oxide (nNOS) (Cell Signaling, Danvers, MA, USA) at 1/200 dilution and anti-tryptophan hydroxylase type 2 (Tph2) (Abcam, Waltham, MA, USA), the main enzyme of 5-HT synthesis at 1/200 dilution, in a solution containing 1% normal goat serum (NGS), 0.5% Triton X-10 in PBS for 48 h at 4 °C. Sections were washed in PBS (10 min, 3 times) and incubated with the following secondary antibodies: anti-goat Alexa 647, anti-rabbit Alexa 488 and anti-mouse Alexa 488 (Abcam, Waltham, MA, USA) at 1/500 dilution for 5 h at room temperature. The sections were then, washed three times with PBS, mounted on glass slides, and imaged using Leica DMi8 fluorescence inverted microscope (Leica Microsystems Inc, Deerfield, IL, USA). The numbers of retrogradely labeled neurons expressing nNOS (nNOS+) and Tph2 were estimated in a defined region of interest (ROI) using Leica software. The percentage of neurons expressing both RFP and nNOS or RPF and Tph2 is the ratio of neurons double-labeled divided by neurons expression RFP.
Data and statistical analysis
Glutamate-mediated oEPSCs were analyzed using Clampfit 10.7 software (Molecular Devices, Union City, CA, USA). The amplitude of oEPSCs was determined by measuring the average current during a 2 ms time window at the peak of each oEPSC and subtracted from the baseline current measured during a 5 ms before optical stimulation. To assess the direct effect of NO donor SNAP, all oEPSC amplitudes were normalized to the mean baseline recorded for at least 5 min before the drug application. For paired-pulse experiments the paired-pulse ratios (PPR = oEPSC2 /oEPSC1) were averaged for at least 60 consecutive trials in PC and PE rats. Statistical analysis was performed using Origin 2019b software (OriginLab Co, Northampton, MA, United States). The results in the text and figures are expressed as mean ± SEM. Parametric paired t-test was used for within-group comparison. For comparison between groups, analysis of variance (ANOVA) using post-hoc Bonferroni test was used. Statistical significance was set at p < 0.05.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Roozen, S. et al. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcohol Clin. Exp. Res. 40, 18–32 (2016).
Lange, S. et al. Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. JAMA Pediatr. 171, 948 (2017).
Hellemans, K. G. C., Verma, P., Yoon, E., Yu, W. & Weinberg, J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann. N Y Acad. Sci. 1144, 154–175 (2008).
Barr, H. M. et al. Binge drinking during pregnancy as a predictor of psychiatric disorders on the structured clinical interview for DSM-IV in young adult offspring. AJP 163, 1061–1065 (2006).
Hellemans, K. G. C. et al. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety‐like behaviors in male and female offspring. Alcoholism Clin. Exp. Res. 34, 633–645 (2010).
Dahlström, A. & Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 20, 398–399 (1964).
Jacobs, B. L. & Müller, C. P. Handbook of the Behavioral Neurobiology of Serotonin (Academic, 2010).
Azmitia, E. C. & Segal, M. An autoradiographic analysis of the differential ascending projections of the dorsal and median Raphe nuclei in the rat. J. Comp. Neurol. 179, 641–667 (1978).
Jacobs, B. L. & Azmitia, E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992).
Hale, M. W., Shekhar, A. & Lowry, C. A. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell. Mol. Neurobiol. 32, 695–708 (2012).
Lowry, C. A., Johnson, P. L., Hay-Schmidt, A., Mikkelsen, J. & Shekhar, A. Modulation of anxiety circuits by serotonergic systems. Stress 8, 233–246 (2005).
Dolzani, S. D. et al. Activation of a Habenulo–Raphe circuit is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat. Eneuro 3, ENEURO0229–162016 (2016).
Graeff, F. G., Guimarães, F. S., De Andrade, T. G. C. S. & Deakin, J. F. W. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 54, 129–141 (1996).
Underwood, M. D. et al. Morphometry of the dorsal Raphe nucleus serotonergic neurons in suicide victims. Biol. Psychiatry. 46, 473–483 (1999).
Tajuddin, N. F. & Druse, M. J. In utero ethanol exposure decreased the density of serotonin neurons. Maternal Ipsapirone treatment exerted a protective effect. Dev. Brain Res. 117, 91–97 (1999).
Zhou, F. C., Sari, Y. & Powrozek, T. A. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcoholism Clin. Experimental Res. 29, 141–149 (2005).
Zhou, F. C., Sari, Y., Zhang, J. K., Goodlett, C. R. & Li, T. K. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Dev. Brain Res. 126, 147–155 (2001).
Kim, E. K. et al. Maternal ethanol administration inhibits 5-hydroxytryptamine synthesis and Tryptophan hydroxylase expression in the dorsal Raphe of rat offspring. Brain Develop. 27, 472–476 (2005).
Druse, M. J., Kuo, A. & Tajuddin, N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcoholism Clin. Exp. Res. 15, 678–684 (1991).
Dylag, K. A., Fidalgo, S. V. S., Gard, P. R. & Patel, B. A. Prenatal alcohol exposure reduces 5-HT concentration in mouse intestinal muscle and mucosa. Environ. Toxicol. Pharmacol. 61, 24–29 (2018).
Sliwowska, J. H. et al. Effects of prenatal ethanol exposure on regulation of basal hypothalamic–pituitary–adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology 33, 1111–1123 (2008).
Oubraim, S. et al. Prenatal ethanol exposure causes anxiety-like phenotype and alters synaptic nitric oxide and endocannabinoid signaling in dorsal Raphe nucleus of adult male rats. Transl Psychiatry. 12, 440 (2022).
Wang, A. L. et al. Prenatal ethanol exposure leads to persistent anxiety-like behavior during adulthood indicated by reduced horizontal and vertical exploratory behaviors. Front. Neurosci. 17, 1163575 (2023).
Ranade, S. P. & Mainen, Z. F. Transient firing of dorsal Raphe neurons encodes diverse and specific sensory, motor, and reward events. J. Neurophysiol. 102, 3026–3037 (2009).
Andrade, R. & Haj-Dahmane, S. Serotonin neuron diversity in the dorsal Raphe. ACS Chem. Neurosci. 4, 22–25 (2013).
Okaty, B. W. et al. A single-cell transcriptomic and anatomic atlas of mouse dorsal Raphe Pet1 neurons. eLife 9, e55523 (2020).
Huang, K. W. et al. Molecular and anatomical organization of the dorsal Raphe nucleus. eLife 8, e46464 (2019).
Wang, H. L. et al. Dorsal Raphe dual serotonin-glutamate neurons drive reward by Establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell. Rep. 26, 1128–1142e7 (2019).
Ren, J. et al. Anatomically defined and functionally distinct dorsal Raphe serotonin sub-systems. Cell 175, 472–487e20 (2018).
Yu, X. D. et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat. Neurosci. 25, 1651–1663 (2022).
Asan, E., Steinke, M. & Lesch, K. P. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem. Cell. Biol. 139, 785–813 (2013).
Cools, R., Roberts, A. C. & Robbins, T. W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 12, 31–40 (2008).
Paul, E. D., Johnson, P. L., Shekhar, A. & Lowry, C. A. The Deakin/Graeff hypothesis: focus on serotonergic Inhibition of panic. Neurosci. Biobehavioral Reviews. 46, 379–396 (2014).
O’Hearn, E. & Molliver, M. E. Organization of raphe-cortical projections in rat: A quantitative retrograde study. Brain Res. Bull. 13, 709–726 (1984).
Muzerelle, A., Scotto-Lomassese, S., Bernard, J. F., Soiza-Reilly, M. & Gaspar, P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct. Funct. 221, 535–561 (2016).
Prouty, E. W., Chandler, D. J. & Waterhouse, B. D. Neurochemical differences between target-specific populations of rat dorsal Raphe projection neurons. Brain Res. 1675, 28–40 (2017).
Weissbourd, B. et al. Presynaptic partners of dorsal Raphe serotonergic and GABAergic neurons. Neuron 83, 645–662 (2014).
Geddes, S. D. et al. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal Raphe nucleus by cannabinoids. Proc. Natl. Acad. Sci. U S A. 113, 5429–5434 (2016).
Warden, M. R. et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432 (2012).
Fernandez, S. P. et al. Multiscale single-cell analysis reveals unique phenotypes of Raphe 5-HT neurons projecting to the forebrain. Brain Struct. Funct. 221, 4007–4025 (2016).
Wilson, M. A. & Molliver, M. E. The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience 44, 555–570 (1991).
Haj-Dahmane, S., Béïque, J. C. & Shen, R. Y. GluA2-Lacking AMPA Receptors and nitric oxide signaling gate spike-timing-dependent potentiation of glutamate synapses in the dorsal Raphe nucleus. eNeuro 4, ENEURO.0116-17.2017 (2017).
Gartside, S. E. et al. A role for nitric oxide in serotonin neurons of the midbrain Raphe nuclei. Eur. J. Neurosci. 51, 1881–1899 (2020).
Lu, Y., Simpson, K. L., Weaver, K. J. & Lin, R. C. Coexpression of serotonin and nitric oxide in the Raphe complex: cortical versus subcortical circuit. Anat. Rec. 293, 1954–1965 (2010).
Yu, Y. et al. Prenatal ethanol exposure increases susceptibility to depression- and anxiety-like behavior in adult female offspring and its underlying mechanism. Reprod. Toxicol. 96, 36–46 (2020).
Cullen, C. L., Burne, T. H. J., Lavidis, N. A. & Moritz, K. M. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS ONE. 8, e54924 (2013).
O’Connor, M. J. & Paley, B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J. Pediatr. Psychol. 31, 50–64 (2006).
Zhang, X. et al. Prenatal alcohol exposure and the risk of depression in offspring: A meta-analysis. International Journal of Clinical Practice 2022, 1–9 (2022).
Easey, K. E., Timpson, N. J. & Munafò, M. R. Association of prenatal alcohol exposure and offspring depression: A negative control analysis of maternal and partner consumption. Alcoholism Clin. Exp. Res. 44, 1132–1140 (2020).
Sliwowska, J. H., Song, H. J., Bodnar, T. & Weinberg, J. Prenatal alcohol exposure results in long-term serotonin neuron deficits in female rats: modulatory role of ovarian steroids. Alcohol Clin. Exp. Res. 38, 152–160 (2014).
Tajuddin, N. F. & Druse, M. J. Treatment of pregnant alcohol-consuming rats with Buspirone: effects on serotonin and 5‐hydroxyindoleacetic acid content in offspring. Alcoholism Clin. Exp. Res. 17, 110–114 (1993).
Waselus, M., Valentino, R. J. & Van Bockstaele, E. J. Collateralized dorsal Raphe nucleus projections: A mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 41, 266–280 (2011).
Teissier, A. et al. Activity of Raphé serotonergic neurons controls emotional behaviors. Cell. Rep. 13, 1965–1976 (2015).
Kawai, H. et al. Median Raphe serotonergic neurons projecting to the interpeduncular nucleus control preference and aversion. Nat. Commun. 13, 7708 (2022).
Grahn, R. E. et al. Activation of serotonin-immunoreactive cells in the dorsal Raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 826, 35–43 (1999).
Meloni, E. G., Reedy, C. L., Cohen, B. M. & Carlezon, W. A. Activation of Raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol. Psychiatry. 63, 832–839 (2008).
Ren, J. et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median Raphe nuclei. eLife 8, e49424 (2019).
Carlisi, C. O. & Robinson, O. J. The role of prefrontal-subcortical circuitry in negative bias in anxiety: translational, developmental and treatment perspectives. Brain Neurosci. Adv. 2, 2398212818774223 (2018).
Zhou, L. et al. Organization of functional long-range circuits controlling the activity of serotonergic neurons in the dorsal Raphe nucleus. Cell. Rep. 18, 3018–3032 (2017).
Dizon, M. L. V. Brain nitric oxide synthase levels increase in response to antenatal ethanol exposure. Alcohol Alcohol. 39, 101–105 (2004).
Hausknecht, K., Shen, Y. L., Wang, R. X., Haj-Dahmane, S. & Shen, R. Y. Prenatal ethanol exposure persistently alters endocannabinoid signaling and endocannabinoid-mediated excitatory synaptic plasticity in ventral tegmental area dopamine neurons. J. Neurosci. 37, 5798–5808 (2017).
Challis, C., Beck, S. G. & Berton, O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal Raphe bidirectionally bias socioaffective choices after social defeat. Front. Behav. Neurosci. 8 (2014).
Cohen, J. Y., Amoroso, M. W. & Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife 4, e06346 (2015).
Kumar, S. et al. Cortical control of affective networks. J. Neurosci. 33, 1116–1129 (2013).
Grizzell, J. A., Clarity, T. T., Graham, N. B., Dulka, B. N. & Cooper, M. A. Activity of a vmPFC-DRN pathway corresponds with resistance to acute social defeat stress. Front. Neural Circuits. 14, 50 (2020).
Berg, L., Eckardt, J. & Masseck, O. A. Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS ONE 14, e0210949 (2019).
Soiza-Reilly, M. et al. SSRIs target prefrontal to Raphe circuits during development modulating synaptic connectivity and emotional behavior. Mol. Psychiatry. 24, 726–745 (2019).
Metzger, M., Bueno, D. & Lima, L. B. The lateral Habenula and the serotonergic system. Pharmacol. Biochem. Behav. 162, 22–28 (2017).
Zhao, H., Zhang, B. L., Yang, S. J. & Rusak, B. The role of lateral habenula–dorsal Raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain. Res. 277, 89–98 (2015).
Winter, C., Vollmayr, B., Djodari-Irani, A., Klein, J. & Sartorius, A. Pharmacological Inhibition of the lateral Habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav. Brain. Res. 216, 463–465 (2011).
Coffey, K. R., Marx, R. E., Vo, E. K., Nair, S. G. & Neumaier, J. F. Chemogenetic Inhibition of lateral Habenula projections to the dorsal Raphe nucleus reduces passive coping and perseverative reward seeking in rats. Neuropsychopharmacology 45, 1115–1124 (2020).
Yang, L. M., Hu, B., Xia, Y. H., Zhang, B. L. & Zhao, H. Lateral Habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal Raphe nucleus. Behav. Brain. Res. 188, 84–90 (2008).
Dolzani, S. D. et al. Activation of a Habenulo-Raphe circuit is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat. eNeuro 3, ENEURO.0229-16.2016 (2016).
Lam, V. Y. Y. et al. Role of corticosterone in anxiety- and depressive-like behavior and HPA regulation following prenatal alcohol exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry. 90, 1–15 (2019).
Brocardo, P. S. et al. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: protective effects of voluntary physical exercise. Neuropharmacology 62, 1607–1618 (2012).
Choong, K. & Shen, R. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience 126, 1083–1091 (2004).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants AA026601 (SHD and RYS) AA 026421 (RYS and SHD) and T32AA007583 (to SO).
Author information
Authors and Affiliations
Contributions
S.O., performed electrophysiological experiments and wrote the initial draft. K.H., performed animal treatment. V.M., performed behavioral tests, S.H-D., and S.O., designed the experiments and performed data analyses. R-YS contributed to the experimental design and manuscript editing. SH-D wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oubraim, S., Hausknecht, K., Micov, V. et al. Chemogenetic inhibition of prefrontal cortex inputs to dorsal raphe reduces anxiety behaviors in male rat model of fetal alcohol spectrum disorder. Sci Rep 15, 14397 (2025). https://doi.org/10.1038/s41598-025-99181-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99181-8