Abstract
Microglia, the brain’s resident macrophages, depend on interleukin-34 and colony-stimulating factor 1 (CSF1) for their development and maintenance, engaging the CSF1 receptor (CSF1R). Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP), a neurodegenerative disorder affecting the brain’s white matter, is caused by heterozygous pathogenic mutations in the CSF1R gene. This study investigated molecular mechanisms underlying ALSP using single-nucleus RNA sequencing on postmortem brain specimens. Results showed a significant reduction in microglia in ALSP brains, with remaining cells exhibiting a unique activation signature. This reduction correlated with decreased myelinating oligodendrocytes (OLs) and increased neuropilin-2+ OLs with a stress-response and anti-apoptotic signature, driven by STAT3 and fibroblast growth factor receptor pathways. Additionally, astrocytes displayed maladaptive activation and stress responses. These findings underscore microglia’s crucial role in supporting OL myelination and limiting astrocyte repair responses, suggesting therapeutic strategies balancing CSF1R, fibroblast growth factor receptor and STAT3 pathways for ALSP and other genetically caused microgliopathies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All requests for human snRNA-seq data are promptly reviewed by the University Hospital Leipzig, Children’s Hospital of Philadelphia and Brain Research Institute at Niigata University to verify if the request is subject to any intellectual property or confidentiality obligations. Human-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a material transfer agreement. The snRNA-seq data generated in this study are available in the Gene Expression Omnibus under accession number GSE295854. The spatial transcriptomic data generated in this study are uploaded to Zenodo and available at: https://doi.org/10.5281/zenodo.15186190. The following datasets were previously published and reanalyzed in this study: snRNA-seq of occipital cortex from control individuals (under accession number GSE190013), snRNA-seq from three control white matter (under accession number GSE180759), and scRNA-seq from Csf1rΔFIRE/ΔFIRE mice (under accession number GSE267545).
Code availability
The code used to analyze scRNA-seq are available upon request.
References
Borst, K., Dumas, A. A. & Prinz, M. Microglia: immune and non-immune functions. Immunity 54, 2194–2208 (2021).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Tremblay, M.-È., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).
Sierra, A. et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 (2010).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020).
Hoffmann, A. & Miron, V. E. CNS macrophage contributions to myelin health. Immunol. Rev. 327, 53–70 (2024).
Kent, S. A. & Miron, V. E. Microglia regulation of central nervous system myelin health and regeneration. Nat. Rev. Immunol. 24, 49–63 (2024).
Irfan, M., Evonuk, K. S. & DeSilva, T. M. Microglia phagocytose oligodendrocyte progenitor cells and synapses during early postnatal development: implications for white versus gray matter maturation. FEBS J. 289, 2110–2127 (2022).
Nemes-Baran, A. D., White, D. R. & DeSilva, T. M. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047 (2020).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
Hagemeyer, N. et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 (2017).
Papapetropoulos, S. et al. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: review of clinical manifestations as foundations for therapeutic development. Front. Neurol. 12, 788168 (2021).
Konno, T. et al. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur. J. Neurol. 24, 37–45 (2017).
Papapetropoulos, S. et al. Clinical presentation and diagnosis of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: a literature analysis of case studies. Front. Neurol. 15, 1320663 (2024).
Muthusamy, K. et al. Adult-onset leukodystrophies: a practical guide, recent treatment updates, and future directions. Front. Neurol. 14, 1219324 (2023).
Rademakers, R. et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 44, 200–205 (2011).
Konno, T., Kasanuki, K., Ikeuchi, T., Dickson, D. W. & Wszolek, Z. K. CSF1R-related leukoencephalopathy: a major player in primary microgliopathies. Neurology 91, 1092–1104 (2018).
Dulski, J., Sundal, C. & Wszolek, Z. K. CSF1R-related disorder. in GeneReviews (eds. M. P. Adam et al.) (University of Washington, 1993).
Dulski, J., Muthusamy, K., Lund, T. C. & Wszolek, Z. K. CSF1R-related disorder: state of the art, challenges, and proposition of a new terminology. Parkinsonism Relat. Disord. 121, 105894 (2024).
Hume, D. A. et al. Phenotypic impacts of CSF1R deficiencies in humans and model organisms. J. Leukoc. Biol. 107, 205–219 (2020).
Stanley, E. R. & Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 6, a021857–a021857 (2014).
Stanley, E. R., Biundo, F., Gökhan, Ş. & Chitu, V. Differential regulation of microglial states by colony stimulating factors. Front. Cell Neurosci. 17, 1275935 (2023).
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012).
Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Pixley, F. J. & Stanley, E. R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14, 628–638 (2004).
Chitu, V., Gökhan, Ş. & Stanley, E. R. Modeling CSF-1 receptor deficiency diseases - how close are we? FEBS J. 289, 5049–5073 (2022).
Wu, J. et al. The phenotypic and genotypic spectrum of CSF1R-related disorder in China. Mov. Disord. 39, 798–813 (2024).
NCBI MedGEn. Brain abnormalities, neurodegeneration, and dysosteosclerosis (concept ID: C5193117); https://www.ncbi.nlm.nih.gov/medgen/C5193117/
Guo, L. & Ikegawa, S. From HDLS to BANDDOS: fast-expanding phenotypic spectrum of disorders caused by mutations in CSF1R. J. Hum. Genet. 66, 1139–1144 (2021).
Tada, M. et al. Characteristic microglial features in patients with hereditary diffuse leukoencephalopathy with spheroids. Ann. Neurol. 80, 554–565 (2016).
Oosterhof, N. et al. Colony-stimulating factor 1 receptor (CSF1R) regulates microglia density and distribution, but not microglia differentiation in vivo. Cell Rep. 24, 1203–1217 (2018).
Konno, T. et al. Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology 82, 139–148 (2014).
Berdowski, W. M. et al. Dominant-acting CSF1R variants cause microglial depletion and altered astrocytic phenotype in zebrafish and adult-onset leukodystrophy. Acta Neuropathol. 144, 211–239 (2022).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Siletti, K. et al. Transcriptomic diversity of cell types across the adult human brain. Science 382, eadd7046 (2023).
Dikmen, H. O. et al. GM-CSF induces noninflammatory proliferation of microglia and disturbs electrical neuronal network rhythms in situ. J. Neuroinflammation 17, 235 (2020).
Yamasaki, R. et al. Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J. Neuroimmunol. 229, 51–62 (2010).
Basso, L. et al. Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis. Proc. Natl Acad. Sci. USA 114, 11235–11240 (2017).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Marschallinger, J. et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194–208 (2020).
Haney, M. S. et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 628, 154–161 (2024).
De Schepper, S. et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat. Neurosci. 26, 406–415 (2023).
Lei, S., Hu, M. & Wei, Z. Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration. Front. Cell Neurosci. 17, 1322451 (2023).
Pandey, S. et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 40, 111189 (2022).
Sadick, J. S. et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805 (2022).
Xiang, X., Zhang, X. & Huang, Q. -L. Plexin A3 is involved in semaphorin 3F-mediated oligodendrocyte precursor cell migration. Neurosci. Lett. 530, 127–132 (2012).
Piaton, G. et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 134, 1156–1167 (2011).
Williams, A. et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130, 2554–2565 (2007).
Kaminski, W. E., Wenzel, J. J., Piehler, A., Langmann, T. & Schmitz, G. ABCA6, a novel a subclass ABC transporter. Biochem. Biophys. Res. Commun. 285, 1295–1301 (2001).
Soane, L., Rus, H., Niculescu, F. & Shin, M. L. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of bcl-2 and mediated by inhibition of caspase-3 activation. J. Immunol. 163, 6132–6138 (1999).
Kögel, D., Linder, B., Brunschweiger, A., Chines, S. & Behl, C. At the crossroads of apoptosis and autophagy: multiple roles of the co-chaperone BAG3 in stress and therapy resistance of cancer. Cells 9, 574 (2020).
McCluggage, F. & Fox, A. H. Paraspeckle nuclear condensates: global sensors of cell stress? Bioessays 43, e2000245 (2021).
Dzwonek, J. & Wilczynski, G. M. CD44: molecular interactions, signaling and functions in the nervous system. Front. Cell Neurosci. 9, 175 (2015).
Tuohy, T. M. F. et al. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia 47, 335–345 (2004).
Steelman, A. J. et al. Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiol. Dis. 91, 336–346 (2016).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Tian, Y. et al. CXCL12 induces migration of oligodendrocyte precursor cells through the CXCR4‑activated MEK/ERK and PI3K/AKT pathways. Mol. Med. Rep. 18, 4374–4380 (2018).
Kang, M. & Yao, Y. Laminin regulates oligodendrocyte development and myelination. Glia 70, 414–429 (2022).
Mierzwa, A. J., Zhou, Y. -X., Hibbits, N., Vana, A. C. & Armstrong, R. C. FGF2 and FGFR1 signaling regulate functional recovery following cuprizone demyelination. Neurosci. Lett. 548, 280–285 (2013).
Rajendran, R., Giraldo-Velásquez, M., Stadelmann, C. & Berghoff, M. Oligodendroglial fibroblast growth factor receptor 1 gene targeting protects mice from experimental autoimmune encephalomyelitis through ERK/AKT phosphorylation. Brain Pathol. 28, 212–224 (2018).
Rajendran, R. et al. Oligodendrocyte-specific deletion of FGFR1 reduces cerebellar inflammation and neurodegeneration in MOG35–55-induced EAE. Int. J. Mol. Sci. 22, 9495 (2021).
Kamali, S. et al. Oligodendrocyte-specific deletion of FGFR2 ameliorates MOG35–55-induced EAE through ERK and Akt signalling. Brain Pathol. 31, 297–311 (2021).
Bansal, R. & Pfeiffer, S. E. FGF-2 converts mature oligodendrocytes to a novel phenotype. J. Neurosci. Res. 50, 215–228 (1997).
Thümmler, K. et al. Polarizing receptor activation dissociates fibroblast growth factor 2 mediated inhibition of myelination from its neuroprotective potential. Acta Neuropathol. Commun. 7, 212 (2019).
Jin, S., Plikus, M. V. & Nie, Q. CellChat for systematic analysis of cell–cell communication from single-cell transcriptomics. Nat. Protoc. 20, 180–219 (2025).
Chadarevian, J. P. et al. Therapeutic potential of human microglia transplantation in a chimeric model of CSF1R-related leukoencephalopathy. Neuron https://doi.org/10.1016/j.neuron.2024.05.023 (2024).
Munro, D. A. D. et al. Microglia protect against age-associated brain pathologies. Neuron https://doi.org/10.1016/j.neuron.2024.05.018 (2024).
McNamara, N. B. et al. Microglia regulate central nervous system myelin growth and integrity. Nature https://doi.org/10.1038/s41586-022-05534-y (2022).
González-Velasco, Ó., Sanchez-Luis, E., Rosa, E. D. L., Sánchez-Santos, J. M. & Rivas, J. D. L. A single-cell clusters similarity measure for different batches, datasets, and samples. Preprint at bioRxiv https://doi.org/10.1101/2022.03.14.483731 (2022).
Rothstein, J. D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Yi, J. -H. & Hazell, A. S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 48, 394–403 (2006).
Zukerberg, L. R. et al. Loss of cables, a cyclin-dependent kinase regulatory protein, is associated with the development of endometrial hyperplasia and endometrial cancer. Cancer Res. 64, 202–208 (2004).
Ohta, Y., Hartwig, J. H. & Stossel, T. P. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 8, 803–814 (2006).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Hou, J. et al. Transcriptomic atlas and interaction networks of brain cells in mouse CNS demyelination and remyelination. Cell Rep. 42, 112293 (2023).
Jacobo-Albavera, L., Domínguez-Pérez, M., Medina-Leyte, D. J., González-Garrido, A. & Villarreal-Molina, T. The role of the ATP-binding cassette A1 (ABCA1) in human disease. Int. J. Mol. Sci. 22, 1593 (2021).
Karasinska, J. M. et al. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 29, 3579–3589 (2009).
Wheeler, M. A. et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596 (2019).
Clark, I. C. et al. Identification of astrocyte regulators by nucleic acid cytometry. Nature 614, 326–333 (2023).
Liu, C. -C. et al. Cell-autonomous effects of APOE4 in restricting microglial response in brain homeostasis and Alzheimer’s disease. Nat. Immunol. 24, 1854–1866 (2023).
Fields, J. & Ghorpade, A. C/EBPβ regulates multiple IL-1β-induced human astrocyte inflammatory genes. J. Neuroinflammation 9, 177 (2012).
Shaftel, S. S., Griffin, W. S. T. & O’Banion, M. K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation 5, 7 (2008).
Ali, Z. S., Van Der Voorn, J. P. & Powers, J. M. A comparative morphologic analysis of adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia-a role for oxidative damage. J. Neuropathol. Exp. Neurol. 66, 660–672 (2007).
Alturkustani, M., Keith, J., Hazrati, L. -N., Rademakers, R. & Ang, L. -C. Pathologic staging of white matter lesions in adult-onset leukoencephalopathy/leukodystrophy with axonal spheroids. J. Neuropathol. Exp. Neurol. 74, 233–240 (2015).
Chen, Y. et al. The functions and roles of sestrins in regulating human diseases. Cell. Mol. Biol. Lett. 27, 2 (2022).
Pereira, B. I. et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 21, 684–694 (2020).
Phipson, B. et al. Propeller: testing for differences in cell type proportions in single cell data. Bioinformatics 38, 4720–4726 (2022).
Teng, X. -Y. et al. OPALIN is an LGI1 receptor promoting oligodendrocyte differentiation. Proc. Natl Acad. Sci. USA 121, e2403652121 (2024).
Kenigsbuch, M. et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat. Neurosci. 25, 876–886 (2022).
Pan, J. et al. Deciphering glial contributions to CSF1R-related disorder via single-nuclear transcriptomic profiling: a case study. Acta Neuropathol. Commun. 12, 139 (2024).
Safaiyan, S. et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Pasquini, L. A. et al. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 18, 1746–1756 (2011).
Thomas, L. & Pasquini, L. A. Galectin-3-mediated glial crosstalk drives oligodendrocyte differentiation and (re)myelination. Front. Cell Neurosci. 12, 297 (2018).
de Jong, C. G. H. M., Gabius, H. -J. & Baron, W. The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell. Mol. Life Sci. 77, 1289–1317 (2020).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Zhou, Y. et al. Human early-onset dementia caused by DAP12 deficiency reveals a unique signature of dysregulated microglia. Nat. Immunol. 24, 545–557 (2023).
Paloneva, J., Autti, T., Hakola, P. & Haltia, M. J. Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy. in GeneReviews (eds. M. P. Adam et al.) (University of Washington, 1993).
Chitu, V. et al. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP). Neurobiol. Dis. 74, 219–228 (2015).
Chitu, V. et al. Microglial homeostasis requires balanced CSF-1/CSF-2 receptor signaling. Cell Rep. 30, 3004–3019 (2020).
Stables, J. et al. A kinase-dead Csf1r mutation associated with adult-onset leukoencephalopathy has a dominant inhibitory impact on CSF1R signalling. Development 149, dev200237 (2022).
Dulski, J. et al. Hematopoietic stem cell transplantation in CSF1R-related leukoencephalopathy: retrospective study on predictors of outcomes. Pharmaceutics 14, 2778 (2022).
Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 23, 580–594 (2023).
Acknowledgements
We thank C. Mirescu (Vigil), S. Brioschi (Washington University in St. Louis) and J. P. Chadarevian (University of California, Irvine) for critical reading and helpful suggestions; C. Chen (Washington University in St. Louis) for providing Oil Red O reagent; A. Watson and the Anatomic and Molecular Pathology Core Labs (Washington University in St. Louis) for helping with histology. We also thank J. Hou (Washington University in St. Louis), J. Han (Washington University in St. Louis) and Z. Miao (University of Pennsylvania) for insightful discussions on transcriptomic analysis. We appreciate the support from L. Spears and M. Castillo (10x Genomics), as well as J. Ponce and the GTAC core (Washington University in St. Louis), for their assistance with spatial profiling. This work was supported by National Institutes of Health grants RF1 AG051485, R21 AG059176 and P01 AG078106; the Cure Alzheimer’s Fund; and Fred and Ginger Haberle Charitable Fund at East Texas Communities Foundation to M. Colonna, and Grant SPP2395 from the German Research Foundation to C.G.B. The Global Leukodystrophy Initiative Clinical Trials Network Pilot Project Career Development Award to J.L.O.-M. M.T. was supported by the Promotion of Science (JSPS, 22H02995). A.K. is supported by the Strategic Research Program for Brain Science from AMED (JP23wm0425019) and the Collaborative Research Project of the Brain Research Institute, Niigata University, Japan.
Author information
Authors and Affiliations
Contributions
S.D., Y.Z. and M. Colonna designed the study and interpreted the results. S.D. and Y.Z. prepared single-nuclei samples and performed computational analyses. D.L., M. Cella, H.H., J.W., J.L., Z.C. and J.K. assisted with the analyses. C.G.B., J.L.O.-M., M.T. and A.K. provided human postmortem brain samples and clinical advice. S.D., Y.Z., J.K. and M. Colonna wrote the manuscript with feedback from the authors.
Corresponding author
Ethics declarations
Competing interests
M. Colonna is a member of the scientific advisory board of Vigil, NGMBio, Cartesian, Cell Signaling Technology and Halyard; receives research support from Vigil and Ono Pharmaceutical; and has patents pending on TREM2 and LILRB4. J.L.O.-M. is a site principal investigator for Vigil Neuroscience clinical trials. J.K. is cofounder of Rho Bio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks David Hume, Clare Pridans and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: S. Houston, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quality controls and cluster characterization of brain cells in control and ALSP samples.
a. Bar graphs of median of number of genes and median of number of UMIs of each sample sequenced in each batch. b. UMAP plot of control and ALSP samples before batch correction. c-e. UMAP plots of control and ALSP samples after batch correction by Harmony. UMAP plots are split by different samples, genders and regions respectively. f. Heatmap showing the expression of marker genes for each cell type. g. Nuclei frequencies for all cell types in each sample. For the brain regions, frontal cortex (FC), occipital cortex (OC), subcortical regions (SC), parietal and temporal lobes, basal ganglia (BG), and white matter (WM) were assessed. h. UMAP plots depicting the expression of feature genes in different cell types. i. Box plots showing average individual expression levels for CSF1R, CSF2RA, CSF3R in the myeloid cell cluster. P values were calculated by two-sided Wilcoxon rank-sum test (n = 13 controls; n = 6 ALSP). j. Scatter plot showing linear regression of average expression levels between CSF1R and CSF2RA, or CSF3R. Gray shading represents the 95% confidence interval, and Pearson’s correlation coefficient and P value are shown.
Extended Data Fig. 2 Cluster characterization of the myeloid cell subsets in control and ALSP samples.
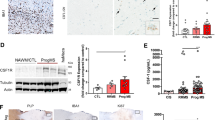
a. UMAP plots of expression of feature genes in different myeloid clusters (re-cluster from the Micro cluster in Fig. 1a). b. Heatmap showing the average gene expression of the top upregulated microglia genes in ALSP versus healthy control samples. c. Box plots showing average individual expression levels for selected DEGs in the myeloid cell cluster. P values were calculated by two-sided Wilcoxon rank-sum test (n = 13 controls; n = 6 ALSP). d. UMAP plot of gene module score of genes related to ferroptosis pathway (Wikipathway: WP4313) (left panel); Violin plot of FTL expression in myeloid cells between the conditions (right panel). P values were determined by two-sided Wilcoxon rank-sum test. e. UMAP plots of control and ALSP brain myeloid cells grouped by different brain regions. For the brain regions, frontal cortex (FC), occipital cortex (OC), subcortical regions (SC), parietal and temporal lobes, basal ganglia (BG), and white matter (WM) were analyzed. f-h. Violin plots displaying the gene set scores of DEGs derived from two AD datasets (f), two MS datasets (g), and LDAM dataset (h) in control and ALSP myeloid cells. The gene set scores were calculated by UCell. P values were determined by two-sided Wilcoxon rank-sum test. Box center lines, bounds of boxes and whiskers indicate median, first and third quartiles and minima and maxima within a 1.5× IQR, respectively. i. Representative images of Oil Red O, CD163 and Hematoxylin staining in the white matter of control and ALSP brain samples. j-k. Scatter plot showing linear regression of average expression levels between CSF1R and selected genes, revealing they are positively (j) or negatively (k) correlated with CSF1R. Gray shading represents the 95% confidence interval; Pearson’s correlation coefficient and P value are shown.
Extended Data Fig. 3 Integration of white matter data with a public dataset, and cluster characterization of myeloid cell subsets in control and ALSP samples.
a. Representative IHC images of CD163 staining in control and ALSP brain samples (left), with corresponding quantification (right). Each data point represents the value from a single image. For each individual, 5 images were taken and analyzed. Statistical significance was assessed using a non-parametric t-test. Data are shown as box-and-whisker plots (min to max) with all data points displayed. Images from individual donors (n = 3 controls; n = 2 ALSP) are denoted by distinct shapes (circle, triangle, or rectangle). Scale bar = 100 µm. b. Representative IHC images of LGALS3 staining in control and ALSP brain samples (left), with corresponding quantification (right). Each data point represents the value from a single image. For each individual, 5 images were taken and analyzed. Statistical significance was assessed using a non-parametric t-test. Data are shown as box-and-whisker plots (min to max) with all data points displayed. Images (n = 15 controls; n = 10 ALSP) from individual donors (n = 3 controls; n = 2 ALSP) are denoted by distinct shapes (circle, triangle, or rectangle). Scale bar = 100 µm. c. UMAP plot of nuclei from the white matter (WM) snRNA data from Absinta et al., and the current study before integration. d. UMAP plot of nuclei isolated from the white matter brains of controls and individuals with ALSP after integration; n = 39,624 total nuclei; n = 5 controls and n = 4 ALSP patients. Ex_neuron, excitatory neurons; In_neuron, inhibitory neurons; Oligo, oligodendrocytes; OPC, oligodendrocyte precursor cells; Astro, astrocytes; Micro, microglia; Endo, endothelial cells; Stroma, stromal cells; IMM, immune cells. e. UMAP plots of nuclei isolated from the white matter brains of controls and individuals with ALSP after integration, grouped by individuals. In this analysis, nuclei from the current study (Control-1 and Control-4) were renamed as Control-1 and Control-2, respectively; nuclei from three control white matter samples from Absinta et al. (CTRL1, CTRL2, CTRL3) were renamed as Control-3, Control-4, and Control-5. f. UMAP plots of control and ALSP white matter brain macrophages. g. Nuclei frequency of each myeloid cell subsets across all samples. h. Venn diagrams of overlapping genes commonly upregulated in ALSP microglia from the white matter and ALSP microglia from all regions.
Extended Data Fig. 4 Cluster characterization of OLs in control and ALSP samples.
a. Dot plot showing expression of marker genes for each OL subset. b. Heatmap showing the gene set scores of the OL subsets from Pandey et al. and Sadick et al., on the OL subset identified in the current study. c. Nuclei frequency of each OL subset across all samples. d. Venn diagrams of overlapping genes commonly upregulated in ALSP OLs and signature genes of Oligo4 subset. e. Heatmap showing the average gene expression of the top upregulated genes in ALSP versus control OLs. f. Violin plots displaying the gene set scores of ALSP, AD and MS OLs, and MAPK signaling pathway in control and ALSP OLs. The gene set scores were calculated by UCell. P values were determined by two-sided Wilcoxon rank-sum test. Box center lines, bounds of boxes and whiskers indicate median, first and third quartiles and minima and maxima within a 1.5× IQR, respectively. g. Representative images of IHC staining of MBP (i), NF200 (ii), TPPP (iii), GFAP (iv) in ALSP brain sections; the grey matter (GM) and white matter (WM) are indicated. Scale bar = 500 µm except for iii where is 50 µm. Arrowheads highlight OLs with strong expression of TPPP/p25 in the subcortex (left) and weak to no expression in the progressing lesion (middle to right) on the same tissue section. h. Dot plot showing expression of marker genes for each subset of OL-lineage cells. i. IPA analysis of OLs identifies FGF2 as a significant upstream regulator activated in ALSP OLs. P-value was determined using Fisher’s exact test. j. Representative immunofluorescent images of GFAP and FGF2 staining in control and ALSP brain samples (left), with corresponding quantification of FGF2⁺ nuclei per image (right). Statistical significance was assessed using a non-parametric t-test. Each data point represents the value from a single image. Statistical significance was assessed using a non-parametric t-test. Data are shown as box-and-whisker plots (min to max) with all data points displayed. Images (n = 15 controls; n = 10 ALSP) from individual donors (n = 3 controls; n = 2 ALSP) are denoted by distinct shapes (circle, triangle, diamond, or rectangle). Scale bar = 100 µm.
Extended Data Fig. 5 Cluster characterization of white matter OLs in control and ALSP samples.
a. UMAP plot of white matter OLs (re-cluster from the Oligo cluster in Extended Data Fig. 3d) from the integrated dataset. b. Donut plots showing nuclei distribution of each OL subset in control or ALSP samples. c. Nuclei frequency of each OL subsets across all samples. d. Venn diagrams of overlapping genes commonly upregulated in ALSP OLs from the white matter and ALSP OLs from all regions. e. Pan-cell type relative information flow showing signaling pathways identified in ALSP (in red) and control (blue) white matter cells from the CellChat analysis. f. Chord diagrams plotting FGF signaling strength with the senders and receivers with their predicted ligands and receptors from the CellChat analysis. The lines represent changes in ligand-receptor interaction strength. g. Circle plot showing directed interaction of selected signaling pathways between cell types from the CellChat analysis.
Extended Data Fig. 6 Cluster characterization of astrocytes in control and ALSP samples.
a. Representative IHC images showing GFAP staining in the frontal cortex of control and ALSP brain samples (left), with corresponding quantification (right). Statistical significance was determined using a non-parametric t-test. Data are presented as box-and-whisker plots with all data points; n = 8 control, n = 3 ALSP. Scale bar = 20 µm. b. Dot plot showing expression of marker genes for each astrocyte subset. c. Nuclei frequencies of each astrocyte subset across all samples. d. Heatmap showing the average gene expression of the top upregulated genes in ALSP versus control astrocytes. e. Molecular Function (MF) pathways enriched in ALSP OLs. f. Box plots showing average individual expression levels for selected DEGs in astrocytes. P values were calculated by two-sided Wilcoxon rank-sum test. g. UMAP plots of expression of IL1B across all cell types. h. Representative IHC images showing ASC staining in early-stage (left) and advanced-stage (right) ALSP white matter lesions. Increased ASC signal is observed in advanced lesions, indicating enhanced inflammasome activation. Scale bar = 50 µm.
Extended Data Fig. 7 Cluster characterization of white matter astrocytes in control and ALSP samples.
a. UMAP plot of the integrated dataset of white matter cells from ALSP and control brains. b. UMAP plot of astrocytes (re-cluster from the Astro cluster in Extended Data Fig. 3d) from the integrated dataset. c. Nuclei frequencies of each astrocyte subset across all samples. d. Dot plot showing expression of marker genes for each astrocyte subset. e. Volcano plot depicting DEGs in ALSP versus control astrocytes in the white matter. f. GO-MF enriched in astrocytes from ALSP individuals. g. GO-MF enriched in astrocytes from controls in comparison to ALSP.
Extended Data Fig. 8 Cluster characterization of endothelial, stromal, and immune cells in control and ALSP samples.
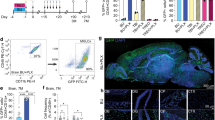
a. Volcano plot depicting DEGs in ALSP versus control capillary blood endothelial cells (cBEC). b. Molecular function (MF) enriched in cBEC from ALSP (upper panel) or control (lower panel) individuals. c. IPA pathways predicted to be activated (upper panel) or inhibited (lower panel) in ALSP cBEC. d. UMAP plot of stromal cells re-cluster from the Stroma and Peri_Fibro clusters. e. Dot plot showing expression of marker genes for each stromal subset. f. Volcano plot depicting DEGs in ALSP versus control pericytes. g. Molecular Function (MF) enriched in perivascular fibroblasts from ALSP compared to controls. h. IPA of pathways predicted to be activated in ALSP pericytes. i. UMAP plots of immune cells re-cluster from the IMM clusters. j. Dot plot showing expression of marker genes for each immune cell subset. k. UMAP plots of immune cells from different individuals. l. Violin plots displaying the expression of selected genes in control and ALSP immune cells.
Extended Data Fig. 9 Xenium in situ spatial characterization in control and ALSP white matter.
a. Representative hematoxylin-stained images and immunofluorescent images of control (left) and ALSP (right) white matter samples used for spatial transcriptomic profiling. The cell boundary staining, aSMA/Vimentin and DAPI staining were performed in the Xenium multimodal Cell Segmentation Staining workflow. Scale bar = 1000 μm. b. Heatmap showing the expression of marker genes for each cell type. c. Dot plot showing expression of marker genes for each OL subset. d. Spatial maps of control (left) and ALSP white matter (right) samples showing MOG and ST18 expression. Scale bar = 1000 μm. e. Spatial maps of control (left) and ALSP white matter (right) samples showing BAG3, SERPINA3, IFITM3 expression. Scale bar = 1000 μm. f. Spatial maps of control (left) and ALSP (right) white matter samples displaying module scores for the glial cell differentiation gene ontogeny pathway. Differences in spatial distribution suggest altered glial maturation in ALSP. Scale bar = 1000 μm. g. Spatial maps of control (left) and ALSP (right) white matter samples showing module scores for the regulation of response to stress gene ontogeny pathway. Increased stress-related transcriptional signatures are evident in ALSP white matter. Scale bar = 1000 μm.
Supplementary information
Supplementary Information
Supplementary Table 1 Participant information and CSF1R mutations carried by individuals assessed in the study. Supplementary mutation information and references.
Supplementary Table 2
DEGs of ALSP versus control in each cell type.
Supplementary Table 3
Raw data used for IHC and immunofluorescence quantification.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, S., Zhou, Y., Li, D. et al. Mutations in the human CSF1R gene impact microglia’s maintenance of brain white matter integrity. Nat Immunol 26, 1198–1211 (2025). https://doi.org/10.1038/s41590-025-02195-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-025-02195-7