Abstract
Previous studies conducted by the same group of researchers found that Traditional Chinese Medicine Astragalus mongholicus Bunge and Hedyotis diffusa Willd (A-H) significantly suppressed the cell proliferation of lung adenocarcinoma (LUAD). MicroRNAs are considered promising candidates for cancer diagnosis and treatment. This study focused on miR-582-3p as the primary subject of investigation to explore the mechanism by which A-H inhibits cell proliferation through miR-582-3p. The overexpressing and silencing miR-582-3p cell models were established by using lentiviral transfection technology. CCK-8 assay (24 h, 48 h, 72 h) and clone formation assay (1 w) were employed to detect the proliferation of A549 cells. Moreover, flow cytometry analysis (24 h) was performed to detect the cell cycle. Western blotting (WB) and a luciferase reporter assay were also used to measure the expression of cell cycle-related proteins and verify the direct interaction between miR-582-3p and p27, respectively. The LV-miR-582-3p inhibitor + shRNA-p27 stable A549 cells were constructed in the same manner to repeat the above-mentioned procedure. The CCK-8 assay was conducted to assess the effects of various concentrations of A-H on the proliferation of A549 cells. A-H-containing serum was prepared to intervene in LV-miR-582-3p and mimic A549 cells. Subsequently, the same procedure was repeated, as described earlier. Results indicated a direct interaction between miR-582-3p and p27. Furthermore, miR-582-3p was found to enhance the proliferation of A549 cells by regulating cell cycle-related proteins, specifically p27. It was also observed that A-H-containing serum inhibited the proliferation of A549 cells through the miR-582-3p-p27 signaling pathway. The study findings revealed the underlying molecular mechanisms of miR-582-3p in the development and prognosis of A549 LUAD cells. In addition, A-H inhibited LUAD proliferation through the miR-582-3p-p27 signaling pathway. These findings may provide a new understanding of the use of Chinese medicine in treating lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is a commonly diagnosed form of cancer and also the leading cause of cancer-related mortality, according to recent epidemiological studies1. Due to its high incidence and mortality rates, lung cancer is the most prevalent malignant tumor in China, affecting approximately 1,060,600 individuals in 2022, posing a serious threat to human health2. Non-small cell lung carcinomas account for more than 85% of lung cancer cases and are divided into lung adenocarcinoma (LUAD), lung squamous cell carcinomas (LSCC), and large-cell lung carcinomas (LCLC) subtypes3. Surgical resection and chemoradiation are the primary treatments for early-stage lung cancer4. However, the 5-year survival rate for lung cancer patients remains low5. Unfortunately, the insidious onset of lung cancer results in nearly 70% of cases being diagnosed at an advanced stage, thereby forfeiting the most promising opportunity for a cure6. Patients with early lung cancer recurrence or enlargement of tumors related to invasion and metastasis in advanced stages have a poor prognosis, which is closely related to the uncontrolled proliferation of malignant cells. Therefore, it is essential to gain a deeper understanding of the mechanisms that underlie the development of lung cancer. Numerous studies have confirmed that cell cycle disarrangement is the predominant cause of unrestrained cell proliferation7. This implies that focusing on specific stages of the cell cycle could be a promising new approach for treating lung cancer.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that are naturally present in eukaryotic organisms, and expressed throughout the body and consist of a single strand of RNA that is 18–25 nucleotides long. By binding to the 3’-untranslated region (3’UTR) of target mRNAs, miRNAs can cause putative protein translation repression or mRNA degradation, negatively regulating gene expression at the post-transcriptional level8. Therefore, the abnormal expression of miRNAs leads to the downregulation or upregulation of target proteins, resulting in a variety of diseases such as cancers9. A growing body of evidence has suggested that miRNAs are abnormally expressed in various tumor types such as lung carcinoma10and play a crucial role in the development of cancer, regulation of tumor cell cycle, cell proliferation, differentiation, apoptosis, migration, and metastasis9. As a result, more comprehensive investigations of cancer-associated miRNAs and the mechanisms underlying their roles may significantly change our understanding of cancer development, progression, and prognosis.
RNA-seq analyses on tumor and normal tissue samples of lung cancer using publicly available data from the Cancer Genome Atlas (TCGA) have revealed significant differences between the two tissue types in the expression of multiple miRNAs. Among these, miR-582-3p has been identified as the most suitable candidate for further investigation due to its clinicopathological characteristics and potential prognostic value. Studies have shown that miR-582-3p plays an important role in bladder cancer11and ovarian cancer12. However, a few studies have focused on LUAD affected by miR-582-3p. This study discovered that miR-582-3p exhibited elevated levels of expression in both LUAD tissues and LUAD cells. It functioned as an oncogene by stimulating the proliferation of LUAD cells. Additionally, it influenced the expression of molecules associated with the cell cycle, including Cyclin D1, CDK4, Cyclin E, CDK2, p-Rb, and E2 F3. As a result, it facilitated the transition from the G1 phase to the S phase of the cell cycle in LUAD.
Bioinformatics analysis has shown that p27 Kip1 (p27) is a direct target of miR-582-3p, which is consistent with the authors’ previous experiments. p27 is a member of the Cip/Kip family of cyclin-dependent kinase inhibitors (CKIs), and is encoded by the CDKN1B gene13. It interacts with Cyclin-CDK complexes to inhibit their enzymatic activity, leading to cell cycle arrest at G0/G1, which is crucial for maintaining normal cell cycle progression and cell number14. Abnormal p27 expression is often associated with tumor development15. This study showed the low expression of p27 in LUAD tissues and cells. Furthermore, it was found that p27 can partially counteract the effects of miR-582-3p on regulating the cell cycle and suppressing cell proliferation in LUAD.
Traditional Chinese medicine, also known as the natural combinatorial chemical library, holds great potential for the treatment of multiple chronic diseases, particularly tumors16. A-H pair drug consists of Astragalus mongholicus and Hedyotis diffusa Willd. Previous research by the authors has found that Feiyiliu Mixture (FYLM), a clinical experience formula of Chinese medicine, combined with erlotinib, significantly inhibited tumor growth in LUAD compared to erlotinib alone17. To effectively illustrate the objective of traditional Chinese medicine and address the challenges posed by complex regulations and unclear mechanisms of excessive use of traditional Chinese medicine formulas, the primary focus of our research was on studying the medicinal properties of FYLM-Astragalus mongholicus and Hedyotis diffusa Willd (A-H). The finding showed that A-H significantly inhibited the A549 cell proliferation and suppressed the expression of Cyclin D118. However, its mechanism of action is still unknown.
Therefore, this study aims to investigate the mechanisms by which miR-582-3p promotes cell proliferation. Specifically, it seeks to determine if miR-582-3p directly targets p27, leading to uncontrolled cell cycle progression and unlimited proliferation of LUAD cells. Additionally, the study aims to investigate whether A-H inhibits A549 cell proliferation through the miR-582-3p-p27 signaling pathway. The study findings may provide scientific foundations for miR-582-3p as a potential target for therapeutics in LUAD.
Materials and methods
Preparation of A-H Decoction
The A-H consisted of two Chinese herbs, including 18 g of Huangqi [Astragalus membranaceus (Fisch.) Bge.] and 20 g of Baihuasheshecao [Oldenlandia diffusa (Willd.) Roxb]. All the Chinese medicine pieces were provided by Bozhou Pharmaceutical Co Ltd (Bozhou, People’s Republic of China) and certified by Prof. Feng Li of Shandong University of Traditional Chinese Medicine. All herbs (38 g in total) were mixed in 10 times the volume of distilled water for 30 min, then decocted to boiling for 1 h, and filtered through filter paper. The residual dregs were boiled again in eight times distilled water for 30 min, and the two filtrates were mixed and concentrated to 1 g/ml in a rotary evaporator.
Preparation of A-H-containing serum
Twenty male Sprague-Dawley rats weighing 180 to 200 g were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) [animal License number: SCXK (Jing) 2021-0006]. The subjects were under specific pathogen-free (SPF) conditions, with a relative humidity of 30–50%, a temperature of 23 ± 2°C, and a 12-hour dark/light cycle. They had free access to food and water at all times [Laboratory use of animal license number: SYXK(Lu)2022-0009].
After one week of adaptive feeding, the subjects were randomly allocated into the control and A-H administration groups. The clinical dosage of A-H in adults is 38 g/60 kg, which is equivalent to the daily dosage of 4 g/kg in rats by gavage, based on body surface area and human-rat dose conversion (human: rat = 1:6.3). The control group was administered only normal saline (0.9% NaCl). After 6 days of continuous gavage, the subjects were anesthetized with sodium pentobarbital (45 mg/kg intraperitoneally) for blood sampling from the abdominal aorta. After clotting for 1 h at room temperature, the blood samples were centrifuged at 3,000 rpm for 15 min at 4°C to collect the serum. The samples were then inactivated by a water bath at 56°C for 30 min, filtered through a 0.22-µm microporous membrane, and stored in a refrigerator at −80°C until in vitro experiments.
The euthanasia method for experimental animals needs to follow animal welfare and ethical principles, ensuring that animals end their lives without pain and with minimal fear. The euthanasia method for this experimental animal is cervical dislocation method.
UPLC-Q-Orbitrap-MS analysis
The serum samples containing A-H were analyzed for their composition using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer, which was coupled with a Dionex UltiMate 3000 LC System [Thermo Fisher Scientific (China) Co. Ltd.]. The resulting 100 µl of medicated serum A-H was mixed with 300 µl of a methanol solution. The mixture was then vortexed for 10 s and centrifuged at 18,000 g for 10 min at 4°C. The chromatographic separation was carried out on an EC-C18 column (100 mm × 2.1 mm, 1.9 μm) at a column temperature of 35°C, and the A-H mixture was chromatographically separated using gradient elution. Mass spectra were acquired using both positive and negative ion modes with an electrospray ionization (ESI) source. The mass spectrum data were acquired by CD 2.1 software (Thermo Fisher) and contrasted by using the online database McCloud.
Cell lines and culture conditions
NSCLC cell lines (A549, H1299, H1703, and NCI-H520) and human bronchial epithelial (16HBE) cells were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in humidified air (37°C, 5% CO2) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone) and 1% penicillin-streptomycin (Gibco).
Lentivirus construction and transfection of cell lines
The shRNA targeting miR-582-3p was inserted into pLKO.1-EGFP-Puro. The target sequences of KD-miR-582-3p were as follows: KD-miR-582-3p: 5’-GGTTCAGTTGGACACCAGTTAATCCGTAGGTTCAGTTGGACACCAGTTACTTC GGTTCAGTTGGACACCAGTTA-3’; The scramble shRNA was used as negative control (KD-NC). The hairpin sequence of shNC was as follows: 5’-CCTAAGGTTAAGTCGCCCTCG-3’; To knock down endogenous miR-582-3p expression, pLKO.1-EGFP-Puro-KD-NC or pLKO.1-EGFP-Puro-KD-miR-582-3p was transfected along with the packaging plasmid psPAX2 and the envelope plasmid pMD2.G into 293 T cells using Turbofect Transfection Reagent (Thermo) according to the manufacturer′s instructions. To overexpress miR-582-3p, pLV or pLV-miR-582-3p was transfected along with the packaging plasmid psPAX2 and the envelope plasmid pMD2.G into 293 T cells using Turbofect Transfection Reagent (Thermo). The target sequences of OE-miR-582-3p were as follows: OE-miR-582-3p: 5’-GTATGTTGCTTCAAGTCATTCATGCACACATTGAAGAGGACAGACAGAGCCCTGCTGAACACCACTAGCAAATCTCTCTCTAGTAGACCACAACAAGTCAATCTGTGCTCTTTGATTACAGTTGTTCAACCAGTTACTAATCTAACTAATTGTAACTGGTTGAACAACTGAACCCAAAGGGTGCAAAGTAGAAACATTTCATTGTGAAGTCGGCTGGGGACAAAGAAAACACCACCAGAACCCCCTTCAATATGCAAAGGGAAAATTCCTTCTCAAGTACAAGCTACATGGTGCCTTT-3’; Forty-eight hours after transfection, the virus particles were collected and filtered, Forty-eight hours later, stable cells were selected using puromycin (2 µg/ml) for 1 week.
A549 cells were transfected with the lentiviral vector (LV-hsa-miR-582-3p-mimic vector, LV-hsa-miR-582-3p-inhibitor vector) and its corresponding control lentivirus (LV-mimic negative control, LV-inhibitor negative control) structured by lentiviral vectors (GenePharma, Shanghai, China). The A549 cells were infected with a lentiviral vector using an appropriate multiplicity of infection (MOI) when cells were grown to 80% confluency, according to the manufacturer’s instructions. Stably transfected cells were selected with puromycin. p27 knockdown was achieved by lentiviral shRNA vectors. The shRNA-p27 sequences were as follows: shRNA-p27#1: CGGTGGACCACGAAGAGTTAA; shRNA-p27#2: GCGCAAGTGGAATTTCGATTT; shRNA-p27#3: TAACTCTGAGGACACGCATTT. The shRNA-p27 and its negative control lentiviral vector encoding scrambled shRNA were also purchased from Shanghai GenePharma Co. Ltd (Shanghai, China).
TCGA
The level 3 RNA sequencing (RNA-seq) data for 535 samples of lung cancer tissues and 59 samples of paracancerous tissues were downloaded from the TCGA database (https://portal.gdc.cancer.gov) up to December 11, 2021. TCGA also provides detailed clinical data, including gene expression, gender, pathologic stage, T stage, N stage, M stage, and vital status. etc. This study employed RNA-seq data and clinical information for further analyses. Since all the data used in this study were obtained from TCGA, there was no need for approval from an ethics committee. This study also complied with the publication guidelines provided by TCGA.
Cell proliferation assay
Cell viability was assessed using Cell Counting Kit (CCK)−8 (Beyotime, Shanghai, China) following the manufacturer’s protocol. A549 cells were seeded into a 96-well plate at a density of 10,000 cells per well and were cultured for another 24, 48, or 72 h. After adding 10 µl of CCK-8 to each well, the plates were incubated once again for 2 h. Finally, the absorbance values (OD value) at a wavelength of 450 nm were detected using a microplate reader (Bio-Rad, USA). The experiment was repeated 3 times.
Colony formation assay
Cells were seeded into 6-well plates at 500 cells/well and cultured under normal conditions for 7 consecutive days. After the formation of colonies, the growth medium was removed by aspiration, and cells were washed three times with phosphate-buffered saline (PBS). Subsequently, the colonies were fixed using methanol and then stained with a crystal violet solution. After rinsing with PBS, the plates were photographed and the number of colonies was counted.
Flow cytometry analysis
A549 cells were dissociated with trypsin (Gibco), and the resulting mixture was centrifuged to remove the supernatant. Cells (1 × 106) were washed and re-suspended with PBS, then fixed in ice-cold 70% ethanol overnight at 4°C, and washed again with PBS. Cells were stained with Propidium Iodide (PI) solution (250 µg/ml RNase A and 40 µg/ml PI in PBS). Subsequently, the mixture was incubated for 30 min at room temperature in the dark. Flow cytometry was performed with a FACSCanto II (BD Biosciences), and the data obtained were analyzed in FlowJo (LLC).
Luciferase assay
P27 3’UTRs were amplified from human genomic DNA. Wild-type and mutant 3’UTRs of p27 containing predicted miR-582-3p-binding sites were cloned into the pmirGLO vector (Promega, USA) to generate the wild-type p27 3′-UTR luciferase reporter vector and mutant p27 3′-UTR luciferase reporter vector. HEK293 T was co-transfected with the reporter vector and a miR-NC or miR-582-3p-mimics using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The luciferase activity was measured 48 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, WI, USA).
RNA pull-down assay
Biotin-labeled oligonucleotide probes targeting the junction site of miR-582-3p and antisense probes were synthesized by RiboBio(Guangzhou, China). One milligram of whole-cell lysates was incubated with 3 µg of purified the biotinylated biotinlabelled miR-582-3p probes and control probes for 1 h at 25 °C, then the complexes were isolated with streptavidin agarose beads (Invitrogen). The RNA pulled down by miR-582-3p was reversely transcribed and detected by qRT-PCR analysis.
Western blot (WB)
The proteins in cells were extracted with RIPA buffer (Beyotime, China) and quantified using a BCA protein assay kit (Beyotime, China). Proteins were separated using SDS-PAGE and then transferred to Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore), which was blocked with 5% milk in TBS with 0.1% Tween (TBST) for 1 h at room temperature. They were then incubated with primary antibodies purchased from Abcam overnight. After three times washing with TBST buffer, the PVDF membrane was incubated with a secondary antibody (Abcam) for 1 h at room temperature. Then the protein signals were detected by the ECL kit and bands were quantified in Image J.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with TRIzol reagents (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s manual. Then the total RNA was converted to cDNA using the reverse transcription reagent kit (Takara, Dalian, China). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (TAKARA, Japan) with a 7300 real-time PCR system (Applied Biosystems). Relative quantification of gene expression was analyzed using the 2−△△Ct method with GAPDH as the internal control for normalization. MiRNA was extracted with the mirVana miRNA Isolation Kit (Qiagen, Germantown, MD, USA), and reverse transcription and miRNA detection were carried out using the miRNA Reverse Transcription kit and miRcute Plus miRNA qPCR Kit (SYBR Green, Qiagen), respectively, with the U6 small nuclear RNA serving as endogenous control for normalization. The sequences of primers used in this study are shown in Table 1.
Bioinformatics analyses
All statistical analyses were conducted in R-3.6.3. The relative expression of miR-582-3p was shown by histogram in LUAD and adjacent normal tissues, and then the association between miR-582-3p expression and Univariate and multivariate Cox regression analysis was employed to determine the effect of miR-582-3p on Overall Survival (OS), as well as other pathological and clinical factors. Receiver operating characteristic (ROC) curves were developed to assess the diagnostic accuracy of miR-582-3p expression and distinguish the stages III, IV and stages I, II together. Two popular public databases, TargetScan (http://www.targetscan.org/vert_71/) and miRDB (http://mirdb.org/miRDB/), were used for miRNA target prediction. Spearman correlation was also performed to analyze the expression correlation between miR-582-3p and p27.
Statistical analysis
All data were analyzed in GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± SD or median with interquartile spacing, Normality is checked using the Shapiro-Wilk test. If the data is normally distributed, the difference between the two groups is represented by Student’s t-test (two-tailed); One-way ANOVA, followed by post-hoc Tukey multiple comparison tests and Dunnett multiple comparison tests as appropriate is used for multiple group comparisons. If the data are not normally distributed, the difference between the two groups is determined by Wilcoxon rank sum test(Mann-Whitney test); three or more groups is Kruskal Wallis. When the variable is categorical, if the data meet the condition that all theoretical frequencies are > 5 and the total sample size is ≥ 40, the Chisq test is used for intergroup comparisons; when the data meet the condition that theoretical frequencies are ≥ 1 but ≤ 5 and the total sample size is ≥ 40, the Yates’ correction is applied for intergroup comparisons; when the data contain theoretical frequencies < 1 or the total sample size is < 40, Fisher’s exact test is employed for intergroup comparisons. P < 0.05 was considered statistically significant.
Results
miR-582-3p is upregulated in LUAD tissues and is closely associated with poor patient prognosis
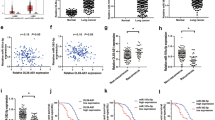
The relevant data obtained from TCGA were analyzed to identify miR-582-3p associated with LUAD progression, patient survival, and clinicopathological features. The miR-582-3p levels were significantly higher in LUAD tissues than in paracancerous tissues (Fig. 1A). The ROC curve was further visualized to confirm the diagnostic value of miR-582-3p in LUAD. The result showed that miR-582-3p may have a certain level of diagnostic accuracy, as evidenced by the area under the curve of 0.606 in the diagnosis of lung cancer (Fig. 1B). The data from the TCGA LUAD indicated that patients in the late stages (III and IV) exhibited greater expression of miR-582-3p compared to those in the early stages (I and II) (Fig. 1C). ROC curve analysis revealed that the AUC of miR-582-3p expression in predicting pathologic grade was 0.617 (Fig. 1D). This indicates that higher expression of miR-582-3p may be indicative of a poor prognosis for patients. The forest plot is consistent with the above conclusion, indicating that miR-582-3p may be a key factor affecting prognosis (Fig. 1E). Univariate and multivariate Cox model analyses were performed to determine the link between miR-582-3p expression and OS in LUAD patients. The univariate analysis showed that the high level of miR-582-3p was significantly correlated with poor OS (HR: 1.382; P = 0.031), and other clinicopathological variables, including Tumor Node Metastasis (TNM) stage, were associated with poor survival (Table 2). The Cox proportional hazards regression model demonstrates that the miR-582-3p level (HR: 1.616; P = 0.007) was an independent prognostic factor for OS, in addition to the TN stage in the multivariate analysis. In addition, the clinical significance of miR-582-3p in LUAD was assessed by examining the correlations between miR-582-3p expression level and the various clinicopathological parameters. LUAD patients were then categorized into high-expression and low-expression groups based on the median levels of miR-582-3p. Results revealed that miR-582-3p was significantly correlated with the pathologic stage (P = 0.027), OS (P = 0.005), and N stage (P = 0.022). Nevertheless, miR-582-3p expression was not correlated with age (P = 0.399), gender (P = 0.311), smoking (P = 0.323), and TM stage (P = 0.083, P = 0.0604) (Table 3).
These results suggest that LUAD patients with high levels of miR-582-3p expression are more prone to develop more advanced tumors and exhibit poor prognosis, compared to those with low levels of miR-582-3p expression. As a result, miR-582-3p is expected to become a potential biomarker for the recurrence and prognosis of LUAD patients.
Relative miR-582-3p expression in LUAD tissues and its clinical significance based on TCGA data. (A) miR-582-3p expression increased in LUAD tissues compared with paracancerous tissues. Wilcoxon rank sum test, 95% CI: 0.080–0.873, (J-I): 0.470. (B) ROC curve of miR-582-3p in the diagnosis of paracancerous and LUAD tissues. 95% CI: 0.539–0.672. (C) miR-582-3p expression increased in stages III and IV compared with stages I and II in LUAD tissues. Wilcoxon rank sum test, 95% CI: −0.937–0.296. (J-I): −0.617. (D) ROC curve of miR-582-3p in distinguishing the stages III and IV together and stages I and II together. 95% CI:0.556–0.678. (E) Forest plot for the multivariate Cox proportional hazard regression model. (*P < 0.05, **P < 0.01, ***P < 0.001).
miR-582-3p was upregulated in lung cancer cell lines and promoted LUAD proliferation by regulating cancer cell cycle progression
Based on the association between miR-582-3p expression and clinical features in LUAD patients, it was speculated that miR-582-3p may play an important role in LUAD progression. Therefore, the mRNA level of miR-582-3p was measured in four lung cancer cell lines, including A549, H1299, NCI-H520, and H1703, as well as the normal human lung epithelial cells (16HBE). The expression of miR-582-3p increased in LUAD cell lines, especially A549 cells (Fig. 2A). As a result, the A549 cell line was selected for the follow-up experiments for its higher expression and better typicality. To achieve more stable overexpression and low expression of miR-582-3p, A549 cells were transfected with lentiviruses that overexpressed or silenced miR-582-3p. The level of miR-582-3p in A549 stable cells was then detected using qRT-PCR (Fig. 2B). The proliferation of A549 cells transfected with LV-miR-582-3p mimic or LV-miR-582-3p inhibitor was measured using the CCK-8 assay at 24 h, 48 h, and 72 h, and the clone formation assay at one week. The CCK-8 assay results showed that the overexpression of miR-582-3p promoted the proliferation of A549 cells, while miR-582-3p knockdown decreased cell proliferation (Fig. 2C). Similarly, the clone formation assay showed that the upregulation and downregulation of miR-582-3p promoted and reduced the capacity of A549 cells in colony formation, respectively (Fig. 2D and E). These results indicated that miR-582-3p significantly promoted the in vitro proliferation of A549 cells. To investigate the molecular mechanism by which miR-582-3p promoted the proliferation of A549 cells, its effect on the cell cycle was examined by a 24-hour flow cytometry analysis. Changes in S-phase cell fraction (SPF) and DNA ploidy are vital features of abnormal tumor cell proliferation19. Cell cycle analysis revealed that LV-miR-582-3p mimic cells had lower frequency in the G0/G1 phase but more in the S phase, while LV-miR-582-3p inhibitor cells increased the G0/G1 phase but decreased the S phase. These findings suggest that silencing the expression of miR-582-3p blocked the proliferation of A549 cells by arresting cells in the G0/G1 phase (Fig. 2F and G). The WB was performed to detect cell cycle-related proteins, such as Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, Rb, and E2 F3, after miR-582-3p overexpression and knockdown. The results showed that the miR-582-3p overexpression significantly increased the levels of Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, and E2 F3, and miR-582-3p silencing led to a sharp decrease in Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, and E2 F3 (Fig. 3). These data suggest that miR-582-3p may promote the proliferation of A549 cells by regulating cell cycle-related proteins.
miR-582-3p was more expressed in lung cell lines and accelerated cell proliferation by promoting G1/S cell cycle transition. (A) miR-582-3p expression increased in lung cancer cells (A549, H1299, NCI-H520) compared with 16HBE. (B) Relative miR-582-3p expression in A549 cells transfected with lentiviruses overexpressing and silencing miR-582-3p by qRT-PCR. (C, D, F) Effects of miR-582-3p on the proliferation of A549 cells were detected by CCK-8 and colony formation. (E, G) Effects of miR-582-3p on regulating the cell cycle in A549 cells were detected by cell-cycle analysis. Data were presented as mean ± SD for three individual experiments. one-way ANOVA, **P < 0.01.
Effects of miR-582-3p on the expression of proteins related to cell cycle regulation in A549 cells. A549 cells were treated with LV-mNC, LV-miR-582-3p mimic, LV-iNC or LV-miR-582-3p inhibitor. The expression of Cyclin D1 (A, B), CDK4 (A, C), Cyclin E (A, D), CDK2 (A, E), p-Rb (A, F), Rb (A, G), E2 F3 (A, H) in A549 cells from the indicated group were detected by western blot assay. Results were mean ± SD for three individual experiments. one-way ANOVA, **P < 0.01.
miR-582-3p directly targeted and downregulated the Cyclin kinase inhibitor p27 in A549 cells
To investigate the mechanism underlying the robust effect of miR-582-3p on the cycle progression of A549 cells, the target genes of miR-582-3p were predicted using two miRNA target prediction algorithms: TargetScan v6.2 and miRDB. Bioinformatic analysis revealed more than 300 potential target genes of miR-582–3p. Among these candidates, p27 was most closely related to cell cycle regulation. TCGA database was used to analyze the expression of p27 in lung adenocarcinoma and paracancerous tissues, and the p27 gene showed low expression in lung adenocarcinoma tissue but exhibited high expression in paracancerous tissue (Fig. 4A). The results also demonstrated that the p27 expression significantly decreased in lung cancer cell lines compared to 16HBE (Fig. 4B, C). Subsequently, information was obtained regarding the binding of miR-582-3p to the sites in the 3′UTR of p27. The binding sites of p27 and miR-582-3p are shown in Fig. 4D. A luciferase reporter assay was performed to validate the direct effect of miR-582-3p on the regulation of p27 expression. The assay contained a p27 wild-type regulatory sequence with a mutation in the binding site. The direct inhibitory binding of miR-582-3p to the 3’UTR of p27 was detected by cloning the 3’UTR into a luciferase reporter plasmid. The results showed that miR-582–3p significantly decreased the luciferase activity of the wild-type p27 3′-UTR reporter plasmid but caused no difference in the mutant type in A549 cells (Fig. 4E). RNA pull-down experiment once again demonstrated miR-582-3p can directly interact with p27 (Fig. 4F). The relationship between miR-582-3p and p27 in LUAD tissues from TCGA was also analyzed by Spearson correlation analysis, and the results indicated an inverse correlation between the expression of miR-582-3p and p27 (Fig. 4G). WB and qRT-qPCR analyses confirmed the elevated levels of p27 expression in miR-582-3p-overexpressed cells and miR-582-3p-silenced cells (Fig. 4H, I,J). All these data indicated that miR-582-3p mediated the downregulation of p27 by directly targeting its 3’UTR sequence. Moreover, p27 was a direct target of miR-582-3p, negatively regulated by miR-582-3p.
p27 is a direct target of miR-582-3p. (A) The expression of p27 decreased in LUAD tissues compared to paracancerous tissues, Wilcoxon rank sum test, 95% CI: −0.317–0.070, (J-I): −0.188. (B, C) The expression of p27 decreased in lung cancer cells compared to 16HBE. (D) The predicted wild-type or mutated miR-582-3p binding sites in the 3’-UTR of p27 mRNA. (E) The luciferase report assay demonstrated that the overexpression of miR-582-3p could reduce the intensity of fluorescence in A549 cells transfected with the p27-WT vector, while it did not affect the p27-MUT vector. (F) RNA pull-down experiment demonstrated miR-582-3p can directly interact with p27. (G) The expression of p27 was negatively correlated with miR-582-3p from TCGA, Spearman’s Rank Correlation Coefficient Test. r=−0.088. (H-J) The expression of p27 in LV-miR-582-3p mimic or LV-miR-582-3p inhibitor A549 cells was measured by WB and RT-qPCR. Data were presented as mean ± SD for three individual experiments. one-way ANOVA, *P < 0.05, **P < 0.01.
miR-582-3p promoted LUAD cell proliferation by downregulating p27
Rescue experiments were carried out to confirm the role of p27 in the miR-582-3p-induced promotion of A549 cell proliferation. LV-miR-582-3p inhibitor + shRNA-p27, LV-miR-582-3p inhibitor + shRNA-NC, LV-iNC + shRNA-p27, and LV-iNC + shRNA-NC were used to transduce A549 cells. The knockdown activity of the three shRNA constructs of p27 was verified, and shRNA-p27#2 was selected for subsequent experiments with the best interference effect (Fig. 5A). CCK-8 assay and clone formation assay showed that the knockdown of p27 reversed the partial function of downregulated miR-582-3p in suppressing cell proliferation (Fig. 5B, C, E). The cell cycle assays demonstrated that the knockdown of miR-582-3p blocked the cell cycle at the GO/G1 phase; however, the downregulation of p27 abolished the cell cycle arrest at the GO/G1 phase induced by miR-582-3p knockdown (Fig. 5D, F). In other words, the miR-582-3p knockdown regulated the proliferation of A549 cells by arresting cells at the G0/G1 phase by upregulating p27. To further explore the possible regulatory mechanism of miR-582-3p-induced cell cycle arrest at the G0/G1 phase, the expression level of cell cycle proteins at the G1/S phase was measured by WB. The results showed that the protein expression of p27 in A549 cells was significantly increased by the miR-582-3p knockdown in comparison to the LV-iNC + shRNA-NC. Conversely, the co-downregulated miR-582-3p and p27 cells exhibited a lower expression of p27 than the downregulated miR-582-3p group (Fig. 6A-C), and the knockdown of miR-582-3p downregulated the expression of Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, and E2 F3, while the knockdown of p27 reversed this effect on the expression of Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, and E2 F3 induced by miR-582-3p (Fig. 6D-K). These findings generally confirmed that miR-582-3p enhanced LUAD cell proliferation by downregulating p27 expression.
miR-582-3p knockdown inhibited the proliferation and arrested cell cycle at the G0/G1 phase in A549 cells by upregulating p27 expression. (A) shRNA-p27#2 construct exhibited the best interference effects. (B, C, E) The CCK-8 and colony formation assays demonstrated that the inhibition of miR-582-3p resulted in reduced proliferation through the upregulation of p27 and flow cytometry analysis revealed that the inhibition of miR-582-3p resulted in the suppression of cell cycle progression through the upregulation of p27 (D, F). Data were presented as mean ± SD for three individual experiments, one-way ANOVA, **P < 0.01.
The miR-582-3p knockdown upregulated the expression of proteins at the G1/S phase through p27. A549 cells were treated with LV-miR-582-3p inhibitor + shRNA-p27, LV-miR-582-3p inhibitor + shRNA-NC, LV-iNC + shRNA-p27 or LV-iNC + shRNA-NC. The expression of p27 (A, B), Cyclin D1 (D, E), CDK4 (D, F), Cyclin E (D, G), CDK2 (D, H), p-Rb (D, I), Rb (D, J), E2 F3 (D, K) in A549 cells from the indicated group were detected by western blot assay. The expression of p27 in A549 cells from the indicated group were detected by qRT-PCR (C). Results were mean ± SD for three individual experiments, one-way ANOVA, **P < 0.01.
Identification of the chemical constituents of A-H pair
An LC-MS/MS analysis was performed to obtain the main components of A-H. As shown in Tables 4 and 32 kinds of compounds from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://old.tcmsp-e.com/tcmsp.php) were identified. The main components of huangqi included L-Asparagine, L-Arginine, Nicotinic acid, Coumarin, 4-Aminobutanoate, Chlorogenic Acid, Folic acid, Isoquercetrin, 9,12,15-octadecatrienoic acid, Formononetin-7-O-glucoside, Formononetin-7-O-glucoside, Quercetin, Kumatakenin, Daidzein, Formononetin, Kaempferol, 5-O-methylvisammioside, Linoleic acid, and docosanoic acid. The main components of baihuasheshecao included P-Coumaric acid, Succinic acid, Ferulic acid, Scopoletin, Deacetylasperulosidic acid, Quercetin, Ursolic acid, Genipin, (3beta)-stigmast-5-en-3-ol, Sitogluside, 4-Methoxycinnamic acid, and 3-Epioleanolic acid. The total ion chromatograms (TCI) of A-H under the positive and negative ion modes are shown in Fig. 7A. In addition, Fig. 7. B-C presents the chemical structural formula of the main active ingredients of A-H.
A-H-containing serum inhibits LUAD cell proliferation through miR-582-3p- p27 signaling pathway
Cell viability in A549 cells was assessed by exposing them to different concentrations of A-H-containing serum for 24 h. The serum samples containing A-H demonstrated a dose-dependent inhibition of A549 cell proliferation (IC50 = 12.08%) (Fig. 8A). To verify that A-H inhibits LUAD proliferation through the miR-582-3p-p27 signaling pathway, a concentration of 10% A-H-containing serum was chosen for rescue experiments. The CCK-8 and clone formation assays showed that the upregulation of miR-582-3p reversed the inhibitory effect of A-H on LUAD cell proliferation (Fig. 8B, D, E). In the cell cycle assays, the overexpression of miR-582-3p abolished cell cycle arrest at the GO/G1 phase induced by A-H (Fig. 8C, F). WB revealed that the upregulation of miR-582-3p reversed this effect on the expression of p27, Cyclin D1, CDK4, CyclinE, CDK2, p-Rb, and E2 F3 induced by miR-582-3p (Fig. 9). These findings generally confirmed that A-H may inhibit LUAD proliferation through the miR-582-3p-p27 signaling pathway. A-H drug pair are traditional Chinese medicines with no significant toxicity. However, Hedyotis diffusa Willd is a cold-natured medicine. Long-term and high-dose use can damage the yang qi of the human body. Therefore, the common clinical dosage is 18 g of Astragalus membranaceus and 30 g of Hedyotis diffusa Willd. The CCK-8 assay showed that the proliferation inhibition rate of A549 cells by A-H was closely related to its concentration. Moreover, the intervention of the miR-582-3p could reverse the proliferation inhibition of A549 cells by A-H, suggesting that the effect of A-H on A549 cell proliferation is related to the pathway mediated by miR-582-3p, rather than its own toxicity.
A-H inhibited cell proliferation and arrested cell cycle at G0/G1 in A549 cells by downregulating miR-582-3p expression. (A) CCK-8 determined the relative viability of A549 cells treated with A-H-containing serum for 24 h; (B, D, E) The CCK-8 and colony formation assays demonstrated that the proliferation of A-H was suppressed by miR-582-3p and flow cytometry analysis revealed that A-H hindered the progression of the cell cycle through miR-582-3p (C, F). Data were presented as mean ± SD for three individual experiments, one-way ANOVA, **P < 0.01.
A-H upregulated the expression of proteins related to the G1/S phase through the miR-582–3p-p27 signaling pathway. A549 cells were treated with LV-mNC or LV-miR-582-3p mimic and incubated with medium containing normal or A-H serum for 24 h. The expression of p27 (A, B), Cyclin D1 (E, F), CDK4 (E, G), Cyclin E (E, H), CDK2 (E, I), Rb (E, J), p-Rb (E, K) and E2 F3 (E, L) in A549 cells from the indicated group were detected by western blot assay. The expression of miR-582-3p (C) and p27 (D) in A549 cells from the indicated group were detected by RT-PCR. Results were mean ± SD for three individual experiments, one-way ANOVA, *P < 0.05, **P < 0.01.
Discussion
Lung cancer is one of the most commonly diagnosed malignant forms of cancer worldwide and the leading cause of cancer-related deaths in both developed and developing countries1. Patients who experience either early-stage recurrence or deterioration at middle and late stages have a poor prognosis and a high mortality rate, which is closely associated with the progression of malignant solid tumors. The malignant proliferation of solid tumors is caused by the unlimited proliferation of lung cancer cells, and this excessive cell proliferation is closely linked to disrupted cell cycle progression7.
It is estimated that more than 60% of protein-coding genes are potentially modulated by various miRNAs19. Several studies have discovered that miRNAs play a diverse range of roles in gene imprinting and mRNA splicing. Specifically, they are involved in suppressing target genes, particularly those related to cancer cell proliferation and tumor advancement, at the post-transcriptional level20. The abnormal expression of miRNAs can influence many critical biological processes, like cell cycle and cell proliferation21. Many studies have shown that miRNAs are abnormally expressed in various common malignancies including lung cancer10. Additionally, their detectability and stability indicate that miRNAs have the potential to be used as biomarkers for tracking tumor progression and predicting patient prognosis22.
This study utilized high-throughput RNA sequencing to analyze both tumor and normal tissues of LUAD at TCGA. The results showed that some miRNAs were differentially expressed. Then miR-582-3p was identified as the most appropriate molecule in a follow-up study for its clinicopathological characteristics and prognosis. The results indicated that miR-582-3p was highly expressed in LUAD tumor tissues and cell lines. To further investigate the biological function of miR-582-3p in lung cancer, models of the miR-582-3p overexpression and miR-582-3p interference were developed using lentiviral vector gene transfer techniques. The overexpression of miR-582-3p accelerated the proliferation of A549 cells, increased cell proportion in the S phase, and contributed significantly to the expression of cell-cycle-related proteins, i.e., Cyclin D1, CDK4, Cyclin E, CDK2, p-Rb, and E2 F3. This means that miR-582-3p may promote the proliferation of A549 cells by regulating cell cycle proteins. Wang et al. found that circRAPGEF5 promoted bladder cancer cell proliferation, migration and invasion by competitively binding to miR-582-3p to upregulate KIF3 A expression11; Zheng et al. reported that NKILA promoted the progression of CCA through NKILA/miR-582-3p/YAP1 axis, which was modulated by NUNS2-mediated m5 C and METTL3-mediated m6 A modifications23. These two studies revealed the biological roles of miR-582-3p in different cancers, and miR-582-3p was meaningful for certain tumors in the proliferation inhibition, and was influenced by endogenous competitive binding patterns regulated by upstream circRNA or lncRNA, which altersed its impact on downstream target genes. Jin found that miR-582-3p was highly expressed in NSCLC tissues and lung cancer cells, by detecting the protein levels of wnt-5a, Axin, and SMAD324, it was inferred that miR-582-3p may regulate the growth process of lung cancer through the Wnt and TGF signaling pathways, which was consistent with my research findings. Except for only a few reports, a few studies have addressed the roles and mechanisms of miR-582-3p in LUAD. Therefore, it is necessary to deeply investigate the numerous biological functions, pathological processes, and the target and mechanism of action of miR-582-3p in lung cancer.
The target genes for miR-582-3p were analyzed using the Targetscan and miRDB databases to further investigate the molecular mechanisms of miR-582-3p in regulating the cycle and proliferation of A549 cells. p27 has attracted extensive research interest, owing largely to its role as a star molecule of the cell cycle. Therefore, p27 was selected as a possible target of the miRNA. This study showed that the expression of p27 significantly increased after miR-582-3p silencing. Moreover, the luciferase reporter assay and RNA pulldown confirmed that p27 directly binds to the miR-582-3p. Consistent with these findings, the study also found a negative correlation between miR-582-3p and p27 in relevant patients from TCGA. Accordingly, it can be concluded that the upregulation of miR-582-3p promotes the proliferation of tumor cells and influences the expression of cell cycle proteins, which may be related to the inhibition of p27.
Cell proliferation is stringently modulated by the cell cycle, which is divided into four stages: G0/G1, S, G2, and M, tightly controlled by a series of cell cycle checkpoints25. The progression of the cell cycle is positively regulated by cyclin-dependent kinases (CDKs), which are bound to and activated by their specific partners, cyclins. Conversely, the cell cycle is negatively regulated by CDK inhibitors (CKIs). The transition from G0/G1 to S phase in normal physiological processes refers to the initiation and completion of DNA replication. This process is precisely regulated by the Cyclin D1/CDK4/p27 and Cyclin E/CDK2/p27 signaling pathways. The kinase activity complexes formed by Cyclin D1/CDK4 and cyclin E/CDK2 catalyze the phosphorylation of the retinoblastoma (RB) protein. Once phosphorylated, RB dissociates from its binding partner, E2 F3. The freed E2 F3 molecules then enter the nucleus and act as transcription factors, participating in DNA replication. This ultimately promotes the progression from the G1 phase to the S phase of the cell cycle26. P27 plays a crucial role in regulating cell cycle progression from G0/G1 to S stage, It achieves this by interacting with the Cyclin D1/CDK4 and Cyclin E/CDK2 complexes, effectively inhibiting the activity of these kinases, which is necessary to maintain the proper balance of cellular number and function27. However, in pathological processes, the development and progression of a variety of human cancers, including lung cancer, are always accompanied by the downregulation of p2728. Gu et al. showed that ubiquitin-associated ___domain-containing protein 2 (UBAC2) promotes the development of bladder cancer proliferation, which is primarily attributed to its ability to regulate p27 by influencing the function of circular RNA BCRC-329. p27 is one of the most important targets for regulating cell cycle and cell proliferation.
Rescue experiments were conducted to demonstrate that p27 was a direct target of miR-582-3p for affecting the progression and proliferation of A549 cells. The results showed that the knockdown of p27 could reverse the favorable effects of downregulated miR-582-3p on cell growth, cell cycle, and cell cycle-related proteins in A549 cells, which demonstrated that miR-582-3p inhibited the protein level of p27, thereby negatively affecting the cell cycle and proliferation of A549 cells. The discovery of this mechanism undoubtedly provided scientific basis for miR-582-3p as a therapeutic target for LUAD, and expanded Dr. Jin’s understanding of miR-582-3p promoting non-small cell lung cancer24. The cell cycle is arrested at the G1/S phase, directly inducing cellular senescence, which is a major characteristic of aging. The senescence-associated secretory phenotype (SASP) regulates the secretion of interleukins and chemokines, collectively accelerating the immune clearance of senescent cells and inhibiting the proliferation of tumor cells30. Drugs that promote senescence have been developed based on this mechanism and are being tested in clinical trials. Therefore, from the perspective of aging, the overactivation of the miR-582-3p-p27 pathway inhibits tumor cell senescence, downregulates the density of macrophages, neutrophils, dendritic cells, mast cells, and other immune cells, suppresses immune clearance, and promotes malignant proliferation of LUAD cells. This undoubtedly adds deeper implications to the role of the miR-582-3p-p27 pathway in LUAD. Therefore, it is necessary to conduct in-depth research on the crosstalk between the cell cycle-based senescence and inflammatory factor pathways. Next, our research team will detect the transcription status of immune cells in the tumor microenvironment. The research group will perform cell analysis on LUAD tissue, and adopt technologies of Cellular Indexing of Transcriptomes and Epitopes by Sequencing(CITE-seq), Single-cell RNA Sequencing(scRNA-seq) and T cell receptor sequencing(TCR-seq) to identify immune cell population31. CITE-seq data will further confirm the cell identity by mature protein cell markers, GeoMx Digital Spatial Profiler(DSP) technology will detect the density, spatial position, and angiogenesis of immune cells aiming to explore the relationship between immune cell types and pathological types of patients, as well as the expression level of miR-582-3p. This will provide a scientific basis for inhibiting LUAD from the aspects of the immune microenvironment and cell interaction. In addition, this research provides scientific basis for A-H to inhibit the proliferation of LUAD cells. However, some patients with LUAD have genetic mutations. CITE-seq and scRNA-seq technology can identify tumor cell mutations, which provides a basis for studying the influence of A-H on drug resistance to targeted therapies. This will be another branch of the research that the team will carry out next. Literature report indicated that quercetin, widely present in both Astragalus mongholicus and Hedyotis diffusa Willd, could inhibit the proliferation of LUAD cells by upregulating miR-16 expression to reduce claudin-2 levels32. Quercetin also showed promise as a dietary phytochemical that may offer preventive and therapeutic possibilities for malignant mesothelioma through the regulation of microRNAs33. The main component of Hedyotis diffusa Willd, ursolic acid, can weaken the stemness and chemotherapy resistance of non-small cell lung cancer cells via the miRNA-149-5p/MyD88 axis34. These findings provided insights into targeting important regulatory molecules like miRNAs, starting from the smallest herbal formula-A-H pair, which could synergistically enhance effects and facilitate better clinical translation. Chinese herbal medicine is known as a natural compound. A-H pair are authentic Chinese medicinal herbs. In terms of dosage, the Pharmacopoeia of the People’s Republic of China states: Astragalus mongholicus is recommended at a dosage of 9–30 g; Hedyotis diffusa Willd at 15–30 g. According to clinical guidelines and preliminary experimental studies, 18 g of Astragalus membranaceus and 30 g of Hedyotis diffusa were selected for mechanism exploration18. These findings generally confirmed that miR-582-3p promoted LUAD cell proliferation by downregulating p27 and A-H may inhibit LUAD proliferation through the miR-582-3p-p27 signal pathway.
Conclusion
The study results suggested that miR-582-3p promoted LUAD cell proliferation by downregulating p27 expression and A-H may inhibited cell proliferation through the miR-582-3p-p27 signaling pathway (Fig. 10). The study findings elucidated not only miR-582-3p acting as a potential predictive biomarker of adverse prognosis and tumor progression for LUAD, but providing a scientific basis for tumor suppression by traditional Chinese medicine and laying a theoretical foundation for the clinical transformation of traditional Chinese medicine outcomes. However, this study predominantly focused on cell experiments, and there was still a relative lack of animal models. Therefore, the authors will conduct in vivo experiments in future studies to validate the findings of this study. Next, our research team will transplant stable A549 cell lines which have been successfully modeled into nude mice to establish A549 LUAD Xenograft Model to investigate the impact of high and low miR-582-3p expression on the growth of LUAD xenografts, to explore the impact of miR-582-3p on the growth of LUAD xenografts by regulating p27 and to observe the impact of A-H on the growth of LUAD xenografts by regulating miR-582-3p. It has been documented in the literature that A549 cells containing luciferase were injected into the tail vein of the mouse metastasis model. After intraperitoneal injection of saline or Astragalus Polysaccharide (PG2), the metastasis of A549-Luc cells was monitored. It was found that the fluorescence intensity of the lungs in mice treated with PG2 was significantly reduced, and the number of pulmonary metastatic nodules was significantly decreased. H&E staining showed that the tumor foci in the PG2 treatment group were less severe. This suggests that PG2 can directly act on lung tissue to inhibit the invasiveness of lung adenocarcinoma cells35; Huang et al. reported that after injection of saline or Hedyotis diffusa Willd injection (HDI) into nude mice with transplanted LUAD, the impact of HDI on the growth of lung adenocarcinoma cells in vivo was monitored. The results showed that HDI caused significant damage to LUAD tumor tissue36. The above experiments indicate that both Astragalus membranaceus and Hedyotis diffusa Willd can directly act on lung tissue and have a significant inhibitory effect on LUAD. The research group will further verify the action pathway of A-H in lung tissue in animal experiments to have a clearer understanding of the pharmacokinetics of the pair. miR-582-3p has several target genes, such as TFRC, CDKN1B (p27), TSPAN19, ZNF627, CMC2, DEC1 (http://www.targetscan.org/vert_72/). It is not clear if p27 is the sole target of miR-582-3p in promoting cell cycle and proliferation in A549 cells. miR-582-3p has numerous upstream regulators including lncRNAs, such as LINC00921, NEAT1, MAGI2-AS3, PRKCQ-AS1, SNHG14 (https://starbase.sysu.edu.cn/).These findings provide critical insights into the complex network formed by the upstream regulators and downstream targets of miR-582-3p, elucidating its role in promoting tumor progression. Moreover, they offer further scientific evidence supporting the potential of miR-582-3p as a therapeutic target in cancer treatment. Specifically, CircSHKBP1 act as “sponges” to absorb miR-582-3p to upregulate HUR and VEGF as well as decoying HSP90 by competing with STUB1 to promote GC progression37, and the dysfunction of certain star transcription factors can enhance the transcription of miR-582-3p and then downregulate the expression of p27, promoting tumor proliferation38. The regulatory mechanism of miR-582-3p upstream transcription has been rarely reported. Finding answers to these questions requires larger experiments studies. Scientific research should serve the clinical needs. Next, the research team will carry out clinical trials under the support of ethics to explore the impact of A-H on the prognosis of LUAD, and rely on the cooperation of pharmaceutical enterprises to develop relevant effective traditional Chinese patent drug to achieve the transformation of scientific research achievements. However, since this clinical trial excludes patients with gene mutations, and some patients with stage III LUAD have gene mutations, targeted drug therapy is a very important mean of treatment. Therefore, is there a conflict between taking targeted drugs and A-H? Or does A-H actively inhibit the drug resistance caused by long-term use of targeted drugs? Of course, this requires a large number of clinical samples and continued basic research.
`
Data availability
The datasets generated and analysed during the current study are available in the FAIRsharing.org repository, [https://doi.org/10.6084/m9.figshare.28528637.v1].
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Han, B. et al. Cancer incidence and mortality in China, Journal of the National Cancer Center 4, 47–53 (2024). (2022). https://doi.org/10.1016/j.jncc.2024.01.006
Nooreldeen, R. & Bach, H. Current and future development in lung Cancer diagnosis. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22168661 (2021).
Donington, J., Schumacher, L. & Yanagawa, J. Surgical issues for operable Early-Stage Non–Small-Cell lung Cancer. J. Clin. Oncol. 40, 530–538. https://doi.org/10.1200/jco.21.01592 (2022).
Bi, J. H. et al. Observed and relative survival trends of lung cancer: A systematic review of population-based cancer registration data. Thorac. Cancer. 15, 142–151. https://doi.org/10.1111/1759-7714.15170 (2023).
Jacobsen, M. M. et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer. 112, 156–164. https://doi.org/10.1016/j.lungcan.2017.08.011 (2017).
Weston, W. A. & Barr, A. R. A cell cycle centric view of tumour dormancy. Br. J. Cancer. 129, 1535–1545. https://doi.org/10.1038/s41416-023-02401-z (2023).
Lee, Y. S. & Dutta, A. MicroRNAs in Cancer. Annu. Rev. Pathol. 4, 199–227. https://doi.org/10.1146/annurev.pathol.4.110807.092222 (2009).
Kousar, K. et al. MiRNAs in regulation of tumor microenvironment, chemotherapy resistance, immunotherapy modulation and MiRNA therapeutics in Cancer. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms232213822 (2022).
Lobera, E. S., Varela, M. A., Jimenez, R. L. & Moreno, R. B. MiRNA as biomarker in lung cancer. Mol. Biol. Rep. 50, 9521–9527. https://doi.org/10.1007/s11033-023-08695-9 (2023).
Wang, C. & Yang, X. CircRAPGEF5 sponges miR-582-3p and targets KIF3A to regulate bladder cancer cell proliferation, migration and invasion. Int. Immunopharmacol. 131 https://doi.org/10.1016/j.intimp.2024.111613 (2024).
Dai, T. et al. The miRNA mir-582-3p suppresses ovarian cancer progression by targeting AKT/MTOR signaling via lncRNA TUG1. Bioengineered 12, 10771–10781 (2021). https://doi.org/10.1080/21655979.2021.2003662
Nilmani. et al. CDK regulators-Cell cycle progression or apoptosis-Scenarios in normal cells and cancerous cells. Adv. Protein Chem. Struct. Biol. 135, 125–177. https://doi.org/10.1016/bs.apcsb.2022.11.008 (2023).
Chu, I. M., Hengst, L. & Slingerland, J. M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 8, 253–267. https://doi.org/10.1038/nrc2347 (2008).
Currier, A. W. et al. p27/Kip1 functions as a tumor suppressor and oncoprotein in osteosarcoma. Sci. Rep. 9 https://doi.org/10.1038/s41598-019-42450-0 (2019).
Li, Z., Feiyue, Z. & Gaofeng, L. Traditional Chinese medicine and lung cancer——From theory to practice. Biomed. Pharmacother. 137 https://doi.org/10.1016/j.biopha.2021.111381 (2021).
Shi, J., Hao, S., Liu, X., Li, Y. & Zheng, X. Feiyiliu mixture sensitizes EGFRDel19/T790M/C797S mutant non-small cell lung cancer to osimertinib by attenuating the PRC1/Wnt/EGFR pathway. Front. Pharmacol. 14 https://doi.org/10.3389/fphar.2023.1093017 (2023).
Sun, H., Ma, S., Zhuang, H., Zhao, Y. & Liu, S. Mechanism of scutellariae Radix-Hedyotidis herba against proliferation of lung adenocarcinoma A549 cells. Chin. J. Experimental Traditional Med. Formulae. 27, 28–35 (2021). (in Chinese).
van Rooij, E. The Art of MicroRNA research. Circul. Res. 108, 219–234. https://doi.org/10.1161/circresaha.110.227496 (2011).
Liu, H. et al. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol. Cancer. 17 https://doi.org/10.1186/s12943-018-0765-5 (2018).
Pedroza-Torres, A. et al. MicroRNAs in tumor cell metabolism: roles and therapeutic opportunities. Front. Oncol. 9 https://doi.org/10.3389/fonc.2019.01404 (2019).
Yang, H. et al. MiRNA-Based Therapies for Lung Cancer: Opportunities and Challenges? Biomolecules 13 (2023). https://doi.org/10.3390/biom13060877
Zheng, H., Zhu, M., Li, W., Zhou, Z. & Wan, X. m5C and m6A modification of long noncoding NKILA accelerates cholangiocarcinoma progression via the miR-582‐3p‐YAP1 axis. Liver Int. 42, 1144–1157. https://doi.org/10.1111/liv.15240 (2022).
Jin, X. Screening of differentially expressed MiRNA and circrna of non-small cell lung cancer and preliminary verification of miR-582-3p function. Jilin Univ. (2021). (in Chinese).
Goldberg, M. L. & Gunaratne, G. H. Cell biology: how to short-circuit the cell cycle. Curr. Biol. 32, R561–R563. https://doi.org/10.1016/j.cub.2022.05.013 (2022).
Kamranvar, S. A., Rani, B. & Johansson, S. Cell cycle regulation by Integrin-Mediated adhesion. Cells 11 https://doi.org/10.3390/cells11162521 (2022).
Gardner, L. B. et al. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 276, 7919–7926. https://doi.org/10.1074/jbc.M010189200 (2001).
Dobashi, Y. et al. Regulation of p27 by ubiquitin ligases and its pathological significance in human lung carcinomas. Hum. Pathol. 66, 67–78. https://doi.org/10.1016/j.humpath.2017.05.022 (2017).
Gu, C. et al. UBAC2 promotes bladder cancer proliferation through BCRC-3/miRNA-182-5p/p27 axis. Cell Death Dis. 11 https://doi.org/10.1038/s41419-020-02935-7 (2020).
Calcinotto, A. et al. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99, 1047–1078. https://doi.org/10.1152/physrev.00020.2018 (2019).
Leader, A. M. et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell. 39, 1594–1609e1512. https://doi.org/10.1016/j.ccell.2021.10.009 (2021).
Sonoki, H. et al. Quercetin decreases Claudin-2 expression mediated by Up-Regulation of MicroRNA miR-16 in lung adenocarcinoma A549 cells. Nutrients 7, 4578–4592. https://doi.org/10.3390/nu7064578 (2015).
Sayeed, M. A. et al. Regulation of MicroRNA using promising dietary phytochemicals: possible preventive and treatment option of malignant mesothelioma. Biomed. Pharmacother. 94, 1197–1224. https://doi.org/10.1016/j.biopha.2017.07.075 (2017).
Chen, Q. et al. The miRNA-149‐5p/MyD88 axis is responsible for ursolic acid‐mediated Attenuation of the stemness and chemoresistance of non‐small cell lung cancer cells. Environ. Toxicol. 35, 561–569. https://doi.org/10.1002/tox.22891 (2019).
Liao, C. H. et al. Astragalus polysaccharide (PG2) suppresses macrophage migration inhibitory factor and aggressiveness of lung adenocarcinoma cells. Am. J. Chin. Med. 48, 1491–1509. https://doi.org/10.1142/s0192415x20500731 (2020).
Huang, F. et al. Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine 104 https://doi.org/10.1016/j.phymed.2022.154319 (2022).
Xie, M. et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer. 19 https://doi.org/10.1186/s12943-020-01208-3 (2020).
Sun, H., Xu, L., Liu, X. & Liu, S. Molecular mechanism research of Astragalus Membranaceus and Hedyotis diffusa regulate Sp1-miR-582-3p-p27 Axis to inhibit lung adenocarcinoma proliferation. Chin. J. Experimental Traditional Med. Formulae. 31, 116–124 (2025). (in Chinese).
Acknowledgements
The authors would like to thank the Shandong Provincial Natural Science Foundation (No. ZR2023QH072) and Science and Technology Development Plan of Shandong Administration of Traditional Chinese Medicine program (No. 2021Q076) for financial their support, the Experimental Center of Shandong University of Traditional Chinese Medicine for providing the experimental platform, the Shandong Chinese Medicine Antiviral Engineering Research Center for providing technical support, and the TCGA network for providing high-throughput data.
Funding
This research was supported by the Shandong Provincial Natural Science Foundation (No. ZR2023QH072) and the Science and Technology Development Plan of the Shandong Administration of Traditional Chinese Medicine program (No. 2021Q076).
Author information
Authors and Affiliations
Contributions
SL and TM designed the experiments and provided technical guidance throughout the experiments. HS performed the experiments and wrote the manuscript. LX analyzed the data. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
The animal experiments were conducted according to established animal welfare guidelines, and the animal study was reviewed and approved by Ethics Review Committee of Shandong University of Traditional Chinese Medicine, ethical approval number was SDUTCM20221130010. The euthanasia method for experimental animals needs to follow animal welfare and ethical principles, ensuring that animals end their lives without pain and with minimal fear. The euthanasia method for this experimental animal was cervical dislocation method.
Research statement
Our research was conducted strictly in accordance with the ARRIVE guidelines, which involved the feeding of experimental animals, animal sampling, cell culture and transfection, result analysis, statistical significance, etc., all of which were conducted in accordance with established scientific methods.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Xu, L., Liu, S. et al. Astragalus mongholicus and Hedyotis diffusa willd inhibit cell proliferation by attenuating the miR-582-3p-p27 signaling pathway in LUAD. Sci Rep 15, 13411 (2025). https://doi.org/10.1038/s41598-025-97996-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97996-z