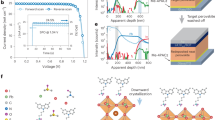
Abstract
Although perovskite solar cells (PSCs) are promising next generation photovoltaics, the production of PSCs might be hampered by complex and inefficient procedures. This Review outlines important advances in materials and methods for the cost-effective manufacturing of PSCs, including precursor synthesis, selection criteria for precursors based on chemistry, additive engineering, and deposition techniques. The goal of these technologies is not only to improve the performance and stability of PSCs, but also to significantly reduce their manufacturing costs. These advances are critical to the commercialization of PSCs, in terms of making them viable and cost-effective.
Similar content being viewed by others
Introduction
Photovoltaic technology is becoming increasingly important in the search for clean and renewable energy1,2,3. Among the various types of solar cells, PSCs are promising next-generation photovoltaics due to their superior optoelectronic properties, including high absorption coefficients, tunable bandgaps, long carrier diffusion lengths, and so on4,5,6,7,8,9,10. These outstanding features, combined with the potential for low-cost and high-throughput manufacturing, have positioned PSCs as strong contenders for future photovoltaics applications11,12,13,14,15. This growing interest is driving substantial research efforts and investments in improving their performance and scalability. However, despite the rapid progress made in the field of PSCs, challenges remain, particularly in perovskite fabrication process14,16,17,18,19,20,21. Traditional fabrication processes often might involve complex and inefficient procedures that hinder the commercialization of these devices14,17,22,23,24,25. Therefore, it is essential to make advancements in materials and methods to simplify and reduce the fabrication costs of PSCs26,27,28,29.
Among the materials and methods explored for fabricating PSCs, the preparation and deposition methods of the perovskite layer are key aspects of the research17,30,31. Through careful selection of precursors, additives and solvents, as well as optimization of deposition techniques, researchers can significantly boost the performance and stability of PSCs while reducing manufacturing costs25,32,33,34,35,36. The selection of precursors, for instance, directly affects the crystallization kinetics, film quality, and ultimately the photovoltaic performance of the PSCs31,37,38,39,40,41. Additive engineering, on the other hand, can stabilize the perovskite phase, inhibition migration, and passivate defects, minimize recombination losses, and improve the overall stability and efficiency of the PSCs32,42,43,44,45. This underscores the importance of precursors and additives in influencing perovskite film quality. A comprehensive understanding of precursor chemistry is essential for achieving high-quality films. Furthermore, deposition techniques should be carefully tailored and optimized to ensure uniform film formation, high coverage, and scalability22,25,46,47. In recent years, significant advancements have been made in materials and methods utilized for PSCs48,49,50,51,52,53,54,55,56,57,58,59. These efforts have led to the development of PSCs with improved efficiency, stability, and reproducibility60,61,62,63,64,65,66.
In this review, we aim to explore the important advancements in materials and methods for the cost-effective fabrication of PSCs based on efficient conventional ink components, including cost-effective precursor synthesis and selection criteria, additive engineering, and deposition techniques. Furthermore, we investigate the challenges faced in this field and explore potential directions for future research and development. By understanding the principles and latest advancements in PSC fabrication, we can provide practical guidelines for cost-effective fabrication procedures.
Precursor selection and additive engineering
Precursor selection18,67,68,69 and additive engineering41,53,70,71,72 are crucial steps for the fabrication of PSCs since they affect the crystallization kinetics36,73, film morphology, and quality41,65, thereby ultimately influencing the photovoltaic performance of the devices71,74,75. Therefore, careful consideration of these factors is essential for eventually achieving the cost-effective fabrication of PSCs.
Precursor development
When it comes to selecting precursors, the choice of organic or inorganic halide salts is crucial. Typically, inorganic salts such as lead iodide (PbI2) and cesium iodide (CsI), as well as organic salts like methylammonium iodide (MAI) and formamidinium iodide (FAI), are used as the precursors4,76. It is noteworthy that the purity of these precursors can significantly affect the quality of the perovskite films72,77,78,79. For instance, impurities in the precursors may result in defects in the perovskite crystal structure, reducing the efficiency and stability of the solar cell80,81. Moreover, the stoichiometric ratio of the precursors is another critical factor that must be precisely controlled82. For example, the ratio of PbI2 to MAI, FAI, or CsI determines the composition and crystallinity of the perovskite film. An imbalance in this ratio can lead to the formation of undesired phases or defects, affecting the optical and electrical properties of the film83. For example, excess PbI2 in the precursor solution can promote the formation of a PbI₂-rich phase within the perovskite film. This excess PbI₂ can act as a passivation layer, potentially improving the film’s electronic properties by reducing surface recombination. However, too excessive PbI₂ can lead to incomplete conversion to the perovskite phase, resulting in poor film quality and lower device performance. Conversely, an excess of MAI or FAI can lead to the formation of perovskite films with better coverage and reduced pinhole density. However, too much organic iodide can result in the formation of non-perovskite phases, which are detrimental to the film’s stability and performance. Consequently, precise control over the stoichiometric ratio is essential for achieving high-quality perovskite films84. Therefore, exploring ways to eliminate precursor impurities and precise control over the stoichiometric ratio could improve PSCs performance82. An effective strategy to address these inherent factors involves the purification of pre-synthesized perovskite microcrystal powders via crystallization. The purified crystals typically achieve a purity level exceeding 99.99%52,85. This process entails redissolving the pre-synthesized microcrystal powders to function as precursors in the subsequent fabrication of perovskite films, thereby ensuring the production of a higher-quality perovskite film. (Fig. 1).
a Synthesis of δ-FAPbI3 powder at room temperature. b Current density-voltage (J–V) curves of devices based on pre-synthesized FAPbI3 powder and the conventional precursor mixture. c (Upper panel) Front and (lower panel) bottom photos of 1-mL vials with different solvents containing 1 mmol of δ-FAPbI3 at room temperature. d (Upper panel) SEM images and (lower panel) XRD pattern of the aqueous synthesized δ-FAPbI3 perovskite microcrystals (ASPM). e Digital image of kilogram-scale high-yield δ-FAPbI3 powder synthesized at pH lower than 1.9. f J–V curves of the conventional precursor mixture-based (control) and the ASPM precursor-based PSCs. a, b adapted with permission from ref. 85. Copyright 2019 American Chemical Society. c–f adapted with permission from ref. 52. Copyright 2024 The American Association for the Advancement of Science.).
For instance, we reported the development of efficient (>21%) and reproducible PSCs using ambient-temperature stable δ-phase FAPbI3 powder synthesized from low-grade PbI2 (purity <99%) and FAI (Fig. 1a, b)85. This innovation overcame challenges associated with non-stoichiometry and impurities in conventional precursor mixtures, resulting in consistent photovoltaic performance. Subsequently, the use of pre-synthesized crystalline perovskite was reported, which enabled the removal of residual compositions and intermediate chemical phases to enhance stability86. This approach significantly improved the operational stability of the PSCs, achieving over 2000 hours of lifetime, demonstrating the potential of pre-synthesized powder engineering in advancing efficient and stable PSCs. Further, the liquid phase reaction and antisolvent crystallization method were reported to reduce impurities87, which led to a high power conversion efficiency (PCE) of 25.1% and excellent stability. For instance, within an N2 atmosphere and at 85 °C, the unencapsulated device was continuously illuminated with one sun (100 mW/cm2) for 1000 h. Impressively, it maintained 97.22% of its initial PCE, demonstrating the durability of the PSCs under thermal stress. Currently, the synthesis of high-quality α-phase single crystals using organic solvents such as acetonitrile (ACN) and 2-methoxyethanol (2-ME) at high temperatures has been validated and adopted by many laboratories. However, α-phase single crystals also face challenges, such as the requirement for high-temperature preparation and instability at room temperature88. Most recently, an aqueous synthesis of highly pure δ-phase formamidinium lead iodide (FAPbI3) and other compositions was reported52, which enabled kilogram-scale precursor (Fig. 1c–e). Since α-phase FAPbI3 needs a high temperature of over 150 °C, aqueous synthesis of δ-phase FAPbI3 at room temperature is a cost-effective way for mass production of precursor material. As shown in Fig. 1f, the synthesized δ-phase FAPbI3 microcrystals achieved a remarkable purity level of up to 99.996%, resulting in a record PCE of 25.6% (25.3% certified). The significant reduction of Ca2+ ions, the major impurity, decreased carrier trap states and greatly improved device performance and stability. This latest development not only addresses the challenge of defects in the perovskite layer but also offers a promising route for cost-effective PSC fabrication, paving the way for further advancements in the field.
Additive engineering
Additive engineering is another effective method for enhancing the properties of perovskite films41,53,70,71,72, which may significantly affect crystallization kinetics, film quality, and the stability of the perovskite material34,89. A wide range of additives, including solvents, functional additives, dopants and interface engineering, have been explored for this purpose32,90.
Solvents
Solvents are crucial in the deposition of perovskite films, as their selection profoundly influences the wettability of the substrate, precursor dissolution, and evaporation rate during the process91,92. However, in this review, given that DMSO and other efficient polar solvents commonly used have been widely accepted as solvents for preparing high-performance devices, the principle of how they enhance device performance has also been extensively studied. Meanwhile, the conventional fabrication process often involves the utilization of a substantial number of toxic solvents, which not only contaminate the environment but also endanger human health, ultimately triggering a considerable escalation in hidden costs93,94. Hence, green solvents exhibiting low toxicity or non-toxicity might possess a more pronounced potential advantage in cost-effective fabrication of PSCs93,94. We will mainly focus on analyzing their toxicity and environmental friendliness. Fig. 2a provides the life cycle assessment of solvents for PSCs that aids in understanding the entire process and potential environmental impacts of perovskite production93. This allows for a systematic and standardized evaluation of solvents, facilitating the large-scale, environmentally friendly, and cost-effective fabrication of PSCs. For example, the estimation for solvent required for a blade-coated perovskite film is around 0.5 mL/m2. Therefore, Fig. 2b estimates that for a module prepared by blade coating with a PCE of 15%, an astonishing volume of 3500 L of solvent would be required to achieve a power generation capacity of 1 GW. However, among the various polar solvents commonly used, such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), γ-butyrolactone, N-methyl-2-pyrrolidone, tetrahydrofuran, dimethylacetamide, only DMSO was evaluated as a non-toxic green solvent by measuring disability-adjusted life years (Fig. 2c)93,94. Besides, the annual production of DMSO is ~100,000 tons. Industrial-grade DMSO costs approximately 1 USD per kilogram. For 3500 L (3.85 tons), the cost would be ~3850 USD. When scaled to the overall cost of producing 1 GW of solar capacity, this represents a minor fraction of the total production costs. Therefore, eco-friendly and low-cost fabrication of PSCs should primarily focus on DMSO as the main solvent, or alternatively, efforts should be made to develop more green solvents for the preparation of high-quality perovskite thin films95,96,97,98. For instance, a method was reported for synthesizing stable α-FAPbI3 that is insensitive to temperature and humidity variations and compatible with large-scale fabrication. This method involves utilizing a vertically aligned PbI2 thin film grown from an ionic liquid green solvent, methylammonium formate (shown in Fig. 2d)97. This kind of PbI2 film facilitates the rapid infiltration and full reaction of FAI through its nanoscale ionic channels to form α-FAPbI3. Consequently, a PSC achieving a PCE of 24.1% was successfully fabricated and exhibited excellent stability under thermal stress. For example, the unencapsulated devices retain 90% of their initial PCEs after 500 h at 85 °C. Based on such ionic liquids as green solvents, the feasibility of fully screen-printing PSC devices was further successfully explored in ambient air. The resulting photovoltaic cells exhibited PCEs of 15.0% and 11.8% for 0.05 cm² and 16.37 cm² (small module), respectively. In addition, the screen-printed PSCs also exhibit excellent operational stability. Specifically, 96.75% of their initial PCE was retained for over 300 h at the maximum power point (MPP)96. Furthermore, a method for fabricating homogenous and dense α-FAPbI3 films without using antisolvent was reported using ethanol-based perovskite precursor solutions. By combining the green solvent DMSO with an alkylammonium chloride (RNH3Cl) in an another green solvent ethanol, an impressive PCE of 25.1% was achieved95. Subsequently, environmentally benign biomass-derived green solvents, specifically γ-valerolactone (GVL) and n-butyl acetate, were reported98. Compared to traditional reagents (DMF and DMSO) that might cause instability of FA+ in the precursor, GVL not only exhibits a strong interaction with FA+, stabilizing it and promoting the formation of high-valence [PbIx]2–x complexes (Fig. 2e). As a result, the FAPbI3 perovskite ink’s stability was notably improved due to the utilization of GVL, lasting up to a year. Moreover, by employing this ink, an impressive PCE of 25.1% was achieved. The scalability of this method was further verified by the successful fabrication of a mini-module (12.25 cm2) with a certified PCE of 20.2%. The aforementioned solvents can serve as non-toxic and biodegradable substitutes for conventional solvents93. Green solvents also strengthen workplace safety, streamline the manufacturing process, and reduce fabrication costs94.
a The life cycle assessment (LCA) of solvents for PSCs. b The schematic illustration of large-scale perovskite films made via blade coating, requiring solvents: 3500 L per 1 GW of perovskite solar module with PCE of 15%. c The toxicity of solvents assessed by measuring disability-adjusted life years (DALYs). d PbI2@MAFa and PbI2@DMF:DMSO solutions showing different colloidal structure. e The benefits of utilizing a γ-valerolactone (GVL) green solvent. (a adapted with permission from ref. 93. Copyright 2020 Springer Nature. b, c adapted with permission from ref. 94. Copyright 2020 Springer Nature. d adapted with permission from ref. 97., Copyright 2021 The American Association for the Advancement of Science. e adapted with permission from ref. 98, Copyright 2023 Springer Nature).
Functional additives
Functional additives, on the other hand, can be used to modify the properties of the perovskite crystals99. By adding additives, the orientation and homogeneity of the perovskite films can be controlled40,65. The commonly used additives are required in very small quantities (their cost is almost negligible) and can significantly enhance the efficiency and stability of the PSCs41,65,100. For instance, our group showed that additives being capable of hydrogen bonding with iodide species in precursor solution led to the creation of polyhedral single crystals with distinct facets (Fig. 3a)65. Our findings indicate that facets with different atomic densities exert a significant influence on the chemical and electronic environments of the perovskite surface, consequently affecting the performance of the final device. For instance, the (100) and (111) facets exhibited comparable carrier mobility and photocurrent, both of which were notably superior to the (110) facet. By increasing the proportion of both (100) and (111) facets among all facets, the corresponding PSC demonstrated a quasi-steady-state PCE as high as 24.6%. Furthermore, the moisture stability of perovskite crystals was discovered to strongly depend on the perovskite facets41. Specifically, the (100) facet is sensitive to moisture, while the (111) facet is tolerant to moisture. Thus, the (100) facet is more susceptible to degradation by moisture (Fig. 3b). Leveraging these crucial insights, we successfully fabricated a (111)-dominant perovskite film using cyclohexyl amine additive by considering the hydrogen bonding at the (111) plane. The PSC based on the (111)-dominant film was quite stable under high humidity conditions.
a (left panel) schematic model depicting different facets of a perovskite cubic crystal and (right panel) the corresponding SEM image displaying a defined (100) and (111) facets. b AFM images of (left panel) the as-prepared sample and (right panel) the sample exposed to 40% RH for 200 h. c Schematic illustration of the inhomogeneous cation distribution across the film. d The cross-sectional transmission electron microscopy (TEM) of perovskite layer and the high-resolution TEM (HRTEM) images captured from the corresponding boxed areas. The third row represents the calculated intensity variations. e Schematic representation of the utilization of additional anion-π interactions as an AX retardant during perovskite fabrication processing. f Schematic representation of intermolecular bonding within the ink. (a adapted with permission from ref. 65, Copyright 2022 Elsevier Inc. b adapted with permission from ref. 41, Copyright 2023 The American Association for the Advancement of Science. c, d adapted with permission from ref. 64, Copyright 2023 Springer Nature. e adapted with permission from ref. 101, Copyright 2023 Springer Nature. f adapted with permission from ref. 104, Copyright 2024 The American Association for the Advancement of Science.).
In terms of the homogeneity of perovskite composition across the layer, FA-rich composition was formed on the perovskite film surface, while Cs-rich composition was dominant at the bottom of the film (Fig. 3c)64. This inhomogeneity is one of the origins of instability and lower efficiency in PSCs. 1-(phenylsulfonyl)pyrrole was developed as an additive to homogenize cation distribution within the perovskite films (Fig. 3d), where the difference in crystallization kinetics and phase transition kinetics between FA cation and Cs cation was overcome. This approach exhibited a certified steady-state PCE of 25.2% along with improved durability and stability. Threading the regulation of crystal growth from the precursor solution, the formation of perovskites relies on the interaction between AX and BX2 components. The traditional methods for regulating film growth have focused solely on the BX2 component using electron donors (D), such as DMSO, for BX2 retardant. An interesting approach was reported to modulate reaction kinetics through anion-π interactions between AX and hexafluorobenzene (HFB), that is, via electron acceptor (A), such as HFB, for AX retardant (Fig. 3e)101. The anion-π bond strength between HFB and I− was estimated to be 51 kJ/mol, that is similar to the interaction energy between DMSO and PbI2. By utilizing the dual-site regulation, no unwanted intermediates were formed, leading to FAPbI3 films with fewer defects and high phase purity. Ultimately, PSCs with active areas of 0.08 cm² showed PCE of 26.1% (25.8% certified) along with improved stability, demonstrating 94% of their initial PCE under light soaking for 1258 h. This method broadens the scope of chemical interactions in perovskite precursors by exploring the AX retardants as well as PbX2 retardants for realizing phase pure perovskite films. In addition, defects arising during the ageing and crystallization of precursor solutions present significant obstacles to the reproducibility and efficiency of solar cells. By inhibiting the oxidation of I⁻ and reducing newly generated I back to I⁻, fluoro-N,N,N″,N″-tetramethylformamidinium hexafluorophosphate (F-(CH3)4CN2PF6, abbreviated as TFFH) stabilizes the precursor solution and passivates under-coordinated Pb²⁺ through interaction with the Pb-I framework. The optimized PSCs achieved a PCE of 42.4% under an illumination of 1002 lux, making them the most efficient among all indoor photovoltaic cells102. During thermal annealing of 3D perovskites, the encapsulated DMSO leaves the bulk film, causing dissolution and recrystallization. This process, balanced by DMSO evaporation, leads to crystal volume collapse and voids at grain boundaries. The use of amidino-based Dion-Jacobson 2-dimensional (2D) perovskite assists in the nucleation and growth of 3D perovskite, reducing voids and bulk defects in the film. This results in efficient charge transport and significantly improved stability. The flexible solar cells retained 92% efficiency after 3000 bending cycles103.
Furthermore, additive engineering can be extended to the Sn-Pb alloyed perovskite, where the utilization of the narrow bandgap Sn-Pb perovskite can replace crystalline Si to make a perovskite-perovskite tandem solar cell. However, unlike the single lead ion, nonuniform nucleation and rapid crystallization are often observed in this binary system, which impedes film quality and overall device performance. By introducing aminoacetamide hydrochloride (AAH) into the precursor (Fig. 3f), the processing window was significantly increased from 10 s to 100 s, thereby facilitating the easier fabrication of high-quality perovskite films without pinholes104. AAH was found to be primarily distributed at the bottom interface of the final perovskite film, serving the additional function of passivating the buried interface. This method enables a large-area perovskite coating via the blade-coating process, where a PCE of 24.5% was demonstrated from a 20-cm2-sized module. The stability test showed that 80% of initial PCE was maintained after 656-hour operation at MPP. This level of stability is remarkable for devices containing a mixed Sn-Pb perovskite layer. Hence, functional additives play a pivotal role in boosting the efficiency and stability of solar cells, thereby enabling the simplified and cost-effective production of PSCs.
It should be emphasized that the additive engineering discussed above can be extended not only to the preparation of Sn-Pb perovskites but also to other types of perovskites in general, such as Pb-free, wide-bandgap, and 2-dimensional (2D) perovskites. However, due to space constraints, we do not go into details again in this review.
Dopants
Dopants are the other type of additive that can be used to modify the electronic properties of perovskite films89. By incorporating dopants into the perovskite, α-phase perovskite can be stabilized36, strain can be regulated105, ion migration is able to be mitigated106,107, defects can be passivated107,108, and thereby the overall stability of the materials and PSCs can be enhanced89,106,108. For instance, the black α-phase of FAPbI3 is often suffers from its intrinsic instability at room temperature due to transition into the inactive non-perovskite yellow δ-phase. Consequently, stabilization of the α-phase at ambient conditions is critical and can be possible by doping. A method utilizing vapour-assisted doping with formamidinium thiocyanate or methylammonium thiocyanate containing SCN− ions has been reported to effectively facilitate the conversion of the yellow phase to the black phase (Fig. 4a)36. The presence of SCN− ions in the perovskite is helpful to maintain the long-term stability of the generated black phase perovskite. Ultimately, PSCs fabricated based on the aforementioned doping method led to a PCE exceeding 23% along with high stability, maintaining 94% of the initial PCE after operating at the MPP for 500 h. Despite these advancements, achieving long-term operational stability under real conditions remains a formidable challenge. Comparative studies on the effectiveness of different stabilization strategies are essential to identify the most promising approaches.
a The δ-to-α phase transition via MASCN or FASCN vapor treatment. b A schematic illustration of strain regulated by FA, MDA, and Cs cations. c Theoretical models for the migration pathway of iodide ions in perovskite lattices depending on different cations doping (Na+, Ca2+, Nd3+). d A comparison of the interaction intensities between different anions (HCOO−, Cl−, Br−, I−, BF4−) and the iodine vacancy. e Schematic representation of the interaction between perovskite and nitrile in polyacrylonitrile. (a adapted with permission from ref. 36, Copyright 2020 The American Association for the Advancement of Science. b adapted with permission from ref. 105, Copyright 2020 The American Association for the Advancement of Science. c adapted with permission from ref. 106, Copyright 2022 Springer Nature. d adapted with permission from ref. 108, Copyright 2021 Springer Nature. e adapted with permission from ref. 107, Copyright 2021 WILEY–VCH).
Additionally, stabilizing the α-phase FAPbI3 involves the incorporation of doped ions such as MA+, Cs+, and Br− into the perovskite lattice. However, this can increase lattice microstrain and bandgap as well. To mitigate these effects, introducing 3.8% methylenediammonium dichloride (MDACl2) was introduced in the FA-based perovskite precursor, where the larger methylenediammonium (MDA2+) can enter the lattice, compensating the Cs+-induced microstrains (Fig. 4b). This method yields perovskites with near-intrinsic bandgaps109 and reduced strain105, enhancing efficiency and thermal stability. Notably, the device maintains a PCE above 90% after heat treatment at 150 °C for 20 h, with a certified PCE of 24.4%.
The aforementioned large cation doping might be limited to use in general due to unwanted low dimensional phase formation. Consequently, it is crucial to further investigate efficient interstitial doping techniques that utilize low dopant concentrations to avoid microstrain. For instance, our study on doping role of cations in suppressing iodide migration shows that trivalent neodymium cations (Nd3+) are more effective than monovalent Na+ at 0.45% and bivalent dopants Ca2+ at 0.25% (Fig. 4c)106. Importantly, the optimal doping concentration of Nd3+ was just 0.08%, which was sufficient to minimize microstrain and maintain perovskite phase stability while suppressing ion migration.
To further maximize the performance of PSCs, anion engineering was proposed. Formate (HCOO−) as a pseudo-halide anion was investigated108, where it was found that the presence of formate suppresses common anion vacancy defects at the grain boundaries and surfaces of the perovskite films (Fig. 4d). The PSCs employing the perovskite films with HCOO− achieved a certified PCE of 25.2%. In addition, polymer materials with pseudo-halogen functional groups were employed for passivating anion defects at the grain boundaries of perovskites107. Compared to the commonly used passivating agents with a carbonyl group (–C=O), the nitrile group (–C≡N) in the polymer (polyacrylonitrile) was found to be stronger in interaction with uncoordinated lead ions (Fig. 4e). This interaction significantly modulated the I/Pb ratio on the perovskite surface, effectively passivating defects and suppressing non-radiative recombination. As a result, in the optimized PSCs the open-circuit voltage (Voc) was increased substantially from 1.12 V to 1.23 V, resulting in one of the highest Voc among the studied similar compositions of halide perovskite.
Interface regulation
In addition to these additives, interface regulation has been considered to further enhance the performance of PSCs17,44,110 In general, the design of the interface is complex. For example, the choice between organic and inorganic charge transport layers (CTLs), as well as the overall interface design, heavily depends on the specific device architecture and intended application. Given the extensive research and numerous reviews on efficient CTLs tailored to various device structures, perovskite compositions, and deposition methods, we will not repeat these discussions here. Instead, this review focuses on the general and effective interface modification layers directly related to the perovskite layer. It was reported that these interface layers formed between the perovskite film and the charge transport layers improved substantially photovoltaic performance and were found to play an important role in protecting the PSCs from degradation111,112,113,114,115. A strategy of precisely adjusting the number of inorganic layer (n) at the interlayer was reported, where the temperature effect of the interlayer formed by post-treatment with oleylammonium iodide was investigated (Fig. 5a)112. Specifically, high-temperature (100 °C) post-treatment mainly leads to the formation of a 2D perovskite passivation layer with n = 1, while a quasi 2D perovskite passivation layer with n = 2 was dominant after overnight storage at room temperature. It was shown that the energy level of the quasi 2D with n = 2 was more compatible with that of C60, which is beneficial for charge transfer and reduces charge accumulation. A PCE exceeding 24% and improved stability was achieved by manipulating the interlayer structure. A selective reaction at interface was proposed by using 3-(aminomethyl) pyridine (3-APy), where 3-APy was selectively reacted with surface FA+, resulting in uniformity of the perovskite surface potential and inhibition of non-radiative recombination (Fig. 5b)113. Eventually, a p-i-n PSC with a PCE exceeding 25% was obtained.
a Schematic diagram and cross-sectional HRTEM image of a 2D perovskite passivation layer. b Schematic representation of the chemical reaction between FA+ and 3-APy. Kelvin probe force microscopy (KPFM) image and height/potential profiles showing homogeneous surface potential generated by the 3-APy surface treatment. c Schematic representation of the facet-dependent passivation depending on passivation materials. d Donor number (DN) and dialectic constant of the various non-polar, polar and polar aprotic solvents for 2D deposition. DMF dimethylformamide, DMSO dimethyl sulfoxide, CHF chloroform, CBZ chlorobenzene, MeCN acetonitrile, EA ethyl acetate, IPA isopropyl alcohol, NMP N-methyl pyrrolidone, TMS tetramethyl sulfone, PC propylene carbonate, EC ethylene carbonate, NME nitromethane, DMAc dimethylacetamide, GBL γ-butyrolactone, THF tetrahydrofuran, DMPU N,N′-Dimethylpropyleneurea. e Schematic illustration of SAM between the FAPbI3 layer and the SnO2 layer, showing silane-based SAM features with a different terminal functional groups (-C3–Cl and -C3≡N). f MPP tracking of the encapsulated PSCs without SAM (ref.) and with -C3–Cl and -C3≡N SAMs. (a adapted with permission from ref. 112, Copyright 2022 The American Association for the Advancement of Science. b adapted with permission from ref. 113, Copyright 2022 Springer Nature. c adapted with permission from ref. 45, Copyright 2023 American Chemical Society. d adapted with permission from ref. 115, Copyright 2022 The American Association for the Advancement of Science. e, f adapted with permission from ref. 116, Copyright 2024 Elsevier Inc.).
Understanding the interaction between the passivation molecules and the different perovskite crystal facets is important to achieve uniform passivation layer on the whole perovskite surface. We studied systematically dependence of crystal facets on passivating materials and verifed a facet-dependent passivation principle45. Specifically, after computational and experimental verification, as shown in Fig. 5c, octylammonium iodide (OAI) tends to interact with only the (100) surface, while phenethylammonium iodide can interact with both (100) and (111) facets but with different adsorption behavior. It was found that the (100) facet is highly sensitive to adsorption of amine, acetic acid and ammonium ion, while the (111) facet is relatively less sensitive to adsorption but can interact with phenyl with Pi (π) electrons and organic anions. 2-amidinopyridine hydroiodide with enhanced Pi (π) electrons and ammonium cations was designed to form a uniform interlayer by simultaneous interactions with both (100) and (111) facets, which eventually led to a PCE of 25.1% along with good light-soaking stability.
Thickness control of the interlayer is hard to control with the traditional precursor solution and spin-coating technique. A method of growing uniform 2D layers on the 3D perovskite layer was reported by adjusting donor number and dielectric constant of solvents for the 2D layer deposition115. As shown in Fig. 5d, polar aprotic solvents with dielectric constant higher than 30 and donor number ranging ~10–20 kcal/mol were found to be appropriate because they dissolve 2D materials but not 3D underneath layer. Among the studied solvents, acetonitrile (MeCN) was found to be the most suitable solvent to make uniform 2D layer on the 3D perovskite film.
For the preparation of multicomponent mixed perovskite films, out-of-plane phase separation and/or uneven cation distribution are often observed, which could result in a weak adhesion interface of the perovskite layer with charge transport layers64,116. A notable study employed an I-terminated silane self-assembled monolayers (SAMs) between the SnO2 and perovskite layers117. This approach was shown to enhance the mechanical adhesion and stability of the interface, thereby improving overall device stability. Based on this, sodium 2-cyanoacetate (SZC) is used as a molecular bridge to establish strong bonds between the SnO₂ transport layer and the perovskite, reducing oxygen vacancy defects and uncoordinated Pb defects at the interface. This changes interfacial energy level alignment and promotes efficient carrier transport118. Additionally, the 2PACz (2-(9H-carbazol-9-yl)ethyl)phosphonic acid) molecule is a popular choice for SAMs in inverted PSCs because of its ability to improve the electronic properties of the interface and enhance device stability119. However, its mechanical adhesion properties are not as robust as those of the iodine-terminated carbazole. Therefore, the mechanical adhesion at the interface of the inverted device was further improved using I-terminated carbazole-based SAM120. This approach effectively inhibited the generation of pores and cracks during the operation of PSCs, leading to the PCE remaining at 96% of the initial PCE after MPP tracking for 1000 h. This demonstrates the close correlation between interfacial adhesion and operational stability. However, as the adhesion of the heterojunction increases, the weak adhesion homojunction interface within the perovskite layer becomes a primary factor limiting further improvements in device stability. The weak adhesion interface was verified by mechanical peeling experiments, which was related to the buried interface of perovskite near the SnO2 layer116. This buried interface was composed of homojunction rendering charges being accumulated due to built-in electric field, which promoted ion migration and thereby led to instability of PSCs117,121. To address this key issue, we introduced silane SAMs with different functional groups with strong chemical coordination (such as -C–Cl and -C≡N) onto the surface of SnO2 layers (Fig. 5e)116. By taking advantage of dipole moments of the SAM layer, the work functions on both sides of the weak adhesion interface could be modulated. Specifically, the SAM layer shifted the work function of the top surface upward from 4.23 eV to 4.04 eV, while a downward shift in the work function of the bottom surface from 3.88 eV to 4.04 eV was attained. Due to the regulation of surface work functions on both sides of the weak adhesion interface, the built-in electric field on the weak adhesion homojunction interface was minimized, which eventually suppressed ion migration. Stability was substantially improved by introducing -C≡N functionalized silane SAM, demonstrating 97% of its initial PCE after 1000 h at MPP (see Fig. 5f). In summary, precursor selection and additive engineering play a crucial role in simplifying and reducing the cost of manufacturing PSCs. Future research in this field should focus on further optimizing and exploring these materials and methods to enhance the scalability of PSCs.
Deposition techniques for large-area fabrication
Advances in the performance and durability of PSCs have triggered their large-scale production67,92,116,122. To achieve this, cost-effective and high-speed roll-to-roll (R2R) fabrication methods are regarded as key deposition technique22,25,46. These methods require accurate control over the formation of the intermediate phase and their conversion into the perovskite structure. Below, we review several deposition techniques, highlighting their advantages, challenges, and the key factors limiting the PCE of devices produced by these methods. For example, R2R gravure printing is highly scalable and flexible but struggles with uniformity and solvent control, limiting PCE due to film defects and crystallization issues. Blade coating is simple and cost-effective, yet maintaining uniform film quality and environmental sensitivity can hinder performance. Slot-die coating offers high throughput and precision but requires costly equipment and precise solution viscosity management, with defect formation and drying dynamics as key PCE limits. Spray coating is versatile and scalable but faces challenges in achieving uniform coverage and environmental sensitivity, affecting PCE through particle size and film defects. Additionally, the choice of solvents presents a certain level of complexity, as different large-area deposition methods typically require distinct solvent combinations. Therefore, the introduction of various deposition techniques is usually accompanied by the optimization of solvent selection. Consequently, when discussing large-area deposition processes for accurate control over the formation of intermediate phases and their conversion into the perovskite structure in this review, we also briefly address the corresponding solvent combinations.
One of the challenges encountered includes a short processing window and uneven crystallization. To overcome these obstacles, a green antisolvent combination consisting of tert-butyl alcohol and ethyl acetate (EA) was introduced into R2R-compatible gravure printing with specific printing patterns (Fig. 6a–c)122. This solvent blend was found to improve the perovskite processing window, facilitating the deposition of uniform and high-crystalline films without PbI2 traces. As a result, a flexible gravure-printed PSC with a PCE of 19.1% was fabricated. However, when compared to the 23.5% PCE of spin-coated PSCs using the same ink, it becomes apparent that the transition from laboratory-scale deposition to large-scale R2R leads to a significant decrease in photovoltaic performance. The challenge lies in maintaining high efficiency during the scale-up of production. Therefore, exploring more scalable perovskite preparation methods and deposition techniques is required.
a Illustration of the R2R technique for the production of flexible PSCs. b Photographs of the R2R-processed PSCs. c Patterns of perovskite printed on PET substrate. d The blade-coating process. e The slot-die coating process. f The ultrasonic-assisted spray-coating process. (a–c adapted with permission from ref. 122, Copyright 2020 Springer Nature. d, e adapted with permission from ref. 123, Copyright 2018 American Chemical Society. f adapted with permission from ref. 124, Copyright 2022 American Chemical Society.).
It was reported that highly crystalline, highly oriented, and scalable smooth thin films were fabricated under ambient conditions at room temperature by utilizing a high-concentration precursor solution and low-boiling point solvents such as a 10% w/w mixture solvent of methylamine in ACN123. This mixture allows the high-concentration solution to rapidly transition into a supersaturated state, enabling the rapid preparation of a high-quality MAPbI3. High-speed in situ XRD monitoring demonstrates that the precursor film can rapidly crystallize within one second at room temperature, achieving a relative crystallinity of over 98%. The method is compatible with various deposition techniques, including blade coating (PCE of 19.6%) (Fig. 6d) and slot-die coating (PCE of 17.3%) (Fig. 6e). Spray-coating is also regarded as an alternative deposition technology, which can be applied to various substrate materials with micro-scale roughness. For instance, CsPbI2Br was prepared by ultrasonic-assisted spray coating (Fig. 6f)124, where other components or layers were also tried to deposit via spray coating. Recently, a detachable, reassemblable tandem perovskite/silicon solar cell has been reported. This modular cell incorporates a spray-coated single-walled carbon nanotube film between the top perovskite cell and the bottom silicon cell. This advancement not only simplifies the assembly, disassembly, and maintenance of the tandem solar cell but also effectively addresses the issue of resource wastage caused by mismatched lifetimes of the top and bottom cells125.
Recently, the entirely R2R fabricated flexible perovskite solar module at ambient conditions was reported126. Through the advancement of scalable deposition methods tailored to each functional layer, such as reverse gravure, slot-die coating, and screen printing, the substitution of vacuum-deposited metal electrodes by perovskite-friendly carbon inks, and the exploitation of a high-throughput R2R preparation platform, a serially connected module (~50 cm2) demonstrated a PCE of 11.0%. Based on the proposed fabrication method, a cost of 0.7 USD/watt peak was predicted for a production rate of 1,000,000 m² per year. It is anticipated that with further optimization of the preparation process and device structure in the future, the cost could be reduced to below 0.50 USD/W. Despite the aforementioned advancements, the scalability of manufacturing techniques for producing high-performance PSCs in laboratory settings remains an issue. Future research should focus on developing robust materials that can withstand environmental stress, optimizing scalable manufacturing processes, and exploring new materials and device architectures that offer intrinsic stability.
Outlook
Despite significant progress in materials and methods for the fabrication of PSCs, its large-scale commercialization is still challenging. For example, the commercialization of PSCs faces several challenges in formulation, deposition, post-processing, performance, and cost-effectiveness. Selecting and optimizing materials is crucial, but maintaining purity and stoichiometry at scale is challenging. Various deposition techniques must scale up while maintaining film quality. Post-processing steps are vital for performance and stability but need to be cost-effective. Achieving PCE during large-scale production and ensuring long-term stability is difficult. The cost-effectiveness relies on low-cost materials and scalable techniques. Synergies between these aspects are essential for successful commercialization, with innovations needed to balance performance and cost. Additionally, since reproducibility is critical for commercialization, a whole series of fabrication processes (additives in precursor solution, pre-and post-treatment methods, and materials) should be well established for mass production. In addition, controlling environmental parameters is also important because the deposition of the perovskite layer is sensitive to environmental conditions such as temperature and humidity127,128. Therefore, standardized processing protocols are crucial. Moreover, long-term stability remains a key issue for the commercial applications of PSCs116,129. Hence, the development of new materials, methods, and advanced encapsulation technologies is essential. Meanwhile, there are concerns about the toxicity of lead-based materials in PSCs, and it is equally important to actively seek non-toxic alternative materials or develop methods to immobilize lead leakage130,131,132,133,134. Nevertheless, the application prospects of PSCs remain bright. By continuously developing new materials and methods to further reduce production costs, PSCs are expected to occupy a more prominent position in the field of renewable energy. As the above challenges are gradually overcome, PSCs will make important contributions to sustainable energy solutions.
Due to the important advancements in materials and methods, the performance and stability of PSCs have been boosted while minimizing costs. These advancements overcome barriers to cost-effective fabrication, bringing PSCs closer to commercial use. Low-cost precursors and materials for not only the perovskite layer but also charge transport layers and electrode materials should be developed to further lower the fabrication cost. Low-cost and reproducible coating methods are important for upscaling PSCs. Finally, long-term stability under operational conditions is pivotal for commercialization. Thus, long-term stability studies on the large-area cells and modules are required. Development of highly effective and low-cost encapsulation materials and methods should be intensively carried out for the perovskite modules.
References
Ashraf, M. et al. Recent trends in sustainable solar energy conversion technologies: mechanisms, prospects, and challenges. Energy Fuels 37, 6283–6301 (2023).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Haegel, N. M. et al. Terawatt-scale photovoltaics: transform global energy. Science 364, 836–838 (2019).
Kim, H.-S. et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012).
Li, H., Shen, N., Chen, S., Guo, F. & Xu, B. Recent progress on synthesis, intrinsic properties and optoelectronic applications of perovskite single crystals. Adv. Funct. Mater. 33, 2214339 (2023).
Zhao, B. et al. High-efficiency perovskite-polymer bulk heterostructure light-emitting diodes. Nat. Photonics 12, 783–789 (2018).
Long, G. et al. Chiral-perovskite optoelectronics. Nat. Rev. Mater. 5, 423–439 (2020).
Chen, B. et al. Enhanced optical path and electron diffusion length enable high-efficiency perovskite tandems. Nat. Commun. 11, 1257 (2020).
Yang, Z. et al. Enhancing electron diffusion length in narrow-bandgap perovskites for efficient monolithic perovskite tandem solar cells. Nat. Commun. 10, 4498 (2019).
Chen, Z. et al. Thin single crystal perovskite solar cells to harvest below-bandgap light absorption. Nat. Commun. 8, 1890 (2017).
Li, T., He, F., Liang, J. & Qi, Y. Functional layers in efficient and stable inverted tin-based perovskite solar cells. Joule 7, 1966–1991 (2023).
Park, N.-G., Huang, J. & Qi, Y. Themed issue on perovskite solar cells: research on metal halide perovskite solar cells towards deeper understanding, upscalable fabrication, long-term stability and Pb-free alternatives. Sustain. Energy Fuels 2, 2378–2380 (2018).
Almora, O. et al. Device performance of emerging photovoltaic materials (version 1). Adv. Energy Mater. 11, 2002774 (2021).
Park, N.-G., Grätzel, M., Miyasaka, T., Zhu, K. & Emery, K. Towards stable and commercially available perovskite solar cells. Nat. Energy 1, 1–8 (2016).
Park, N.-G. Halide perovskite photovoltaics: history, progress, and perspectives. MRS Bull. 43, 527–533 (2018).
Niu, B. et al. Mitigating the lead leakage of high-performance perovskite solar cells via in situ polymerized networks. ACS Energy Lett. 6, 3443–3449 (2021).
Kim, J. Y., Lee, J.-W., Jung, H. S., Shin, H. & Park, N.-G. High-efficiency perovskite solar cells. Chem. Rev. 120, 7867–7918 (2020).
Park, N. G. Research direction toward scalable, stable, and high efficiency perovskite solar cells. Adv. Energy Mater. 10, 1903106 (2020).
Seok, S. I., Grätzel, M. & Park, N. G. Methodologies toward highly efficient perovskite solar cells. Small 14, 1704177 (2018).
Lee, J. W., Lee, D. K., Jeong, D. N. & Park, N. G. Control of crystal growth toward scalable fabrication of perovskite solar cells. Adv. Funct. Mater. 29, 1807047 (2019).
Rong, Y. et al. Challenges for commercializing perovskite solar cells. Science 361, eaat8235 (2018).
Li, D. et al. A review on scaling up perovskite solar cells. Adv. Funct. Mater. 31, 2008621 (2021).
Zhang, C. et al. Efficient stable graphene-based perovskite solar cells with high flexibility in device assembling via modular architecture design. Energy Environ. Sci. 12, 3585–3594 (2019).
Lee, J. W. & Park, N. G. Chemical approaches for stabilizing perovskite solar cells. Adv. Energy Mater. 10, 1903249 (2020).
Park, N.-G. & Zhu, K. Scalable fabrication and coating methods for perovskite solar cells and solar modules. Nat. Rev. Mater. 5, 333–350 (2020).
Alberi, K. et al. The 2019 materials by design roadmap. J. Phys. D: Appl. Phys. 52, 013001 (2018).
Kim, H.-S. et al. Sustainable green process for environmentally viable perovskite solar cells. ACS Energy Lett. 7, 1154–1177 (2022).
Zhang, C. et al. Ti1-graphene single-atom material for improved energy level alignment in perovskite solar cells. Nat. Energy 6, 1154–1163 (2021). This paper details a single-atom material with a well-defined structure that enables precise regulation of the electrical properties of graphene back electrodes, leading to highly efficient and stable modular cell by reducing series resistance.
Zhang, C. et al. CNT-based bifacial perovskite solar cells toward highly efficient 4-terminal tandem photovoltaics. Energy Environ. Sci. 15, 1536–1544 (2022). This paper proposes bifacial C-PSCs with transparent CNT network films in the rear electrode to enhance power output per unit area of PSCs by optimizing CNT types and sizes.
Djurišić, A. et al. Perovskite solar cells–an overview of critical issues. Prog. Quantum Electron. 53, 1–37 (2017).
Lee, D.-K. & Park, N.-G. Additive engineering for highly efficient and stable perovskite solar cells. Appl. Phys. Rev. 10, 011308 (2023).
Chen, J. & Park, N.-G. Materials and methods for interface engineering toward stable and efficient perovskite solar cells. ACS Energy Lett. 5, 2742–2786 (2020).
Jung, H. S., Han, G. S., Park, N.-G. & Ko, M. J. Flexible perovskite solar cells. Joule 3, 1850–1880 (2019).
Kim, H.-S., Hagfeldt, A. & Park, N.-G. Morphological and compositional progress in halide perovskite solar cells. Chem. Commun. 55, 1192–1200 (2019).
bin Mohd Yusoff, A. R. et al. Passivation and process engineering approaches of halide perovskite films for high efficiency and stability perovskite solar cells. Energy Environ. Sci. 14, 2906–2953 (2021).
Lu, H. et al. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 370, eabb8985 (2020).
Halder, A., Yeom, K.-W. & Park, N.-G. Strategies toward suppression of Sn (II) oxidation for stable Sn-Pb perovskite solar cells. ACS Energy Lett. 8, 4267–4277 (2023).
Chen, J., Zhao, X., Kim, S. G. & Park, N. G. Multifunctional chemical linker imidazoleacetic acid hydrochloride for 21% efficient and stable planar perovskite solar cells. Adv. Mater. 31, 1902902 (2019).
Liu, C., Cheng, Y.-B. & Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 49, 1653–1687 (2020).
Ma, C., Grätzel, M. & Park, N.-G. Facet engineering for stable, efficient perovskite solar cells. ACS Energy Lett. 7, 3120–3128 (2022).
Ma, C. et al. Unveiling facet-dependent degradation and facet engineering for stable perovskite solar cells. Science 379, 173–178 (2023). This paper reports that FA-based lead iodide perovskite degrades more quickly when exposed to moisture on the (100) facet compared to the (111) facet and demonstrates that ligand-assisted growth can produce more stable (111)-dominated films that resist moisture and thermal stress.
Zhou, H. et al. Interface engineering of highly efficient perovskite solar cells. Science 345, 542–546 (2014).
Graetzel, M., Janssen, R. A., Mitzi, D. B. & Sargent, E. H. Materials interface engineering for solution-processed photovoltaics. Nature 488, 304–312 (2012).
Cho, A. N. & Park, N. G. Impact of interfacial layers in perovskite solar cells. ChemSusChem 10, 3687–3704 (2017).
Ma, C. et al. Facet-dependent passivation for efficient perovskite solar cells. J. Am. Chem. Soc. 145, 24349–24357 (2023). This paper present a facet-dependent surface passivation principle for perovskite solar cells, demonstrating that passivation selectively occurs on different facets based on their atomic arrangements.
Kim, H.-J., Kim, H.-S. & Park, N.-G. Progress of perovskite solar modules. Adv. Energy Sustain. Res. 2, 2000051 (2021).
Lee, D.-K., Lim, K.-S., Lee, J.-W. & Park, N.-G. Scalable perovskite coating via anti-solvent-free Lewis acid-base adduct engineering for efficient perovskite solar modules. J. Mater. Chem. A 9, 3018–3028 (2021).
Kim, H. S. & Park, N. G. Future research directions in perovskite solar cells: exquisite photon management and thermodynamic phase stability. Adv. Mater. 35, 2204807 (2023).
Jung, S.-K., Park, N.-G. & Lee, J.-W. Light management in perovskite solar cells. Mater. Today Energy 37, 101401 (2023).
Kim, S.-G. et al. Nanographene coupled with interfacial pyrene derivatives for thermally stable perovskite solar cells. ACS Energy Lett. 8, 2267–2275 (2023).
Kim, S. G. & Zhu, K. Chemical design of organic interface modifiers for highly efficient and stable perovskite solar cells. Adv. Energy Mater. 13, 2300603 (2023).
Zhu, P. et al. Aqueous synthesis of perovskite precursors for highly efficient perovskite solar cells. Science 383, 524–531 (2024). This paper presents a aqueous method for synthesizing FAPbI₃ microcrystals, offering an economical and effective route for producing high-purity precursors. This approach eliminates the need for organic solvents and significantly reduces the presence of impurity ions.
McMeekin, D. P. et al. Intermediate-phase engineering via dimethylammonium cation additive for stable perovskite solar cells. Nat. Mater. 22, 73–83 (2023).
Lee, S.-U., Park, H., Shin, H. & Park, N.-G. Atomic layer deposition of SnO2 using hydrogen peroxide improves the efficiency and stability of perovskite solar cells. Nanoscale 15, 5044–5052 (2023).
Wang, C. et al. Enhancing the inherent stability of perovskite solar cells through chalcogenide-halide combinations. Energy Environ. Sci. 17, 1368–1386 (2024).
Wang, F., Yu, H., Xu, H. & Zhao, N. HPbI3: a new precursor compound for highly efficient solution‐processed perovskite solar cells. Adv. Funct. Mater. 25, 1120–1126 (2015).
Yan, K. et al. Hybrid halide perovskite solar cell precursors: colloidal chemistry and coordination engineering behind device processing for high efficiency. J. Am. Chem. Soc. 137, 4460–4468 (2015).
Duan, C. et al. Precursor engineering for ambient‐compatible antisolvent‐free fabrication of high‐efficiency CsPbI2Br perovskite solar cells. Adv. Energy Mater. 10, 2000691 (2020).
Yoon, S. M. et al. Surface engineering of ambient-air-processed cesium lead triiodide layers for efficient solar cells. Joule 5, 183–196 (2021).
Li, D., Wu, P. & Zhang, F. Alkylammonium chloride promotes the record efficiency of perovskite solar cells. Joule 7, 628–630 (2023).
Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021).
Min, H. et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 598, 444–450 (2021).
Peng, J. et al. Centimetre-scale perovskite solar cells with fill factors of more than 86 percent. Nature 601, 573–578 (2022).
Liang, Z. et al. Homogenizing out-of-plane cation composition in perovskite solar cells. Nature 624, 557–563 (2023).
Ma, C. et al. Photovoltaically top-performing perovskite crystal facets. Joule 6, 2626–2643 (2022). This work reports that the carrier mobility and photocurrent of the (100) crystal facet are almost comparable to those of the (111) facet, which are much higher than those of the (110) facet, and provides a deep understanding of the optoelectronic properties of halide perovskite facets.
Lee, J. H. et al. Enhancing intermolecular interaction of Spiro-OMeTAD for stable perovskite solar cells with efficiencies over 24%. ACS Energy Lett. 8, 3895–3901 (2023).
Lee, D.-K., Jeong, D.-N., Ahn, T. K. & Park, N.-G. Precursor engineering for a large-area perovskite solar cell with>19% efficiency. ACS. Energy Lett. 4, 2393–2401 (2019).
Yuan, L. et al. Volatile perovskite precursor ink enables window printing of phase-pure FAPbI3 perovskite solar cells and modules in ambient atmosphere. Angew. Chem. Int. Ed. 63, e202316954 (2024).
Zhao, J. et al. Efficient and stable formamidinium-caesium perovskite solar cells and modules from lead acetate-based precursors. Energy Environ. Sci. 16, 138–147 (2023).
Tan, Q. et al. Inverted perovskite solar cells using dimethylacridine-based dopants. Nature 620, 545–551 (2023).
Ding, B. et al. Dopant-additive synergism enhances perovskite solar modules. Nature 628, 299–305 (2024).
Zhang, Y. & Park, N. G. Tailoring the functionality of additives for enhancing the stability of perovskite solar cells. Adv. Funct. Mater. 33, 2308577 (2023).
Wu, Z. et al. Crystallization kinetics of hybrid perovskite solar cells. Angew. Chem. Int. Ed. 63, e202319170 (2024).
Jiang, X. et al. Molecular dipole engineering of carbonyl additives for efficient and stable perovskite solar cells. Angew. Chem. Int. Ed. 62, e202302462 (2023).
Li, G. et al. Highly efficient pin perovskite solar cells that endure temperature variations. Science 379, 399–403 (2023).
Shin, G. S., Zhang, Y. & Park, N.-G. Stability of precursor solution for perovskite solar cell: mixture (FAI+ PbI2) versus synthetic FAPbI3 Crystal. ACS Appl. Mater. Interfaces 12, 15167–15174 (2020).
Levchuk, I. et al. Deciphering the role of impurities in methylammonium iodide and their impact on the performance of perovskite solar cells. Adv. Mater. Interfaces 3, 1600593 (2016).
Zhang, Y. et al. Stabilizing α-phase FAPbI3 perovskite induced by an ordered solvated quasi-crystalline PbI2. ACS Energy Lett. 9, 159–167 (2023).
Ozaki, M. et al. A purified, solvent‐intercalated precursor complex for wide‐process‐window fabrication of efficient perovskite solar cells and modules. Angew. Chem. Int. Ed. 58, 9389–9393 (2019).
Zhang, W. et al. Anti-solvent engineering to rapid purify PbI2 for efficient perovskite solar cells. Chem. Eng. J. 479, 147838 (2024).
Jiang, R. et al. Low-cost fabrication of efficient perovskite photovoltaics using low-purity lead iodide via powder engineering. ACS Appl. Mater. Interfaces 15, 20958–20965 (2023).
Zhang, Y., Kim, S. G., Lee, D. K. & Park, N. G. CH3NH3PbI3 and HC(NH2)2PbI3 powders synthesized from low-grade PbI2: single precursor for high‐efficiency perovskite solar cells. ChemSusChem 11, 1813–1823 (2018).
Kang, D.-H., Park, Y.-J., Jeon, Y.-S. & Park, N.-G. Extended X-ray absorption fine structure (EXAFS) of FAPbI3 for understanding local structure-stability relation in perovskite solar cells. J. Energy Chem. 67, 549–554 (2022).
Shin, S. et al. Enhancing stability of efficient perovskite solar cells (PCE≈24.5%) by suppressing PbI2 inclusion formation. Adv. Funct. Mater. 33, 2301213 (2023).
Zhang, Y. et al. Achieving reproducible and high-efficiency (> 21%) perovskite solar cells with a presynthesized FAPbI3 powder. ACS. Energy Lett. 5, 360–366 (2019).
Tong, G. et al. Removal of residual compositions by powder engineering for high efficiency formamidinium-based perovskite solar cells with operation lifetime over 2000 h. Nano Energy 87, 106152 (2021).
Mandal, T. N., Heo, J. H., Im, S. H. & Kim, W. S. Highly efficient and stable inverted perovskite solar cell using pure δ‐FAPbI3 single crystals. Small 19, 2305246 (2023).
Chen, L., Yoo, J. W., Hu, M., Lee, S. U. & Seok, S. I. Intrinsic phase stability and inherent bandgap of formamidinium lead triiodide perovskite single crystals. Angew. Chem. 134, e202212700 (2022).
Lee, J.-W., Tan, S., Seok, S. I., Yang, Y. & Park, N.-G. Rethinking the A cation in halide perovskites. Science 375, eabj1186 (2022).
Zhang, F. & Zhu, K. Additive engineering for efficient and stable perovskite solar cells. Adv. Energy Mater. 10, 1902579 (2020).
Cao, X. et al. A review of the role of solvents in formation of high-quality solution-processed perovskite films. ACS Appl. Mater. Interfaces 11, 7639–7654 (2019).
Chao, L. et al. Solvent engineering of the precursor solution toward large‐area production of perovskite solar cells. Adv. Mater. 33, 2005410 (2021).
Vidal, R. et al. Assessing health and environmental impacts of solvents for producing perovskite solar cells. Nat. Sustain. 4, 277–285 (2021).
Park, N.-G. Green solvent for perovskite solar cell production. Nat. Sustain. 4, 192–193 (2021).
Yun, H.-S. et al. Ethanol-based green-solution processing of α-formamidinium lead triiodide perovskite layers. Nat. Energy 7, 828–834 (2022). This paper report a green method for depositing dense and uniform α-FAPbI3 films using ethanol-based solvents without antisolvent dripping, where a combination of a Lewis base (dimethylacetamide or DMSO) and alkylammonium chloride (RNH3Cl) in ethanol stabilizes FAPbI3, leading to high-crystallinity films after spin-coating and annealing.
Chen, C. et al. Perovskite solar cells based on screen-printed thin films. Nature 612, 266–271 (2022).
Hui, W. et al. Stabilizing black-phase formamidinium perovskite formation at room temperature and high humidity. Science 371, 1359–1364 (2021).
Miao, Y. et al. Green solvent enabled scalable processing of perovskite solar cells with high efficiency. Nat. Sustain. 6, 1465–1473 (2023).
Bai, Y., Hao, M., Ding, S., Chen, P. & Wang, L. Surface chemistry engineering of perovskite quantum dots: strategies, applications, and perspectives. Adv. Mater. 34, 2105958 (2022).
Luo, D., Su, R., Zhang, W., Gong, Q. & Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 5, 44–60 (2020).
Huang, Z. et al. Anion–π interactions suppress phase impurities in FAPbI3 solar cells. Nature 623, 531–537 (2023).
Wu, N. et al. Stabilizing precursor solution and controlling crystallization kinetics simultaneously for high‐performance perovskite solar cells. Adv. Mater. 35, 2304809 (2023).
Yang, T. et al. Amidino-based Dion-Jacobson 2D perovskite for efficient and stable 2D/3D heterostructure perovskite solar cells. Joule 7, 574–586 (2023).
Gao, H. et al. Homogeneous crystallization and buried interface passivation for perovskite tandem solar modules. Science 383, 855–859 (2024).
Kim, G. et al. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 370, 108–112 (2020).
Zhao, Y. et al. Suppressing ion migration in metal halide perovskite via interstitial doping with a trace amount of multivalent cations. Nat. Mater. 21, 1396–1402 (2022).
Chen, C. et al. Polyacrylonitrile‐coordinated perovskite solar cell with open‐circuit voltage exceeding 1.23 V. Angew. Chem. Int. Ed. 61, e202113932 (2022).
Jeong, J. et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Isikgor, F. H. et al. Molecular engineering of contact interfaces for high-performance perovskite solar cells. Nat. Rev. Mater. 8, 89–108 (2023).
Yu, S. et al. Homogenized NiOx nanoparticles for improved hole transport in inverted perovskite solar cells. Science 382, 1399–1404 (2023).
Azmi, R. et al. Damp heat-stable perovskite solar cells with tailored-dimensionality 2D/3D heterojunctions. Science 376, 73–77 (2022).
Jiang, Q. et al. Surface reaction for efficient and stable inverted perovskite solar cells. Nature 611, 278–283 (2022).
Park, S. M. et al. Engineering ligand reactivity enables high-temperature operation of stable perovskite solar cells. Science 381, 209–215 (2023).
Sidhik, S. et al. Deterministic fabrication of 3D/2D perovskite bilayer stacks for durable and efficient solar cells. Science 377, 1425–1430 (2022).
Zhang, C. et al. Work function tuning of a weak adhesion homojunction for stable perovskite solar cells. Joule 8, p1394-1411 (2024). This paper reports that tuning the work function at the weak adhesion homojunction interface with self-assembled monolayer functional molecules significantly reduces the built-in electric field, thereby decreasing ion migration, enhancing stability, and improving the photovoltaic performance of perovskite solar cells.
Dai, Z. et al. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 372, 618–622 (2021).
Liu, C. et al. Flexible indoor perovskite solar cells by in situ bottom‐up crystallization modulation and interfacial passivation. Adv. Mater. 36, 2311562 (2024).
Jiang, Q. et al. Towards linking lab and field lifetimes of perovskite solar cells. Nature 623, 313–318 (2023).
Dai, Z. et al. Connecting interfacial mechanical adhesion, efficiency, and operational stability in high performance inverted perovskite solar cells. ACS Energy Lett. 9, 1880–1887 (2024).
Tan, S. et al. Stability-limiting heterointerfaces of perovskite photovoltaics. Nature 605, 268–273 (2022).
Kim, Y. Y. et al. Roll-to-roll gravure-printed flexible perovskite solar cells using eco-friendly antisolvent bathing with wide processing window. Nat. Commun. 11, 5146 (2020).
Dou, B. et al. Roll-to-roll printing of perovskite solar cells. ACS Energy Lett. 3, 2558–2565 (2018).
Lee, D. S. et al. Fully scalable and stable CsPbI2Br solar cells realized by an all-spray-coating process. ACS Appl. Mater. Interfaces 14, 7926–7935 (2022).
Zhang, C., Choi, Y. J., Kim, J. Y. & Park, N.-G. Detachable, reassemblable two-terminal perovskite/silicon tandem solar cells. ACS Energy Lett. 9, 4280–4282 (2024). A detachable, reassemblable modular tandem solar cell with a spray-coated carbon nanotube film simplifies assembly and maintenance while addressing resource wastage from mismatched cell lifetimes.
Weerasinghe, H. C. et al. The first demonstration of entirely roll-to-roll fabricated perovskite solar cell modules under ambient room conditions. Nat. Commun. 15, 1656 (2024).
Said, A. A. et al. Sublimed C60 for efficient and repeatable perovskite-based solar cells. Nat. Commun. 15, 708 (2024).
Al-Ashouri, A. et al. Wettability improvement of a carbazole-based hole-selective monolayer for reproducible perovskite solar cells. ACS Energy Lett. 8, 898–900 (2023).
Kang, D.-H., Lee, S.-U. & Park, N.-G. Effect of residual chloride in FAPbI3 film on photovoltaic performance and stability of perovskite solar cell. ACS Energy Lett. 8, 2122–2129 (2023).
Hossain, M. K. et al. Achieving above 24% efficiency with non-toxic CsSnI3 perovskite solar cells by harnessing the potential of the absorber and charge transport layers. RSC Adv. 13, 23514–23537 (2023).
Luo, H. et al. Sustainable Pb management in perovskite solar cells toward eco-friendly development. Adv. Energy Mater. 12, 2201242 (2022).
Shalan, A. E., Kazim, S. & Ahmad, S. Lead‐free perovskites: metals substitution towards environmentally benign solar cell fabrication. ChemSusChem 12, 4116–4139 (2019).
Zhang, H. et al. Lead immobilization for environmentally sustainable perovskite solar cells. Nature 617, 687–695 (2023).
Fu, G., Lee, D.-K., Ma, C. & Park, N.-G. Disulfidation interfacial engineering toward stable, lead-immobilizable perovskite solar cells. ACS Energy Lett. 8, 4563–4571 (2023).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) under contract NRF-2021R1A3B1076723 (Global Research Leader Program), NRF-2022M3J1A1085280 (Carbon Neutral Technology Program), and RS-2023-00259096 (GRDC Cooperative Hub). This work was, in part, edited by ERNIE Bot versions 4.0 and Microsoft Copilot.
Author information
Authors and Affiliations
Contributions
N.-G.P. conceived the idea and edited the manuscript. C.Z. wrote the draft of the review under the supervision of N.-G.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Thad Druffel, Jacek Jasieniak, and the other anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. Primary Handling Editor: John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Park, NG. Materials and methods for cost-effective fabrication of perovskite photovoltaic devices. Commun Mater 5, 194 (2024). https://doi.org/10.1038/s43246-024-00636-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00636-8
This article is cited by
-
Distributed wireless power transfer based on secondary-side-direct-series topology for integration with building-attached photovoltaic system
Scientific Reports (2025)
-
Solvothermal Synthesis of CsKTiCl6 Halide Double Perovskite and Its Application as a Photocatalyst for the Degradation of Rhodamine B and Latent Fingerprint Development Analysis in Forensic Science
Journal of Inorganic and Organometallic Polymers and Materials (2025)
-
Unveiling and optimizing the role of ALD-SnO2 in Perovskite solar cells
Advances in Industrial and Engineering Chemistry (2025)