Abstract
Th1/Th2 balances may play a vital role in the processes of inflammation and fibrosis. The Th1/Th2 paradigm can be evaluated by representing IFN-γ for Th1 and IL-4 for Th2. OM-85 BV encouraged preferential development of the Th1-type immunity characterized by amplified IFN-γ and decreased IL-4 production. This study aimed to evaluate the inhibitory effect of OM85 on bleomycin (BLM)-induced pulmonary fibrosis in C57 and its possible mechanisms. In vitro experiments demonstrated that OM85 exhibited no significant toxicity to HELF cells. OM-85 inhibited the TGF-β1-induced protein expression of Notch1 and Hes1 and reduced the fibrosis-related marker profiles, such as collagen I, collagen III, fibronectin, P21, and α-SMA, following TGF-β1 treatment of these cells. Immunofluorescence also revealed that OM-85 decreased the expression of α-SMA induced by TGF-β1 in HELF cells. In the vivo experiments, a pulmonary fibrosis model was established by administering three intratracheal doses of BLM (1 mg/kg). The BLM-OM85 group was exposed to an aerosol containing 10.5 mg of OM-85 dissolved in 10 mL of sterile PBS on days 42, 44, 46, 49, 51, and 53. BLM-induced pulmonary fibrosis, leading to increased levels of lung hydroxyproline, total cell count, macrophages, neutrophils, lymphocytes, and the expression of TGF-β1 as well as Notch1 and Hes1 in lung tissue, along with fibrosis-associated proteins such as collagen I, collagen III, fibronectin, P21, and α-SMA. Additionally, the Th1 response was suppressed, as evidenced by decreased IFN-γ in the bronchoalveolar lavage fluid (BALF), while the Th2 response was amplified, marked by increased IL-4 levels in BALF. Moreover, morphological assessments showed that BLM caused increased Ashcroft scores, relative collagen content, and an expanded damaged area, as well as an increased optical density (OD) of collagen I. The administration of OM-85 significantly mitigated these effects. These findings suggest that OM-85 holds therapeutic potential for BLM-induced pulmonary fibrosis in female C57 mice, partly due to the inhibition of Notch1 and Hes1 expression and the modulation of the IFN-γ/IL-4 ratio.
Similar content being viewed by others
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a chronic interstitial lung disease characterized by the progressive scarring of alveolar-capillary units. This scarring process leads to alveolar collapse, impaired gas exchange, reduced lung compliance, and ultimately, respiratory failure. Distinguished as a specific form of interstitial pneumonia, IPF’s cause remains elusive. Statistically, the median survival period post-diagnosis of IPF spans merely 3 to 5 years, predominantly impacting the elderly population1,2,3.
Pulmonary fibrosis exhibits a notably poor response to traditional anti-inflammatory treatments4,5. The exact mechanisms underlying Idiopathic Pulmonary Fibrosis (IPF) remain unclear; however, the balance between Th1 and Th2 cells is thought to play a critical role in inflammation and fibrosis. Animal model studies show that a Th2-dominant response to tissue damage increases susceptibility to pulmonary fibrosis after lung injury, compared to a Th1-dominant response. Under normal physiological conditions, a dynamic equilibrium between Th1 and Th2 cells is crucial for maintaining cellular immunity and humoral functions6. Research indicates that the secretion of Th1/Th2 cytokines can either suppress or facilitate the progression of pulmonary fibrosis, contingent upon their regulatory impacts7,8.
The Notch signaling pathway, first identified by Morgan et al. in the fruit fly Drosophila melanogaster in 1917, is named for the notched appearance of the wing margins in mutants with partial loss of function9. It is now well-established that the Notch signaling pathway is pivotal in cell fate decisions, including survival or apoptosis, as well as in proliferation, differentiation, and the maintenance of stem cell quiescence and identity. There is an increasing consensus that Notch signaling is vital for the development and maintenance of various organs and tissues, notably the lungs and immune system. Given these insights, targeting the inhibition of Notch signaling and modulating the IFN-γ/IL-4 ratio—emerges as a promising strategy for therapeutic intervention in fibrotic diseases10. Based on these concepts, targeted inhibition of Notch and regulation of the IFN-γ/IL-4 ratio represent possible strategic points for therapeutic intervention of fibrosis disease.
Anti-inflammatory treatments have proven ineffective for Pulmonary Fibrosis, occasionally exacerbating the condition. Conversely, we posit that patients with Idiopathic Pulmonary Fibrosis (IPF) might derive benefit from treatments aimed at enhancing the immune response rather than from immunosuppressive therapies, which have been ineffective or detrimental5,6,11. Current strategies for managing IPF emphasize the inhibition of extracellular matrix production, with Nintedanib (a tyrosine kinase inhibitor) and Pirfenidone (with an incompletely understood mechanism) as leading examples12. Nonetheless, the efficacy of these agents is limited, offering merely a modest slowdown in the progressive deterioration of lung function in IPF patients. The tolerability of Nintedanib and Pirfenidone is often poor, particularly among elderly patients or those with advanced fibrotic interstitial lung diseases, which can hinder treatment adherence. Therefore, there is a continuing need to explore new therapeutic approaches that can either cure or significantly alleviate symptoms5.
The OM-85 BV preparation, comprising lysates from eight bacterial pathogens frequently implicated in respiratory tract infections—Haemophilus influenza, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans, and Neisseria catarrhalis—has been noted for its role in promoting a Th1-type immune response in newborn animals. This response is characterized by increased production of IFN-γ and reduced IL-4 levels13. Research indicates that OM-85 enhances the body’s immune defenses, boosting pulmonary resistance to various lethal microbial infections in a non-specific manner. There is substantial evidence supporting the safety and effectiveness of OM-85 in preventing respiratory infections across all ages, including individuals with chronic bronchitis or mild chronic obstructive pulmonary disease (COPD), independent of the infection’s etiology14,15,16,17. Its application in clinical settings has shown significant therapeutic benefits in managing respiratory infections, asthma, and allergic rhinitis, conditions typically dominated by Th2-type immune responses5,18,19. Interestingly, OM-85 administration has been observed to skew the immune response towards Th1 dominance, which may underlie its therapeutic efficacy. In the context of Idiopathic Pulmonary Fibrosis (IPF), clinical observations suggest that OM-85 administration may decelerate disease progression in some patients, hinting at its potential utility in modulating immune responses in pulmonary fibrosis20. These findings highlight OM-85’s regulatory role in lung fibrosis; however, the exact mechanisms and its broader implications for fibrosis management are yet to be fully understood.
In the present study, we investigated the protective effect of OM85 ameliorating BLM-induced pulmonary fibrosis to further elucidate the underlying mechanism of OM85-antagonized pulmonary fibrosis: inhibition of Notch and regulation of IFN-γ/IL-4 ratio.
Materials and methods
Animals, model of BLM-induced pulmonary fibrosis and OM85 treatment
Female (The efficacy of OM85 is not gender-specific. Female mice were selected for this study primarily to maintain experimental consistency, as well as because they are generally more docile and easier to handle for modeling purposes) C57BL/6 mice, specific pathogen-free (SPF), weighing 18.50 ± 0.88 g and aged 8 weeks, were obtained from Vital River Laboratory Animal Technology Co., Ltd., Beijing, China. Upon their arrival, the mice were accommodated in an SPF facility, with the environment controlled at a temperature of 22–26 °C and humidity between 60–70%. Their health status was closely monitored for three days before their random allocation into four experimental groups as follows: (1) NaCl-PBS, n = 12; (2) NaCl-OM85, n = 12; (3) BLM-NaCl, n = 28; and (4) BLM-OM85, n = 20. During the modeling process, anesthesia and tracheal instillation are required. Despite careful handling, there is a risk of asphyxiation and death in mice. Furthermore, BLM induces IPF, with fibrosis progressively worsening, leading to inevitable mortality. However, deceased mice are excluded from the statistical analysis. To ensure adequate sample size for data analysis, each group includes 10 mice.
The animal experiments were carried out within two weeks, strictly adhering to the laboratory animal management regulations of Tongji Medical College, Huazhong University of Science and Technology. The Institutional Animal Care and Use Committee (IACUC) of Tongji Medical College approved all experimental procedures, ensuring compliance with relevant guidelines and regulations, including the ARRIVE guidelines. Furthermore, all anesthesia and euthanasia procedures were conducted in line with the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020 edition), reflecting the highest standards of veterinary care21.
Pulmonary fibrosis was induced via three intratracheal instillations of bleomycin (1 mg/kg) administered bi-weekly (on days 0, 14, and 28) under isoflurane anesthesia, minimizing distress to the animals. An exposure chamber crafted from transparent polyethylene, with dimensions of 35 × 25 × 20 cm3, was linked to a nebulizer’s outlet (model NE-C900, Omron, Dalian, China) to facilitate the aerosolized OM85 exposure. The exposure process was conducted until the liquid was completely nebulized, which generally took about 30 min. This duration ensured that the mice were adequately exposed to the aerosolized OM85 for the entirety of the nebulization process.
Subsequently, the mice were exposed to an aerosol containing 10.5 mg of OM85 dissolved in 10 mL of sterile PBS solution, or a control aerosol of 10 mL sterile PBS solution alone, on days 42, 44, 46, 49, 51, and 53. The schematic representation of the experimental procedure is depicted in Fig. 1.
Schematic representation of the experimental procedure. Pulmonary fibrosis was induced via three intratracheal instillations of bleomycin (1 mg/kg) administered bi-weekly (on days 0, 14, and 28) under isoflurane anesthesia. Subsequently, the mice were exposed to an aerosol containing 10.5 mg of OM85 dissolved in 10 mL of sterile PBS solution, or a control aerosol of 10 mL sterile PBS solution alone, on days 42, 44, 46, 49, 51, and 53. Our experiment was structured every week to accommodate the need for rest days for the experimenters. Consequently, we decided to administer the treatment on Monday (Day 42), Wednesday (Day 44), and Friday (Day 46), continuing the same pattern in the following week with treatment on Monday (Day 49), Wednesday (Day 51), and Friday (Day 53). This approach inadvertently led to the omission of aerosol delivery on Day 48, a Sunday.
Our experiment was structured every week to accommodate the need for rest days for the experimenters. This scheduling aligns with a previous study on OM85, which indicated that administering the treatment every three days was effective14. Consequently, we decided to administer the treatment on Monday (Day 42), Wednesday (Day 44), and Friday (Day 46), continuing the same pattern in the following week with treatment on Monday (Day 49), Wednesday (Day 51), and Friday (Day 53). This approach inadvertently led to the omission of aerosol delivery on Day 48, a Sunday, when no treatments were scheduled due to our adherence to a weekly operational protocol.
The study concluded on day 56, at which point ten mice from each group were humanely euthanized following an intraperitoneal injection of sodium pentobarbital (100 mg/kg). Euthanasia was achieved by exsanguination through severing the abdominal aorta, in strict accordance with AVMA guidelines.
Agents
Bleomycin hydrochloride was purchased from Hi Sun Pharmaceutical Co., Ltd, Zhejiang, China. Broncho-Vaxom™ (batch no. S20150042, OM Pharma, Switzerland), a polyvalent bacterial lysate mixture, was used as the immunostimulant. Drawing from pertinent studies in humans, it has been observed that the inhaled dose of an aerosolized medication deposited in the lungs constitutes only about 20–30% of the nominal dose, even when using a facial mask22. Consequently, determining the appropriate dose of OM85 for mice involved not just the conversion of the equivalent oral dose from humans to mice but also accounting for the fraction lost during aerosol inhalation23. As such, the quantity of OM85 estimated to be deposited in a mouse’s lung with each exposure was calculated to be approximately 200–300 μg. To simplify the process, we dissolved three pills of OM85, totaling 10.5 mg, in phosphate-buffered saline (PBS), and this aerosol solution was distributed among 10 mice. This approach was adopted for both the NaCl-OM85 and BLM-OM85 groups, without distinction based on the animals’ body weight.
TGF-β1(batch no. ELK2792, Biotechnology Co., Ltd., Wuhan, China), Interleukin-4(batch no. ELK1153, Biotechnology Co., Ltd., Wuhan, China), and interferon-γ (batch no. ELK1132, Biotechnology Co., Ltd., Wuhan, China) levels in the BALF were measured using an enzyme-linked immunosorbent assay. Other chemicals and reagents were of analytical grade.
Bronchoalveolar lavage
The trachea of each animal was intubated with an 18-gage catheter soon after the animal was euthanized. Bronchoalveolar lavage fluid (BALF) was obtained by the injection of 0.8 ml phosphate-buffered saline (PBS; pH 7.4, three times, yielding a total of 2.4 ml) followed by gentle aspiration of the fluid. The fluid recovered was filtered and centrifuged at 395 g for 10 min at 4 °C, and the supernatant was collected and stored at − 80 °C for the subsequent measurement of cytokines. Cells in the BALF were resuspended in PBS and counted using a hemocytometer. Smears of the cellular precipitate were examined under a microscope after Wright staining to differentiate between neutrophils, lymphocytes, and macrophages.
Determination of the hydroxyproline content in lung tissues
Hydroxyproline is a major component of fibrillar collagen of all types. It can be used as a reliable biomarker for fibrosis quantitation. The hydroxyproline content in lung tissues was measured using a hydroxyproline assay kit (batch no. A030-2, Jian Cheng.
Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. Briefly, a precisely weighed lung homogenate was hydrolyzed with 5% sodium hydroxide, mixed with color-indicative reagent, and titrated to pH 6.0–6.8 when the mixture became flavescent. After centrifugation, the contents of supernatants were measured at 560 nm using a spectrophotometer The quantification of hydroxyproline was performed referring to the established standard curve.
Pulmonary morphology and morphometry
The histological procedures were carried out using previously reported methods. Briefly, the animals were intratracheally intubated, and the right lungs were removed then bloc by ligation of the right main bronchus. The left lungs were infused with 4% paraformaldehyde (PFA) solution via a tracheal catheter at a constant hydrostatic pressure of 20 cm for at least 20 min until the pleural surface became smooth. The left lungs were fully immersed in PFA solution for at least 4 h before further histological processing. The right lungs were stored at 0 °C for further biochemical measurements. For morphological and morphometric analysis, the PFA-fixed lungs were paraffin-embedded, and sagittal sections with a thickness of 4 µm were cut for each lung sample. The sections were then deparaffinized and stained with Hematoxylin & Eosin (H&E), Sirius red, and Masson’s trichrome, respectively.
The morphometric evaluation in this study was conducted to assess the extent of fibrosis in the lung using a modified Ashcroft scale. This scale assigned grades ranging from 0 to 8 based on the severity of fibrosis in alveolar septa and lung structure. The higher the grade, the more severe the fibrosis. The assessment was conducted on five sections from the central region of a lung specimen. For each section, ten distinct pulmonary microscopic fields were randomly chosen. These fields were examined at 20X magnification using an Olympus DP72 camera system. Overall, the morphometric evaluation using the modified Ashcroft scale provides a quantitative assessment of fibrosis in the lung, which is crucial for understanding the pathophysiology of pulmonary fibrosis and developing effective treatments.
Immunohistochemical detection of collagen I in lung tissue
For immunohistochemistry assays, paraffin-embedded lung tissue sections were deparaffinized and subjected to antigen retrieval. The paraffin sections were successively deparaffinized with xylene and dehydrated with ethanol. Experimental procedures followed the instructions provided in the immunohistochemical staining kit (Santa Cruz Biotechnology, USA). Staining was conducted using DAB solution (Santa Cruz Biotechnology, USA), and counterstaining was performed with hematoxylin. Image Pro Plus 6.0 was utilized to quantify five images per slide for each of the four groups, assessing integral optical density (IOD) and positive area (Area). The mean optical density (MOD = IOD/Area) was then calculated to evaluate collagen I expression.
Western blot
We extracted proteins from mouse lung tissues or cells using a special buffer with added protective agents. We measured the amount of protein with a BCA kit. Then, we separated the proteins using a gel method (SDS-PAGE) and moved them onto special membranes. We blocked unwanted sites on the membranes with skim milk, then added specific antibodies to detect proteins like Notch1, Hes1, collagen I, collagen III, fibronectin, P21, and α-SMA overnight in a cold environment. We used a secondary antibody for 2 h at room temperature to help see our proteins, which we then made visible with a special developing solution after washing. We cut the membranes to look at each protein separately, running them on gels based on their size. The full membrane Western blot and related experimental materials are presented in the Supplementary Material.
Cell viability assay
The CCK-8 assay was performed according to the manufacturer’s instructions. HELF cells were seeded in 96-well plates at a density of 1 × 104 cells per well. Following treatment with different doses of OM85, 10 μl of CCK-8 reagent was added and incubated for 4 h. Absorbance was measured at 450 nm using a microplate reader (DR-200Bs, USA).
Cell culture of HELF
The HELF cells (IM-H219) were obtained from Wuhan Procell Life Technology Co., Ltd. (Wuhan, China), with the passage number below 10. A fibroblast cell line was maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2 with 10% fetal bovine serum. The cells were plated into 6-, 12-, or 96-well plates one day before the experiment.
Cell treatment of HELF
The cells were seeded into plates and divided into three groups: (1) the control group, (2) the TGF-β1 group, in which cells were treated with 5 ng/mL TGF-β1 and (3) the TGF-β1 + OM85 group, in which cells were treated with OM85 (320 μg/mL) followed by TGF-β1 (5 ng/mL) 30 min later. After 48 h of TGF-β1 treatment, cells were collected for subsequent experiments.
α-SMA immunofluorescent staining
HELF cells were cultured on sterile glass slides in 24-well plates for 24 h until they reached 60%–70% confluency and then subjected to treatment with 320 μg/mL of OM85 and/or 5 ng/mL of TGF-β1 for 48 h. Following treatment, the cells were washed thrice with cold PBS and fixed with Immunol Staining Fix Solution for 30 min. Thereafter, the cells were permeabilized using an Immunostaining Permeabilization Buffer with Triton X-100 for 15 min and blocked with Quick Block TM Blocking Buffer at 37 °C for 30 min. The cells were incubated overnight at 4 °C with primary antibodies against α-SMA, followed by incubation with Cy3-labeled goat anti-rabbit (Aspen, China) for 2 h at 37 °C. Cell nuclei were stained with DAPI for 5 min at room temperature. The sections were then observed using an Olympus laser scanning confocal microscope with imaging software.
Statistical analysis
Statistical analyses were conducted in a blinded manner by a statistician using GraphPad Prism software version 9.5 (GraphPad Software Inc.). The test of normality was the Shapiro–Wilk test. If the Shapiro–Wilk test suggests a non-normal distribution, we use the Kruskal–Wallis H test. For normal distribution, after calculating the sample’s SD, we conduct ordinary ANOVA for equal SDs, and Welch’s ANOVA for unequal SDs. A multiple comparison post-hoc test was used, following the recommendation of GraphPad Prism software. P < 0.05 was considered statistically significant. All data are expressed as mean ± standard deviation.
Results
The impact of OM85 on the survival rate of IPF mice induced by BLM
Figure 2 presents the survival curves for mice throughout the study. Our aim for each experimental group is to ensure robust data analysis by maintaining a minimum of 10 mice per group. Initially, we allocated 12 mice to both the NaCl-PBS and NaCl-OM85 groups. Regrettably, due to anesthesia complications and suffocation risks, two mice from each group did not survive. Furthermore, in the BLM-NaCl and BLM-OM85 groups, BLM toxicity significantly influenced mouse mortality, compounding the aforementioned issues. By day 42 of the experiment, there were 14 surviving mice in this group that started with 28 mice, but only 10 survived until tissue collection. Similarly, the BLM-OM85 group started with 20 mice, and by day 42, 10 mice had survived, with all 10 surviving until the time of tissue collection.
shows survival curves throughout the study. Initially, 12 mice were placed in both NaCl-PBS and NaCl-OM85 groups, with two from each group dying due to anesthesia and suffocation. In the BLM-NaCl and BLM-OM85 groups, BLM toxicity further increased mortality. The BLM-NaCl group started with 28 mice, decreasing to 14 by day 42, and then to 10 by tissue collection. The BLM-OM85 group started with 20 mice, with 10 surviving to day 42, all of whom made it to tissue collection. Kaplan–Meier survival analysis in GraphPad Prism 9.5 indicated significance levels: *P < 0.05, **P < 0.01, and ns for P > 0.05.
In the initial phase of modeling, mice in both the NaCl-PBS and NaCl-OM85 groups experienced fatalities primarily due to asphyxiation during instillation or failure to recover from anesthesia, displaying similar mortality rates. This similarity suggests that these groups could be merged for analytical purposes. Upon comparing survival rates across groups, the NaCl group’s mortality was significantly higher than those of the BLM-PBS and BLM-OM85 groups, indicated by P-values of < 0.01 and < 0.05, respectively. Notably, no mortality was observed in the BLM-OM85 group post-nebulization with OM85, despite no statistical difference in mortality rates between the BLM-PBS and BLM-OM85 groups. This outcome highlights the potential protective effect of OM85 nebulization against BLM-induced mortality.
OM85 reduces BLM-induced lung cell accumulation in mice
Figure 3 illustrates the impact of OM85 on both total and differential cell counts in bronchoalveolar lavage fluid (BALF) across four groups. The BLM-PBS group induces a significant increase (P < 0.0001) in total cell counts in BALF compared to the NaCl-PBS group. In the BLM-OM85 group, a significant decrease (P < 0.01) in total cell counts is observed compared to the BLM-PBS group, signifying that treatment with OM85 reversed this change. The differential cell counts reveal a noteworthy increase in macrophages (P < 0.0001), lymphocytes (P < 0.001), and neutrophils (P < 0.001) in the BLM-PBS group compared to the NaCl-PBS group. Furthermore, the total counts of macrophages (P < 0.01), lymphocytes (P < 0.05), and neutrophils (P < 0.01) in the BLM-OM85 group are significantly lower when compared to the BLM-PBS group.
OM85 reduces BLM-induced lung cell accumulation in mice. In IPF-induced pulmonary lavage, there is an increase in the total number of cells, macrophages, lymphocytes, and neutrophils. Administration of OM85 decreases the numbers of these four cell types, alleviating the accumulation of inflammation in the lungs. After assessing the sample distribution’s normality and calculating the sample’s standard deviation (SD), ordinary ANOVA tests were conducted for total cell count and macrophages, while one-way Welch ANOVA tests were used for lymphocytes and neutrophils. Data represent means ± SD of 6 mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and ns for P > 0.05.
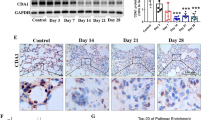
OM85 attenuated the pulmonary morphology changes induced by BLM in mouse lung
In the NaCl-PBS and NaCl-OM85 groups, lung tissues exhibited a normal histological architecture without discernible pathological changes under light microscopy, as evidenced by HE staining (Fig. 4). Conversely, the BLM-PBS group manifested notable histopathological alterations, including pronounced fibrosis as indicated by Masson staining, along with collapsed alveolar spaces, traction, and bronchiectasis in the subpleural space revealed by Sirius Red staining. Notably, the BLM-OM85 group demonstrated a reduced lesion burden compared to the BLM-PBS group across all three staining modalities (Figs. 4, 5, 6).
OM85 improves Ashcroft’s scores induced by BLM in IPF. Representative histology of Hematoxylin and eosin (H&E) staining at 20X magnification from four groups (A) and the analysis of lung tissue structure changes through Ashcroft’s scores (B). After assessing the normality of the sample distribution and calculating the standard deviation (SD) of the sample, one-way Welch ANOVA tests were conducted. Data represent means ± SD of 6 mice. *P < 0.05, **** P < 0.0001, and ns for P > 0.05.
OM85 reduces the relative collagen content induced by BLM in IPF. Representative histology of Masson staining at 20X magnification from four groups (A) and the analysis of relative collagen content (B). After assessing the normality of the sample distribution and calculating the standard deviation (SD) of the sample, ordinary ANOVA tests were conducted. Data represent means ± SD of 6 mice. *P < 0.05, **** P < 0.0001, and ns for P > 0.05.
OM85 reduces the damaged area induced by BLM in IPF. Representative histology of Sirius Red staining at 20X magnification from four groups (A) and the analysis of the damaged area (B). After assessing the normality of the sample distribution and calculating the standard deviation (SD) of the sample, one-way Welch ANOVA tests were conducted. Data represent means ± SD of 6 mice. **P < 0.01, **** P < 0.0001, and ns for P > 0.05.
To validate the impact of OM85 on BLM-induced lung fibrosis, assessments were made using Ashcroft’s scores, relative collagen content, and damaged area. Significantly lower Ashcroft’s scores, relative collagen content, and damaged area were observed in the BLM-OM85 group compared to the BLM-PBS group (Figs. 4, 5, 6), suggesting that OM85 effectively mitigates BLM-induced fibrotic changes in the lung parenchyma.
OM85 reduced the collagen disposition and HYP induced by BLM in mouse lung
The impact of OM85 on pulmonary fibrosis was thoroughly assessed by quantifying the hydroxyproline content in lung homogenates and measuring the mean intensity of collagen I through immunohistochemical staining (Fig. 7). Hydroxyproline levels (P < 0.0001) and the optical density (OD) of collagen I (P < 0.0001) exhibited a significant increase in the lungs of the BLM-PBS group compared to the NaCl-PBS group. Treatment with OM85 in the BLM-OM85 group led to a substantial reduction in hydroxyproline content (P < 0.01) and the OD of collagen I (P < 0.01) relative to the BLM-PBS group. These findings underscore the robust therapeutic potential of OM85 against BLM-induced pulmonary fibrosis, emphasizing its efficacy in inhibiting collagen deposition.
OM85 reduced the expression of collagen I in C57 lung tissue and lowered the hydroxyproline content of lung homogenates in C57 induced by BLM. Representative histology of immunohistochemistry for type I collagen in lung tissue at 20X magnification from four groups (A), the analysis of the optical density (OD) of collagen I (B), and the analysis of hydroxyproline content in lung homogenates (C). After assessing the normality of the sample distribution and calculating the standard deviation (SD) of the sample, ordinary ANOVA tests were conducted (B) and one-way Welch ANOVA tests were conducted (C). Data represent means ± SD of 6 mice (B). Data represent means ± SD of 10 mice (C). ** P < 0.01, **** P < 0.0001, and ns for P > 0.05.
OM85 reduced the expressions of Hes1, Notch1, α-SMA, P21, fibronectin, collagen I, collagen III, induced by BLM in mouse lung
In our study of pulmonary fibrosis, we explored the potential role of Notch signaling.
Using Western blot analysis, we evaluated the protein expression levels of Notch1 and Hes1 (Fig. 8). The results unveiled a notable increase in the expression of Notch1 and Hes1 in the BLM-PBS group compared to the NaCl-PBS group (P < 0.0001). and NaCl-OM85 group (P < 0.0001). Intriguingly, treatment with OM85 in the BLM-OM85 group significantly suppressed the expression of Notch1 (P < 0.01).and Hes1 (P < 0.001). in comparison to the BLM-PBS group. The Western blot data were precisely quantified and visually presented for comprehensive interpretation.
OM85 mitigates the decline of IPF-related proteins induced by BLM. Panel A represents the Western blot experiments for various proteins. Panel B analyzes Notch1 protein levels, Panel C for Hes1, Panel D for collagen I, Panel E for collagen III, Panel F for α-SMA, Panel G for fibronectin, and Panel H for P21. After assessing normality and calculating the standard deviation (SD) of the sample, ordinary ANOVA tests were performed for Notch1 and α-SMA, whereas one-way Welch ANOVA tests were utilized for Hes1, collagen I, collagen III, fibronectin, and P21. Data represent means ± SD for 10 mice. **P < 0.01, ***P < 0.001, ****P < 0.0001, and ns for P > 0.05.
α-SMA is a critical signaling molecule in idiopathic pulmonary fibrosis (IPF). Concurrently, P21 is instrumental in the disease’s pathogenesis. Bleomycin (BLM) induction of IPF results in the significant upregulation of both α-SMA and P21 (P < 0.0001 for both). Treatment with OM85 markedly reduces their expression levels, with α-SMA (P < 0.0001) and P21 (P < 0.001) both showing notable decreases. Figure 8A. graphically presents the reduction trend of these proteins, illustrating the impact of OM85 on their expression in the context of IPF.
Idiopathic pulmonary fibrosis (IPF) involves an excessive buildup of collagen products such as collagen I, collagen III, and fibronectin, which play a pivotal role in fibrosis development. Compared to the control NaCl-PBS group, a significant elevation in the levels of these proteins is noted in the BLM-PBS group, with each protein demonstrating a high degree of statistical significance (P < 0.0001). OM85 treatment notably reduces the concentration of these proteins, evidenced by P-values of P < 0.01 for both collagen I and III, and P < 0.001 for fibronectin. This reduction suggests OM85’s potential to attenuate fibrosis in IPF by influencing specific biochemical pathways. Detailed data analysis and Western blot visuals are available in Fig. 8 for further examination.
OM85 improves IFN-γ levels and the IFN-γ/IL-4 ratio in the BALF of mice with BLM-induced IPF, while also reducing IL-4 levels
Changes in the concentrations of IL-4 and IFN-γ in bronchoalveolar lavage fluid (BALF), along with the IFN-γ/IL-4 ratio, are depicted in Fig. 9A–C. Compared to the NaCl-PBS group, the BLM-PBS group exhibited a significant increase in IL-4 levels (P < 0.0001), with OM85 treatment showing a tendency to mitigate the BLM-induced rise in IL-4 (P < 0.01).
Effects of OM85 on the levels of IFN-γ, IL-4, IFN-γ/IL-4 ratio, and TGF-β1 in BLM-induced pulmonary fibrosis in C57 BALF. OM85 increased the levels of IFN-γ, TGF-β1, and the IFN-γ/IL-4 ratio, while simultaneously decreasing IL-4 in the BALF of IPF induced by BLM. Notably, in healthy mice, OM85 intake promoted the secretion of IFN-γ. Panel A represents IFN-γ, Panel B represents IL-4, Panel C represents the IFN-γ/IL-4 ratio, and Panel D represents TGF-β1. After assessing the normality and calculating the standard deviation (SD) of the sample, ordinary ANOVA tests were performed for IFN-γ, IL-4, the IFN-γ/IL-4 ratio, and TGF-β1. Data represent means ± SD for 10 mice. **P < 0.01, ***P < 0.001, ****P < 0.0001, and ns for P > 0.05.
Conversely, compared to the NaCl-PBS group, IFN-γ levels in the BALF of the BLM-PBS group significantly decreased (P < 0.0001), but OM85 administration significantly counteracted this decrease in IFN-γ levels (P < 0.01). The IFN-γ/IL-4 ratio drastically decreased in the BLM group compared to the NaCl-PBS group (P < 0.0001), while OM85 treatment significantly facilitated the reversal of this decrease (P < 0.01). Additionally, compared to the NaCl-PBS group, the PBS-OM85 group showed an increase in IFN-γ levels, an enhanced IFN-γ/IL-4 ratio (P < 0.0001), and a non-significant decrease in IL-4 levels, though the downward trend should not be disregarded. Overall, OM85 may promote a shift towards Th1 immunity in patients with IPF.
OM85 treatment significantly lowers TGF-β1 concentrations in the BALF of mice affected by BLM-induced IPF
Transforming growth factor-β1 (TGF-β1) levels in bronchoalveolar lavage fluid (BALF) supernatant were measured using a commercial ELISA kit. The results, displayed in Fig. 9D, show that TGF-β1 concentrations in BALF significantly increased in mice exposed to BLM-PBS compared to the NaCl-PBS control group (P < 0.0001). OM85 treatment effectively reduced the elevated TGF-β1 levels in BALF of BLM-exposed mice (P < 0.0001).
Assay to determine the optimal concentration and exposure time of OM85 for inhibiting the proliferation of HELF cells
We utilized the CCK8 assay to assess the effects of varying concentrations of OM85 on the proliferation of HELF cells over specific time intervals, as illustrated in Fig. 10. Initially, the HELF cells underwent treatment with OM85 at concentrations of 40 µg/ml, 80 µg/ml, 160 µg/ml, 320 µg/ml, and 640 µg/ml. Subsequent measurements of cell proliferation occurred at 0, 8, 16, 24and 48 h post-treatment. Previous studies have established OM85 as non-toxic, showing no significant impact on proliferation rates. Based on these findings, and leveraging insights from prior research24, we selected a 320 µg/ml concentration of OM85 for evaluation at the 48-h mark.
Proliferation rates of HELF cells at 0, 8, 16, 24, and 48 h for concentrations of 0, 40, 80, 160, 320, and 640 μg/ml were measured using the CCK-8 assay (n = 6). OM85 was found to be non-toxic, and it is evident from the graph that there were no significant differences in proliferation rates with varying time and OM85 concentrations.
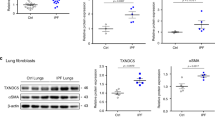
OM-85 down-regulated the expression of α-SMA In TGF-β1-treated HELF cells
We assessed the impact of OM85 on TGF-β1-induced differentiation in HELF cells through an immunofluorescence assay, which demonstrated alterations in α-SMA expression levels, indicative of myofibroblast differentiation (Fig. 11). Exposure to TGF-β1 elevated α-SMA expression in HELF cells; however, OM85 treatment counteracted this increase by reducing α-SMA expression.
OM85 attenuates α-SMA immunofluorescence in HELF cells treated with TGF-β1. Representative results of each group stained with immunofluorescent staining for α-SMA are shown. After assessing the normality and calculating the standard deviation (SD) of the sample, one-way Welch ANOVA tests were conducted. Data represent means ± SD from 6 experiments. ***P < 0.001, ****P < 0.0001.
OM85 inhibited the activation of the notch pathway in TGF-β1-treated HELF cells
To clarify OM85’s action mechanism in TGF-β1-treated HELF cells, we measured the protein expression of Notch1 and Hes1 through Western blot analysis, as shown in Fig. 12. The analysis revealed that exposure to TGF-β1 significantly increased the expression levels of Notch1 (P < 0.01) and Hes1 (P < 0.001) compared to the control group. Importantly, OM85 administration led to a statistically significant reduction in the expression of Notch1 (P < 0.05) and Hes1 (P < 0.01).
OM85 counteracts the TGF-β1-induced reduction of IPF-related proteins in HELF cells. Panel A represents the Western blot experiments for various proteins. Panel B analyzes Notch1 protein levels, Panel C for Hes1, Panel D for collagen I, Panel E for collagen III, Panel F for α-SMA, Panel G for fibronectin, and Panel H for P21. After assessing normality and calculating the standard deviation (SD) of the sample, ordinary ANOVA tests were performed for collagen I, collagen III, and fibronectin, whereas one-way Welch ANOVA tests were utilized for Notch1, Hes1, α-SMA, and P21. Data represent means ± SD from 6 experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
OM85 mitigates the increase in α-SMA, P21, collagen I, collagen III, and fibronectin induced by TGF-β1-treated HELF cells
Following treatment of HELF cells with TGF-β1, these cells transition into activated myofibroblasts, characterized by a marked increase in the secretion of collagen types I and III, as well as fibronectin. Concurrently, P21, a protein implicated in cell differentiation, exhibits a similar upregulation. Our in vitro studies demonstrate that OM85 effectively ameliorates this response, with detailed protein expression data presented in Fig. 12.
Discussion
The use of animal models to replicate human diseases presents significant challenges, and this is particularly true for idiopathic pulmonary fibrosis (IPF). The conventional method of a single intratracheal bleomycin dose has been foundational in pulmonary fibrosis research, shedding light on the mechanisms of fibrosis25. Yet, this approach does not fully capture the chronic and progressive nature of IPF, thus limiting its utility for in-depth mechanistic explorations. To bridge this gap, Elizabeth F. developed a more refined protocol involving three doses of bleomycin instillation26. This method allows for a thorough assessment of lung function, radiological and histological changes, and the accumulation of inflammatory cells. Studies utilizing this protocol have shown that fibrosis not only persists but also intensifies over 24 weeks27, especially in the subpleural regions. Such progression, along with changes in alveolar epithelial cells that mirror those seen in IPF, has led to the adoption of the three-dose bleomycin model in our research to more accurately induce lung fibrosis in mice. This model represents a significant step forward in mimicking the complex pathology of IPF, offering a more relevant framework for studying the disease’s mechanisms and potential treatments28,29.
IPF is a progressive and fatal interstitial lung disease, characterized by the gradual deterioration of pulmonary fibrosis as observed in chest imaging, accompanied by restrictive changes in spirometry, and declining functional status, ultimately leading to respiratory failure and death1. Treatment options for IPF remain limited, making the exploration of OM85, a promising avenue for therapeutic intervention. OM85 has been clinically used to treat individuals with chronic bronchitis or mild chronic obstructive pulmonary disease (COPD), showing efficacy in preventing the progression of lung disease14,15,16,17. Key findings from these studies include the observation that interferon-γ, a principal cytokine produced by Th1 cells, can inhibit fibroblast proliferation and reduce collagen synthesis. Conversely, interleukin-4 (IL-4), a cytokine predominantly associated with Th2 cells, has been identified as a significant promoter of extracellular matrix production by fibroblasts20. This dichotomy in cytokine function underscores the complexity of the immune response in pulmonary fibrosis. Furthermore, research has documented a shift in the Th1/Th2 balance towards a Th2 dominance in patients with pulmonary fibrosis, characterized by elevated levels of IL-430,31. IL-4 and IFN-γ are important regulatory factors for pulmonary fibroblasts. The Th1/Th2 paradigm, which can be evaluated by representing IFN-γ for Th1 and IL-4 for Th230. OM-85 BV encouraged preferential development of the Th1-type immunity characterized by amplified IFN-γ and decreased IL-4 production31. It is essential to conduct a comprehensive investigation into the antifibrotic mechanisms of OM-85 as a potential treatment for IPF patients.
Additionally, to evaluate the potential protective effects of OM85 on BLM-induced idiopathic pulmonary fibrosis, the concentrations of TGF-β1, IL-4, and IFN-γ, as well as the IFN-γ/IL-4 ratio, were measured in the BALF. The study utilizes a simplified three-dose protocol for bleomycin instillation, which results in pathophysiological changes and processes in the model that are largely similar to those observed in human pulmonary fibrosis. Compared to the BLM-PBS group, the BLM-OM85 group showed reduced states of alveolitis and collagen deposition in the lungs, suggesting that OM85 had an inhibitory effect on bleomycin-induced IPF. TGF-β1 is a key cytokine involved in the pathogenesis of pulmonary fibrosis31. In the BLM-OM85 group, the concentration of TGF-β1 in BALF was significantly lower compared to the BLM-PBS group. This suggests that OM85 may inhibit the expression of TGF-β1 and subsequently reduce the collagen deposition in the lung tissues32.
In this study, we examine the protective effects of OM85 on bleomycin (BLM)-induced pulmonary fibrosis in C57 mice, suggesting that OM85 alleviates the fibrotic response.This hypothesis is supported by evidence indicating that OM85 administration reduces the levels of hydroxyproline, IL-4, collagen I, collagen III, fibronectin, P21, and α-SMA in lung tissues of C57, all of which are markers closely associated with fibrosis. This investigation not only supports the anti-fibrotic properties of OM85 but also contributes to our understanding of its underlying mechanisms in combating pulmonary fibrosis.
Previous studies have demonstrated that TGF-β1 treatment in HELF cells induces collagen deposition and the expression of fibrogenic proteins32,33,34. In our study, OM-85 significantly decreased the expression of collagen I, collagen III, fibronectin, P21, and α-SMA, demonstrating its strong anti-fibrotic effects.
Inflammation is widely recognized as a critical factor in the development of idiopathic pulmonary fibrosis (IPF)34,35. OM85 has been identified for its anti-inflammatory effects36,37. OM85 has been shown to reduce inflammation via the TLR4- and TLR2-mediated ERK1/2/NF-κB pathway36. Similarly, in our study, OM85 alleviates fibrosis through its anti-inflammatory properties in IPF mice (36). In our research, the induction of pulmonary fibrosis was achieved through the intratracheal delivery of bleomycin (BLM), a method proven effective by the subsequent quantification of lung hydroxyproline content and the detailed enumeration of total cell counts, including neutrophils and macrophages. The efficacy of this model was further validated by these measures. Notably, administration of OM85 resulted in a marked decrease in lung hydroxyproline levels and a reduction in both total cell counts and specifically, the counts of neutrophils and macrophages. Histological analyses, including H&E, Masson’s trichrome, and Sirius Red staining, complemented these findings, showing that OM85 treatment significantly reduced inflammatory cell infiltration and effectively slowed the fibrotic process within the lungs. In the present study, OM85 also demonstrated therapeutic effects in IPF rats, with its anti-inflammatory action observed as a reduction in total cell count, macrophages, lymphocytes, and neutrophils.
Macrophages, through their critical involvement in inflammation and fibrosis processes, as well as their polarization states, are closely linked to the development of Idiopathic Pulmonary Fibrosis (IPF)38. Specifically, macrophages can polarize into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes based on different signals in the microenvironment39,40. Within this complex context, OM85—a bacterial lysate-based immunomodulator—acts on macrophages to exert a dual-regulatory effect. On one hand, OM85 can promote the production of Interferon-beta (INF-β)41, a cytokine with antiviral, antiproliferative, and anti-fibrotic functions, which works by inhibiting the activity of fibroblasts and the accumulation of collagen, thereby exerting an anti-fibrotic effect. In the future, we intend to develop a comprehensive experimental protocol focused on macrophages, aiming to elucidate the mechanisms and efficacy of using OM85 as a therapeutic option for treating Idiopathic Pulmonary Fibrosis (IPF).
Since its initial discovery, the Notch signaling pathway has been recognized as a highly conserved mechanism across various species, playing an essential role in regulating a multitude of cellular processes such as cell proliferation, differentiation, and apoptosis during development. In mammals, the Notch family comprises four receptors (Notch1-4) and five ligands, which include Jagged 1 and 2 (Jag1, Jag2—homologs of Drosophila’s Serrate) and the Delta-like ligands (Dll1, Dll3, and Dll4)42,43.
In the mammalian respiratory system, the expression of all Notch ligands and receptors at the transcriptional level has been documented, highlighting their ubiquitous presence in lung tissue43,44. Over the last ten years, the Notch signaling pathway has been the focus of intensive research, particularly regarding its involvement in the pathogenesis of various lung conditions42. One significant finding from this body of research is the role of Notch signaling in promoting the differentiation of myofibroblasts from lung fibroblasts1,45. Building on these insights, our study was specifically designed to further investigate the role of Notch1 and its associated proteins in pulmonary fibrosis. Our findings demonstrate that OM-85 significantly reduces the expression of Notch1 and Hes1 in BLM-treated C57 mice and in TGF-β1-stimulated HELF cells, demonstrating its potent antifibrotic effects. These investigations aim to elucidate the molecular mechanisms by which Notch1 contributes to pulmonary fibrosis, potentially paving the way for novel therapeutic interventions.
Conclusion
In conclusion, our findings elucidate the pronounced protective efficacy of OM85 in the pathogenesis of bleomycin-induced pulmonary fibrosis in female C57 mice. This protective effect may be attributed to the inhibition of Notch expression and the modulation of the IFN-γ/IL-4 ratio. Further studies are essential to explore the detailed clinical applications of OM85.Moreover, future studies will seek to elucidate whether OM85 can also mitigate the fibrotic response induced by chronic environmental irritants, such as asbestos and silica, thereby suggesting its potential for broader therapeutic utility across diverse manifestations of pulmonary fibrosis.
Data availability
Every co-author of this research has consented to reveal all data collected during the study. All data produced or analyzed during this research are contained within this manuscript and its supplementary materials. Upon a reasonable request, any additional supporting data related to the article can be obtained from the corresponding author.
References
He, H. et al. Medicine targeting epithelial-mesenchymal transition to treat airway remodeling and pulmonary fibrosis progression. Can. Respir. J. 2023, 3291957 (2023).
Bluth, T. et al. Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA 321(23), 2292–2305 (2019).
Marzec, J. & Nadadur, S. S. Inflammation resolution in environmental pulmonary health and morbidity. Toxicol. Appl. Pharmacol. 459, 116343 (2023).
Darawshy, F., Molyneaux, P. L. & Segal, L. N. Looking beyond the lower airways for microbes affecting pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 209, 1054–1056 (2024).
Tseng, C. C. et al. The role of macrophages in connective tissue disease-associated interstitial lung disease: Focusing on molecular mechanisms and potential treatment strategies. Int. J. Mol. Sci. 24(15), 11995 (2023).
Pu, S. et al. Fermented cordyceps powder alleviates silica-induced pulmonary inflammation and fibrosis in rats by regulating the Th immune response. Chin. Med. 18(1), 131 (2023).
Qin, W., et al. A distinctive insight into inorganic sonosensitizers: Design principles and application domains. Small e2311228 (2024).
Spagnolo, P. et al. The role of immune response in the pathogenesis of idiopathic pulmonary fibrosis: Far beyond the Th1/Th2 imbalance. Expert Opin. Ther. Targets 26(7), 617–631 (2022).
Ding, M. et al. Silica-exposed macrophages-secreted exosomal miR125a-5p induces Th1/Th2 and Treg/Th17 cell imbalance and promotes fibroblast transdifferentiation. Ecotoxicol. Environ. Saf. 267, 115647 (2023).
Xing, X. Y. et al. Jinbei Oral Liquid ameliorates bleomycin-induced idiopathic pulmonary fibrosis in rats via reversion of Th1/Th2 shift. Chin. Herb. Med. 12(3), 273–280 (2020).
Barratt, S. L. et al. Idiopathic pulmonary fibrosis (IPF): An overview. J. Clin. Med. 7(8), 201 (2018).
Marzec, J. M. & Nadadur, S. S. Inflammation resolution in environmental pulmonary health and morbidity. Toxicol. Appl. Pharmacol. 449, 116070 (2022).
Evans, S. E. et al. Stimulated innate resistance of lung epithelium protects mice broadly against bacteria and fungi. Am. J. Respir. Cell. Mol. Biol. 42(1), 40–50 (2010).
Zhu, X. et al. Pre-exposure to aerosolized polyvalent bacterial lysates protects against bleomycin-induced pulmonary fibrosis in mice. Inflammation 45(4), 1692–1699 (2022).
Go, N. et al. A quantitative systems pharmacology workflow toward optimal design and biomarker stratification of atopic dermatitis clinical trials. J. Allergy Clin. Immunol. 153, 1330–1343 (2024).
Choi, J. Y. et al. Effect of Broncho-Vaxom (OM-85) on the frequency of chronic obstructive pulmonary disease (COPD) exacerbations. BMC Pulm. Med. 23(1), 378 (2023).
Yao, S. et al. Bacterial lysate add-on therapy in adult and childhood asthma: A systematic review and meta-analysis. J. Thorac. Dis. 15(6), 3143–3157 (2023).
Guler, S. A. et al. Azithromycin for the treatment of chronic cough in idiopathic pulmonary fibrosis: A randomized controlled crossover trial. Ann. Am. Thorac. Soc. 18(12), 2018–2026 (2021).
Moss, B. J., Ryter, S. W. & Rosas, I. O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 17, 515–546 (2022).
Collet, J. P. et al. Stimulation of nonspecific immunity to reduce the risk of recurrent infections in children attending day-care centers. The Epicrèche Research Group. Pediatr. Infect. Dis. J 12(8), 648–652 (1993).
Nolen, R. S. AVMA board approves Panel on Euthanasia report: Updated guidelines cover more species and methods. J. Am. Vet. Med. Assoc. 239(10), 1269 (2011).
Boe, J. et al. European respiratory society guidelines on the use of nebulizers. Eur. Respir. J. 18(1), 228–242 (2001).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 22(3), 659–661 (2008).
Paupe, J. Immunotherapy with an oral bacterial extract (OM-85 BV) for upper respiratory infections. Respiration 58(3–4), 150–154 (1991).
Jenkins, R. G. et al. An official American thoracic society workshop report: Use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 56(5), 667–679 (2017).
Seibold, M. A. et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One 8(3), e58658 (2013).
Degryse, A. L. et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 299(4), L442–L452 (2010).
Redente, E. F. et al. Persistent, progressive pulmonary fibrosis and epithelial remodeling in mice. Am. J. Respir. Cell Mol. Biol. 64(6), 669–676 (2021).
Williamson, J. D., Sadofsky, L. R. & Hart, S. P. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp. Lung Res. 41(2), 57–73 (2015).
Akenroye, A. et al. Ratio of plasma IL-13/TNF- ∝ and CXCL10/CCL17 predicts mepolizumab and omalizumab response in asthma better than eosinophil count or immunoglobulin E level. Sci. Rep. 14(1), 10404 (2024).
Bowman, L. M. & Holt, P. G. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect. Immun. 69(6), 3719–3727 (2001).
Sun, C. et al. Improvement of idiopathic pulmonary fibrosis through a combination of Astragalus radix and Angelica sinensis radix via mammalian target of rapamycin signaling pathway-induced autophagy in rat. J. Thorac. Dis. 16(2), 1397–1411 (2024).
Gao, L. et al. Glycyrrhizic acid alleviates bleomycin-induced pulmonary fibrosis in rats. Front. Pharmacol. 6, 215 (2015).
Zhang, Y. et al. Knockdown of FBLN2 suppresses TGF-β1-induced MRC-5 cell migration and fibrosis by downregulating VTN. Tissue Cell 81, 102005 (2023).
Singh, S., Jatana, N. & Goel, V. HELF (Haptic Encoded Language Framework): a digital script for deaf-blind and visually impaired. Univers. Access Inf. Soc. 22(1), 121–131 (2023).
Luan, H., et al. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells. J. Interferon. Cytokine Res. 34(7), 526–36 (2014).
Fang, L. et al. OM-85 Broncho-Vaxom(®), a bacterial lysate, reduces SARS-CoV-2 binding proteins on human bronchial epithelial cells. Biomedicines 9(11), 1544 (2021).
Popova, B. et al. HelF, a putative RNA helicase acts as a nuclear suppressor of RNAi but not antisense mediated gene silencing. Nucleic Acids Res. 34(3), 773–784 (2006).
Wang, T. et al. Unmasking the dynamics of Mycoplasma gallisepticum: Deciphering HD11 macrophage polarization for innovative infection control strategies. Poult. Sci. 103(5), 103652 (2024).
Wang, X. et al. the role of macrophages in lung fibrosis and the signaling pathway. Cell Biochem. Biophys. 82, 479–488 (2024).
Dang, A. T. et al. OM-85 is an immunomodulator of interferon-β production and inflammasome activity. Sci. Rep. 7, 43844 (2017).
Guo, R. et al. Context-dependent regulation of Notch signaling in glial development and tumorigenesis. Sci. Adv. 9(45), eadi2167 (2023).
Wang, X. C. et al. New aspects of the epigenetic regulation of EMT related to pulmonary fibrosis. Eur. J. Pharmacol. 956, 175959 (2023).
Zhao, J. et al. HuangQi decoction ameliorates renal fibrosis via TGF-β/smad signaling pathway in vivo and in vitro. Cell Physiol. Biochem. 38(5), 1761–1774 (2016).
Ling, H. et al. Role of ferroptosis in regulating the epithelial-mesenchymal transition in pulmonary fibrosis. Biomedicines 11(1), 163 (2023).
Acknowledgements
We express our gratitude to the International Research Fund of Philips Respironics for their generous support.
Author information
Authors and Affiliations
Contributions
Y.Y. conceptualized the study , led the software development, data curation, visualization, and the writing of the original draft, prepared all figures,and also led the investigation. Z.L. supported the software development, visualization, writing of the original draft, and the investigation. Z.H. provided support in data curation, visualization, writing of the original draft, and the investigation. R.N. supported data curation, visualization, and the writing of the original draft. P.S. contributed to visualization, writing of the original draft, and the investigation as a supporting role. X.W. contributed to the conceptualization, took an equal role in writing-review and editing, and led the investigation. J.Z. equally conceptualized the study, engaged in writing-review and editing, led the supervision, and the investigation.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, Y., Li, Z., Hu, Z. et al. OM85 ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting Notch expression and modulating the IFN-γ/IL-4 ratio. Sci Rep 15, 5436 (2025). https://doi.org/10.1038/s41598-025-89874-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89874-5