Abstract
Natural killer (NK) cells are essential for controlling tumor metastasis, but their protective capacity diminishes when entering an exhaustion state. The mechanisms underlying NK cell exhaustion are incompletely understood. Here, we show that the susceptibility of NK cells to exhaustion is predetermined early during their development and is governed by the transcription factor IRF4. Notably, IRF4 is highly expressed in CD27−CD24+ NK precursors but is nearly absent in immature and mature NK cells. Deletion of IRF4 redirects NK cell development, enabling NK precursors to generate more mature NK cells that resist exhaustion, thereby decreasing melanoma lung metastasis. This resistance to exhaustion is evident by increased effector molecule production and decreased expression of inhibitory receptors such as TIGIT and Pik3ip1. Deleting Pik3ip1 also enhances NK cell ability to counteract melanoma lung metastasis. These findings enhance our understanding of NK cell exhaustion and have implications for preventing cancer metastasis using NK cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq, scRNA-seq and ATAC-seq data have been deposited in the Gene Expression Omnibus under accession codes GSE249188, GSE249230 and GSE283690. Raw Illumina sequencing reads of RNA-seq, scRNA-seq and ATAC-seq datasets were aligned to reference mouse genome mm10 (Ensembl 93), http://jul2018.archive.ensembl.org/Mus_musculus/Info/Index. All other data supporting the findings of this study are available within the article and Supplementary Information. Source data are provided with this paper.
Code availability
ScRNA-seq and ATAC-seq data analyses were conducted using publicly available codes and open-source software packages. No new algorithms were developed for this study.
References
Lopez-Soto, A., Gonzalez, S., Smyth, M. J. & Galluzzi, L. Control of metastasis by NK cells. Cancer Cell 32, 135–154 (2017).
Liu, S. et al. NK cell-based cancer immunotherapy: from basic biology to clinical development. J. Hematol. Oncol. 14, 7 (2021).
Wu, S. Y., Fu, T., Jiang, Y. Z. & Shao, Z. M. Natural killer cells in cancer biology and therapy. Mol. Cancer 19, 120 (2020).
Menard, C. et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 69, 3563–3569 (2009).
Smyth, M. J. et al. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 162, 6658–6662 (1999).
Street, S. E., Cretney, E. & Smyth, M. J. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97, 192–197 (2001).
Seaman, W. E., Sleisenger, M., Eriksson, E. & Koo, G. C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J. Immunol. 138, 4539–4544 (1987).
Liu, Y. et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 36, 6143–6153 (2017).
Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018).
Judge, S. J., Murphy, W. J. & Canter, R. J. Characterizing the dysfunctional NK cell: assessing the clinical relevance of exhaustion, anergy, and senescence. Front. Cell Infect. Microbiol. 10, 49 (2020).
Bi, J. & Tian, Z. NK cell exhaustion. Front. Immunol. 8, 760 (2017).
Ardolino, M. et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Invest. 124, 4781–4794 (2014).
da Silva, I. P. et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res 2, 410–422 (2014).
Komita, H. et al. Expression of immune checkpoint molecules of T cell immunoglobulin and mucin protein 3/galectin-9 for NK cell suppression in human gastrointestinal stromal tumors. Oncol. Rep. 34, 2099–2105 (2015).
Benson, D. M. Jr. et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010).
Kondo, M., Weissman, I. L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Abel, A. M., Yang, C., Thakar, M. S. & Malarkannan, S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 9, 1869 (2018).
Chiossone, L. et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood 113, 5488–5496 (2009).
Fu, B. et al. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology 133, 350–359 (2011).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Ohteki, T. et al. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-alpha/beta+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187, 967–972 (1998).
Lohoff, M. et al. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J. Exp. Med. 192, 325–336 (2000).
Taki, S., Nakajima, S., Ichikawa, E., Saito, T. & Hida, S. IFN regulatory factor-2 deficiency revealed a novel checkpoint critical for the generation of peripheral NK cells. J. Immunol. 174, 6005–6012 (2005).
Adams, N. M. et al. Transcription factor IRF8 orchestrates the adaptive natural killer cell response. Immunity 48, 1172–1182.e1176 (2018).
Santosa, E. K. et al. Control of nutrient uptake by IRF4 orchestrates innate immune memory. Nat. Immunol. 24, 1685–1697 (2023).
Geary, C. D. et al. Non-redundant ISGF3 components promote NK cell survival in an auto-regulatory manner during viral infection. Cell Rep. 24, 1949–1957.e1946 (2018).
Yan, H. et al. The transcription factor IRF4 determines the anti-tumor immunity of CD8+ T cells. iScience 26, 108087 (2023).
Yu, A. et al. Continuous expression of interferon regulatory factor 4 sustains CD8+ T cell immunity against tumor. Research (Wash. DC) 6, 0271 (2023).
Wang, G. et al. IRF4 ablation in B cells abrogates allogeneic B cell responses and prevents chronic transplant rejection. J. Heart Lung Transpl. 40, 1122–1132 (2021).
Rosmaraki, E. E. et al. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 31, 1900–1909 (2001).
Fathman, J. W. et al. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood 118, 5439–5447 (2011).
Mittrucker, H. W. et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 275, 540–543 (1997).
Huntington, N. D. et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J. Immunol. 178, 4764–4770 (2007).
Malaise, M. et al. KLRG1+ NK cells protect T-bet-deficient mice from pulmonary metastatic colorectal carcinoma. J. Immunol. 192, 1954–1961 (2014).
Alvarez, M. et al. Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway. JCI Insight 5, e127729 (2019).
Uche, U. U. et al. PIK3IP1/TrIP restricts activation of T cells through inhibition of PI3K/Akt. J. Exp. Med. 215, 3165–3179 (2018).
Hsu, J. et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018).
Chauvin, J. M. et al. IL15 Stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin. Cancer Res. 26, 5520–5533 (2020).
He, Y. et al. Contribution of inhibitory receptor TIGIT to NK cell education. J. Autoimmun. 81, 1–12 (2017).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 20, 477–494 (2004).
Gordon, S. M. et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36, 55–67 (2012).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Sciume, G. et al. CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood 117, 4467–4475 (2011).
Wijaya, R. S. et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J. Hepatol. 71, 252–264 (2019).
Chen, Y. et al. Pik3ip1 is a negative immune regulator that inhibits antitumor T-cell immunity. Clin. Cancer Res. 25, 6180–6194 (2019).
Narni-Mancinelli, E. et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl Acad. Sci. USA 108, 18324–18329 (2011).
Wu, J. et al. Ablation of transcription factor IRF4 promotes transplant acceptance by driving allogenic CD4+ T cell dysfunction. Immunity 47, 1114–1128 e1116 (2017).
Wang, G. et al. The RNA helicase DHX15 is a critical regulator of natural killer-cell homeostasis and functions. Cell Mol. Immunol. 19, 687–701 (2022).
Zou, D. et al. T cell exhaustion is associated with antigen abundance and promotes transplant acceptance. Am. J. Transpl. 20, 2540–2550 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e1821 (2019).
Acknowledgements
We thank E. Vivier (Aix Marseille University) for the Ncr1Cre mouse strain and L. L. Lanier (University of California San Francisco) for the RMA-S cell line. We would like to thank the Single Cell Genomics Core at Baylor College of Medicine, the Biostatistics and Bioinformatics (BBI) shared resources at Houston Methodist Neal Cancer Center and the Houston Methodist Flow Cytometry Core Facility for their excellent services. This work was supported by internal fund from Houston Methodist Research Institute (to W.C.).
Author information
Authors and Affiliations
Contributions
X.Z., J.W., X.X. and W.C. designed the study. X.Z., X.C.L. and W.C. wrote the manuscript. X.Z. performed core experimental work and data analysis. Z.Y. performed computational analyses. J.W., X.X., D.Z., G.W., J.F., P.L., L.J.M., X.C.L. and W.C. performed supporting experimental work and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Dagmar Gotthardt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
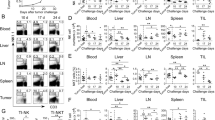
Extended Data Fig. 1 IRF4 deletion in T cells, dendritic cells, or macrophages does not reduce melanoma metastasis.
(a) Representative image of lung metastasis in Irf4fl/fl and Irf4fl/flCd4-Cre mice 20 days post B16-F10 cell intravenous injection, and a graph showing percentage survival rates for the injected mice. n = 6 mice per group; ns, not significant. (b) Representative lung metastasis image (day 20 post B16-F10 cell injection) in Irf4fl/fl and Irf4fl/flLysM-Cre mice, and percentage survival rates. n = 7 mice per group; ns, not significant. (c) Percentage survival rates of Irf4fl/flCd11c-Cre and Irf4fl/fl mice post B16-F10 cell intravenous injection. n = 5 mice per group; ns, not significant. (d) Percentages of remaining CD3‒NK1.1+ cells within splenocytes of WT and Irf4–/– mice three days post anti-NK1.1 mAb (clone PK136) treatment. Results are representative of two or three independent experiments. Data were analyzed by a log-rank test.
Extended Data Fig. 2 Irf4‒/‒ NK cells exhibit a more mature phenotype.
(a) Representative flow cytometry plots of CD3‒NK1.1+ cells and bar graphs showing NK cell numbers in the liver and blood (per ml) of naïve WT and Irf4−/− mice. For the liver, n = 6 mice per group, **P = 0.0022; for the blood, n = 5 mice per group, ***P = 0.0001. (b) Representative flow cytometry plots show CD27 and CD11b expression in NK cells. The bar graph shows the percentage of CD27‒CD11b+ cells among total NK cells in the indicated tissues of naïve WT and Irf4−/− mice. n = 6 mice per group; from left to right: ****P < 0.0001, ***P = 0.0009, ***P = 0.0010, * P = 0.0128. (c) Sorted NK cells from naïve WT and Irf4−/− mice were labeled with CTV and stimulated in vitro with IL-12 (10 ng/ml), IL-18 (50 ng/ml), and IL-15 (50 ng/ml) for 3 days to assess proliferation. (d) Sorted NK cells from naïve WT and Irf4−/− mice were stimulated in vitro with IL-12, IL-18, and IL-15 for 3 days, followed by apoptosis assessment. n = 5 biologically independent samples per group; ****P < 0.0001. Data are presented as mean ± SD (a,b,d). Results are representative of two or three independent experiments. Data were analyzed by a two-tailed unpaired Student’s t-test.
Extended Data Fig. 3 Irf4–/– BM cell reconstitution does not enhance the generation of other immune cells.
(a) Flow cytometry analysis confirming that WT and Irf4–/– BM cells were mixed at a 1:1 ratio prior to transfer into lethally irradiated Rag1–/– mice. (b) Flow cytometry analysis of the percentage of NKP cells in the bone marrow of WT and Irf4–/– mice. n = 3 mice per group; ns, not significant. (c and d) Lethally irradiated Rag1−/− mice were reconstituted with a 1:1 mixture of CD45.1+ WT and CD45.2+ Irf4–/– BM cells. Flow cytometry was performed 6 weeks post transfer to assess the percentage of live cells (c) and CD3+NK1.1– T cells (d) derived from transferred WT or Irf4–/– BM cells. n = 3 mice; for c: ***P = 0.0003, ns, not significant; for d: **P = 0.0052, ns, not significant. Results are representative of two or three independent experiments. Data are presented as mean ± SD (b,c,d). Data were analyzed by a two-tailed unpaired Student’s t-test.
Extended Data Fig. 4 IRF4 expression in NKP and NK cells during melanoma metastasis.
(a and b) Irf4GFP reporter mice were intravenously injected with B16-F10 cells. Shown are the percentages of IRF4.GFP+ cells within NK cells in spleen and lung (a) and the percentages of IRF4.GFP+CD27‒ cells within BM NKP cells (b) at different time points post B16-F10 injection. n = 3 mice per time point. (c and d) Rag2−/−γc−/− mice were transferred with a 1:1 mixture of WT and Irf4−/− NKP cells. (c) Gating strategy used to sort BM NKP cells for transfer. (d) Percentages of NK cells derived from the transferred WT or Irf4−/− NKP cells at 6 weeks post-transfer. n = 4 mice; **P = 0.0021. Data are presented as mean ± SD (a,b,d). Data were analyzed by a two-tailed unpaired Student’s t-test (d).
Extended Data Fig. 5 Identification of IRF4-expressing CD24+CD27– NKP cells.
(a and b) scRNA-seq analysis of a total of 20,836 cells, including 12,740 WT NKP and 8,096 Irf4−/− NKP cells. UMAP plots (a) illustrating the normalized expression of Irf4 and Cd24a in NKP cells. Bubble plots (b) illustrating the normalized expression of Irf4 and Cd24a across identified NKP cell clusters (matching cluster IDs in Fig. 3a). The color intensity corresponds to the average expression level within the cluster. The size of each bubble indicates the percentage of cells expressing the gene within the cluster. (c–e) CD24‒CD27+ NKP cells from WT CD45.1+ BM and CD24+CD27‒ NKP cells from WT CD45.2+ BM were adoptively co-transferred into Rag2−/−γc−/− mice in a 1:1 ratio, followed by flow cytometry analysis six weeks later. Shown are percentages of CD45.1+ and CD45.2+ cells among CD3–NK1.1+ splenocytes (c, n = 3 mice, **P = 0.0036), expression of CD27 versus CD11b among CD3–NK1.1+ splenocytes derived from the transferred NKP cells (d), and expression of CD27 in CD3–NK1.1– splenocytes derived from the transferred NKP cells (e). (f) CD27–CD24med NKP cells from WT CD45.1+ BM and CD27–CD24high NKP cells from WT CD45.2+ BM were adoptively co-transferred into Rag2−/−γc−/− mice in a 1:1 ratio. The percentages of splenic CD3‒NK1.1+ NK cells derived from these transferred NKP cells were assessed two weeks post-transfer (n = 3 mice; ****P < 0.0001). Data are presented as mean ± SD (c,f). Data were analyzed by a two-tailed unpaired Student’s t-test (c,f).
Extended Data Fig. 6 Evaluation of NK cells from WT and Irf4‒/‒ mice.
(a,b) Splenic NK cells from naïve WT and Irf4‒/‒ mice were stimulated with IL-12, IL-15, and IL-18. Shown are the percentages of IFN-γ+ and perforin+ cells (a), as well as granzyme B+ cells (b), within WT and Irf4‒/‒ NK cells. For IFN-γ, n = 5 mice per group; for perforin, n = 4 mice per group; for granzyme B, n = 3 mice per group; ns, not significant. (c) Splenic NK cells from naïve WT and Irf4‒/‒ mice were cocultured with RMA/S-GFP target cells at the indicated ratios. Percentage killing of target cells were illustrated. n = 3 biologically independent samples per group. (d) Percentages of granzyme B+ cells within lung NK cells, comparing WT versus Irf4–/– mice 20 days post B16-F10 cell intravenous injection. n = 3 mice per group; **P = 0.0038. (e) Expression of TIGIT in NK cells from the spleens of naïve WT and Irf4−/− mice. n = 4 mice per group; ns, not significant. (f) NK cells sorted from the spleens of naïve WT and Irf4−/− mice were stimulated in vitro with plate-bound anti-NK1.1 (PK136) antibody (20 µg/ml), IL-2 (10 ng/ml), and IL-15 (5 ng/ml) for 3 days, followed by assessment of TIGIT expression. n = 6 biologically independent samples per group; ****P < 0.0001. Data are presented as mean ± SD. Data were analyzed by a two-tailed unpaired Student’s t-test.
Extended Data Fig. 7 Deleting IRF4 in Ncr1-expressing NK cells does not prolong survival in the melanoma metastasis model.
(a) Irf4fl/fl mice from Jackson Laboratory contain an eGFP gene positioned in the opposite orientation upstream of the Irf4 promoter. Upon Cre-mediated Irf4 deletion, eGFP is expressed, although at low levels in some cells. Shown is GFP expression in NK cells from the spleens of naïve Irf4fl/fl and Irf4fl/flNcr1Cre+/‒ mice. (b) Expression of Nkp46 in NK cells from the spleens of naïve Irf4fl/fl and Irf4fl/flNcr1Cre+/‒ mice. (c) Percentages of Nkp46+ cells among CD3‒NK1.1+ splenocytes in naïve Irf4fl/fl and Irf4fl/flNcr1Cre+/‒ mice. n = 3 mice per group; ns, not significant. (d) Percentage survival rates of Irf4fl/fl, Irf4w/wNcr1Cre+/‒, and Irf4fl/flNcr1Cre+/‒ mice following intravenous injection of B16-F10 cells. n = 5 mice per group; ns, not significant. Data are presented as mean ± SD (c). Data were analyzed by a two-tailed unpaired Student’s t-test (c) and a log-rank test (d).
Extended Data Fig. 8 Irf4 deficiency in NKP cells renders mature NK cells less prone to exhaustion.
(a and b) Rag2−/−γc−/− mice were transferred with a 1:1 mixture of CD45.1+ WT NKP and CD45.2+ Irf4−/− NKP cells. Two weeks later, the mice were intravenously injected with B16-F10 cells. Splenic NK cells derived from the transferred NKP cells were analyzed 14 days after B16-F10 injection. Shown are the percentages of WT and Irf4–/– NK cells among total splenic NK cells (a) and the percentages of TIGIT+ cells within WT and Irf4–/– NK cells (b). For a: n = 3 mice, ***P = 0.0002; for b: n = 4 mice, ***P = 0.0008. (c) Lethally irradiated Rag1−/− mice were reconstituted with a 1:1 mixture of CD45.1+ WT BM and CD45.2+ Irf4–/– BM cells. Six weeks later, the mice were injected with B16-F10 cells. NK cells derived from the transferred BM NKP cells were analyzed 25 days after B16-F10 injection. The top flow plots show the percentages of WT and Irf4–/– NK cells, and the bottom flow plots show TIGIT expression in WT and Irf4–/– NK cells in the indicated tissues. The bar graph shows the percentages of TIGIT+ cells within WT and Irf4–/– NK cells. n = 6 mice; from left to right: ****P < 0.0001 ****P < 0.0001, *P = 0.0162, ****P < 0.0001, ****P < 0.0001. Data are presented as mean ± SD. Data were analyzed by a two-tailed unpaired Student’s t-test.
Extended Data Fig. 9 Chromatin accessibility at indicated loci in NK cells.
ATAC-seq was performed on NK cells sorted from naïve WT, Irf4–/–, Irf4fl/fl, and Irf4fl/flNcr1Cre+/‒ mice (related to Fig. 6). Genome tracks show chromatin accessibility at the Prf1, Ifng, Tigit and Gzmb loci across the indicated NK cell groups.
Extended Data Fig. 10 Pik3ip1 deficiency reduces NK cell exhaustion during melanoma metastasis.
(a) Percentages of NK cells in the spleen of naïve WT and Pik3ip1‒/‒ mice. n = 5 mice per group; ns, not significant. (b) Percentages of TIGIT+ cells within spleen and lung NK cells, comparing WT vs. Pik3ip1‒/‒ mice 20 days post B16-F10 cell injection. n = 3 mice per group; *P = 0.0169, **P = 0.0050. (c) Percentages of IFN-γ+ and perforin+ cells within lung NK cells, comparing WT vs. Pik3ip1‒/‒ mice 20 days post B16-F10 injection. n = 3 mice per group; **P = 0.0023, *P = 0.0155. Data are presented as mean ± SD. Results are representative of two or three independent experiments. Data were analyzed by a two-tailed unpaired Student’s t-test.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Cluster proportion source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Yin, Z., Wu, J. et al. IRF4 expression by NK precursors predetermines exhaustion of NK cells during tumor metastasis. Nat Immunol 26, 1062–1073 (2025). https://doi.org/10.1038/s41590-025-02176-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-025-02176-w