Abstract
Almost half of the human genome consists of retrotransposons—‘parasitic’ sequences that insert themselves into the host genome via an RNA intermediate. Although most of these sequences are silenced or mutationally deactivated, they can present opportunities for evolutionary innovation: mutation of a deteriorating retrotransposon can result in a gene that provides a selective advantage to the host in a process termed ‘domestication’1,2,3. The PNMA family of gag-like capsid genes was domesticated from an ancient vertebrate retrotransposon of the Metaviridae clade at least 100 million years ago4,5. PNMA1 and PNMA4 are positively regulated by the master germ cell transcription factors MYBL1 and STRA8, and their transcripts are bound by the translational regulator DAZL during gametogenesis6. This developmental regulation of PNMA1 and PNMA4 expression in gonadal tissue suggested to us that they might serve a reproductive function. Through the analysis of donated human ovaries, genome-wide association studies (GWASs) and mouse models, we found that PNMA1 and PNMA4 are necessary for the maintenance of a normal reproductive lifespan. These proteins self-assemble into capsid-like structures that exit human cells, and we observed large PNMA4 particles in mouse male gonadal tissue that contain RNA and are consistent with capsid formation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
PNMA4-IP particle sequencing data generated and analyzed in this study were deposited in the Gene Expression Omnibus (GEO) under accession number GSE287603. Published datasets analyzed in this paper are also available in the GEO: human ovary snRNA-seq data under accession number GSE202601; human oocyte single-cell RNA-seq data under accession numbers GSE107746 and GSE155179; human testes snRNA-seq data under accession number GSE106487; and mouse ovary snRNA-seq data under accession numbers GSE267729 and GSE232309. All other data, including source data, supporting the conclusions in this paper are available in the published article and extended data. Further information and requests for data, resources and reagents should be directed to, and will be fulfilled by, the corresponding author (L.E.B.).
References
Kaneko-Ishino, T. & Ishino, F. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals. Front. Microbiol. 3, 262 (2012).
Jangam, D., Feschotte, C. & Betrán, E. Transposable element domestication as an adaptation to evolutionary conflicts. Trends Genet. 33, 817–831 (2017).
Naville, M. et al. Not so bad after all: retroviruses and long terminal repeat retrotransposons as a source of new genes in vertebrates. Clin. Microbiol. Infect. 22, 312–323 (2016).
Kokošar, J. & Kordiš, D. Genesis and regulatory wiring of retroelement-derived domesticated genes: a phylogenomic perspective. Mol. Biol. Evol. 30, 1015–1031 (2013).
Henriques, W. S. et al. The diverse evolutionary histories of domesticated metaviral capsid genes in mammals. Mol. Biol. Evol. 41, msae061 (2024).
Laureau, R. et al. Meiotic cells counteract programmed retrotransposon activation via RNA-binding translational repressor assemblies. Dev. Cell 56, 22–35 (2021).
Pang, S. W., Lahiri, C., Poh, C. L. & Tan, K. O. PNMA family: protein interaction network and cell signalling pathways implicated in cancer and apoptosis. Cell. Signal. 45, 54–62 (2018).
Devine, M. F., Kothapalli, N., Elkhooly, M. & Dubey, D. Paraneoplastic neurological syndromes: clinical presentations and management. Ther. Adv. Neurol. Disord. 14, 1756286420985323 (2021).
Zhang, X.-L. et al. Pnma5 is essential to the progression of meiosis in mouse oocytes through a chain of phosphorylation. Oncotarget 8, 96809–96825 (2017).
Soumillon, M. et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 3, 2179–2190 (2013).
Yuan, L. et al. Single‐cell transcriptome analysis of human oocyte ageing. J. Cell. Mol. Med. 25, 6289–6303 (2021).
Zhang, Y. et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol. Cell 72, 1021–1034 (2018).
Wang, M. et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 23, 599–614 (2018).
Jin, C. et al. Molecular and genetic insights into human ovarian aging from single-nuclei multi-omics analyses. Nat. Aging 5, 275–290 (2024).
Zagore, L. L. et al. DAZL regulates germ cell survival through a network of polyA-proximal mRNA interactions. Cell Rep. 25, 1225–1240 (2018).
Rosario, R., Smith, R. W. P., Adams, I. R. & Anderson, R. A. RNA immunoprecipitation identifies novel targets of DAZL in human foetal ovary. Mol. Hum. Reprod. 23, 177–186 (2017).
Yang, C.-R. et al. The RNA-binding protein DAZL functions as repressor and activator of mRNA translation during oocyte maturation. Nat. Commun. 11, 1399 (2020).
Lonsdale, J. et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Isola, J. V. V. et al. A single-cell atlas of the aging mouse ovary. Nat. Aging 4, 145–162 (2024).
Ashley, J. et al. Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262–274 (2018).
Pastuzyn, E. D. et al. The neuronal gene Arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell 172, 275–288 (2018).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Xu, J. et al. PNMA2 forms immunogenic non-enveloped virus-like capsids associated with paraneoplastic neurological syndrome. Cell 187, 831–845 (2024).
Dodonova, S. O., Prinz, S., Bilanchone, V., Sandmeyer, S. & Briggs, J. A. G. Structure of the Ty3/Gypsy retrotransposon capsid and the evolution of retroviruses. Proc. Natl Acad. Sci. USA 116, 10048–10057 (2019).
Peyret, H. A protocol for the gentle purification of virus-like particles produced in plants. J. Virol. Methods 225, 59–63 (2015).
Mountjoy, E. et al. An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nat. Genet. 53, 1527–1533 (2021).
Ghoussaini, M. et al. Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 49, D1311–D1320 (2021).
Consortium, T. E. C. A. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 26, 252–258 (2020).
Armstrong, N. S.- et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 53, 185–194 (2021).
Barton, A. R., Sherman, M. A., Mukamel, R. E. & Loh, P.-R. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat. Genet. 53, 1260–1269 (2021).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104, 65–75 (2019).
Hollis, B. et al. Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat. Commun. 11, 1536 (2020).
Conine, C. C. & Rando, O. J. Soma-to-germline RNA communication. Nat. Rev. Genet. 23, 73–88 (2022).
Coticchio, G. et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 21, 427–454 (2015).
Clarke, H. J. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip. Rev. Dev. Biol. https://doi.org/10.1002/wdev.294 (2018).
Macaulay, A. D. et al. Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation. Biol. Reprod. 94, 16 (2014).
Macaulay, A. D. et al. The gametic synapse: RNA transfer to the bovine oocyte. Biol. Reprod. 91, 90 (2014).
Wang, H. et al. Rejuvenation of aged oocyte through exposure to young follicular microenvironment. Nat. Aging 4, 1194–1210 (2024).
Tan, C. T. et al. MOAP-1 mediates Fas-induced apoptosis in liver by facilitating tBid recruitment to mitochondria. Cell Rep. 16, 174–185 (2016).
Vos, M. D. et al. The RASSF1A tumor suppressor activates Bax via MOAP-1. J. Biol. Chem. 281, 4557–4563 (2006).
Fu, N. Y., Sukumaran, S. K. & Yu, V. C. Inhibition of ubiquitin-mediated degradation of MOAP-1 by apoptotic stimuli promotes Bax function in mitochondria. Proc. Natl Acad. Sci. USA 104, 10051–10056 (2007).
Tan, K. O. et al. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J. Biol. Chem. 276, 2802–2807 (2001).
Berchowitz, L. E. et al. Regulated formation of an amyloid-like translational repressor governs gametogenesis. Cell 163, 406–418 (2015).
Elguindy, M. M. & Mendell, J. T. NORAD-induced Pumilio phase separation is required for genome stability. Nature 595, 303–308 (2021).
Martins, J. P. S. et al. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J. Cell Sci. 129, 1271–1282 (2016).
Cao, Q., Padmanabhan, K. & Richter, J. D. Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. RNA 16, 221–227 (2010).
Deo, V. K., Kato, T. & Park, E. Y. Chimeric virus-like particles made using GAG and M1 capsid proteins providing dual drug delivery and vaccination platform. Mol. Pharm. 12, 839–845 (2015).
Madigan, V. et al. Human paraneoplastic antigen Ma2 (PNMA2) forms icosahedral capsids that can be engineered for mRNA delivery. Proc. Natl Acad. Sci. USA 121, e2307812120 (2024).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Scheffler, K., Giannini, F., Lemonnier, T. & Mogessie, B. The prophase oocyte nucleus is a homeostatic G-actin buffer. J. Cell Sci. 135, jcs259807 (2022).
Mogessie, B. Visualization and functional analysis of spindle actin and chromosome segregation in mammalian oocytes. Methods Mol. Biol. 2101, 267–295 (2019).
Andersen, K. R., Leksa, N. C. & Schwartz, T. U. Optimized E. coli expression strain LOBSTR eliminates common contaminants from His-tag purification. Proteins 81, 1857–1861 (2013).
Gallagher, J. R., Kim, A. J., Gulati, N. M. & Harris, A. K. Negative‐stain transmission electron microscopy of molecular complexes for image analysis by 2D class averaging. Curr. Protoc. Microbiol. 54, e90 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A.cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Gaspary, A. et al. Rie1 and Sgn1 form an RNA-binding complex that enforces the meiotic entry cell fate decision. J. Cell Biol. 222, e202302074 (2023).
Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ottoz, D. S. M., Tang, L., Jovanovic, M., Dyatel, A. & Berchowitz, L. E. Assembly and function of the amyloid-like translational repressor Rim4 is coupled with nutrient conditions. EMBO J. 42, e113332 (2023).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Yang, M. et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J. Neurosci. 32, 6525–6541 (2012).
Acknowledgements
We thank V. Brandt, S. Goff and S.-O. Park for guidance and critical reading of the manuscript. We thank E. Xu for providing unpublished data as a personal communication. We thank I. Adams, R. Reid and G. Karsenty for technical assistance and helpful discussions. We thank the histology cores at CUIMC and at Rutgers, the mouse behavior core at CUIMC, the ligand core at the University of Virginia, B. Mayfield and J. T. George for technical assistance. We thank C.-S. Li at the Genetically Modified Mouse Model Shared Resource at CUIMC for generation of knockout mice. We thank Y. Sabo for plasmids, C.-M. Ho for preliminary negative stain TEM, H. Chung for sharing use of her microscope and S. Sternberg for sharing use of his FPLC. Research in the Berchowitz laboratory is supported by the Schaefer Research Scholars Program, the Irma T. Hirschl Family Trust and the NIH (R35 GM124633 to L.E.B.). Research in the Wiedenheft laboratory is supported by the NIH (R35 GM134867 to B.W.), the M.J. Murdock Charitable Trust and the Montana State University Agricultural Experimental Station (US Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA)). Research in the Suh laboratory is supported by the NIH (R01 AG069750 to Y.S.) and a grant (GCRLE-1320) from the Global Consortium for Reproductive Longevity and Equality at the Buck Institute, made possible by the Bia-Echo Foundation. Research in the Schindler laboratory is supported by the NIH (R35 GM136340 to K.S.). Research in the Mogessie laboratory is supported by the Wellcome Trust (213470/A/18/Z) and the NIH (R35 GM146725 to B.M.). Research in the Jain laboratory is supported by the NIH (R35 GM147130 to D.J.) and Human Genetics Institute of New Jersey laboratory startup funds. Research in the Gennarino laboratory is supported by the NIH (National Institute of Neurological Disorders and Stroke R01 NS109858 to V.A.G.), the Neurodegeneration Challenge Network/Chan Zuckerberg Initiative, the Paul A. Marks Scholar Program, the TIGER Award at CUIMC and the Columbia Stem Cell Initiative. Research in the Stout laboratory is supported by the NIH (R01 AG069742 to M.B.S) and the Global Consortium for Reproductive Longevity and Equality (GCRLE-4501 to M.B.S).
Author information
Authors and Affiliations
Contributions
Conceptualization: T.W.P.W., R.L. and L.E.B. Methodology: T.W.P.W., W.S.H., H.B.C., R.L., M.B.S., V.A.G., B.M., D.J., K.S., Y.S., B.W. and L.E.B. Formal analysis: T.W.P.W., W.S.H., M.R., S.S., J.S., C.J., S.K. and J.V.V.I. Investigation: T.W.P.W., W.S.H., H.B.C., M.R., C.S.B., S.S., J.S., H.B., C.J., A.C., R.C.K., J.V.V.I., B.M. and L.E.B. Original draft: T.W.P.W. and L.E.B. Review and editing: all authors. Visualization: T.W.P.W., W.S.H., S.S., J.S., H.B., C.J., J.V.V.I., B.M., D.J. and L.E.B. Supervision and funding acquisition: M.B.S., V.A.G., B.M., D.J., K.S., Y.S., B.W. and L.E.B.
Corresponding author
Ethics declarations
Competing interests
T.W.P.W., H.B.C. and L.E.B. are co-inventors on a PCT international patent application filed by Columbia University related to this work (PCT/US2024/053329). B.W. is the founder of SurGene, LLC and an inventor on patent applications related to CRISPR–Cas systems and applications thereof. All other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Diana Laird and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Human gonadal expression of PNMA2, PNMA3, and PNMA5.
(a) Analysis of uniquely mapping single-cell RNAseq reads for PNMA2, PNMA3, and PNMA5 loci expressed in spermatogonial stem cells (SSC), differentiating spermatogonia (Dif. Sp.), leptotene spermatocytes (Lepto.), grouped zygotene/pachytene/diplotene spermatocytes (ZPD), transitional spermatocytes (Trans.), post-meiotic spermatids (PMS), Leydig cells, Sertoli cells, and macrophages (data from Wang et al.13, N = 8 donors 27–60 years of age). (b) Ovaries from reproductively young (23–29 years, magenta, N = 4) and reproductively old (49–54 years, gray, N = 4) donors were analyzed by single-nuclei RNAseq. Uniquely mapping reads for PNMA2, PNMA3, and PNMA5 loci were assigned to ovarian tissue types based on clustering analysis. Statistical significance was determined by Mann-Whitney test (all comparisons were not significant).
Extended Data Fig. 2 PNMA1 and PNMA4 can be post-transcriptionally regulated by DAZL and are evolutionarily conserved.
(a) 3V5-tagged PNMA1, PNMA4, or SMC1B (positive control DAZL target) expression plasmids were transfected into HEK-293T cells with and without DAZL co-transfection. (b, c) Protein levels (b) of PNMA1, PNMA4, SMC1B, DAZL, and α-tubulin (loading) were analyzed by immunoblot and PNMA1 and PNMA4 mRNA levels (c) were analyzed by Northern blot. This experiment was repeated independently 3 times with similar results. (d, e) Architecture of the human PNMA1 and PNMA4. Loci are colored according to domains: capsid ___domain (CA, magenta), linker (L, lilac), and RNA-binding ___domain (RBD, purple). The promoter (green) and transcription start sites are shown using arrows. A black triangle on the phylogenetic tree (left) indicates the point of the first expansion of the ancestral PNMA locus leading to PNMA1-5. PNMA1 is universally retained across placental mammals as an intact gene, whereas PNMA4 has experienced lineage-specific pseudogenization (boxed x’s). Conservation at each amino acid is shown with a vertical black line for fifteen eutherian mammals, three marsupials, and two vertebrate outgroups. The histone modification for active transcription (H3K27Ac) in humans is shown along the bottom.
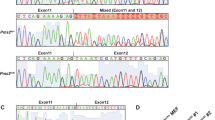
Extended Data Fig. 3 Generation of Pnma1−/− and Pnma4−/− mouse knockouts.
(a) Pnma1 and Pnma4 deletions were generated by CRISPR/Cas9 genome editing (sgRNA target sites shown in dashed lines). Successful deletion was assessed by PCR/sequencing. Shown are chromosomal coordinates, annotated transcripts (CDS in navy, UTRs in cyan, and introns in white), deleted regions, and sequencing reads (below, maroon) confirming deletion of Pnma1 and Pnma4. (b, c) Analysis of mRNA and protein produced in the Pnma1−/− and Pnma4−/− mutants. A testis was dissected from 3-month wild type (C57BL/6 J), Pnma1−/−, and Pnma4−/− mice. Lysate was prepared and split for mRNA and protein analysis. (b) Analysis of Pnma1 and Pnma4 mRNA levels by qPCR (n = 3 replicates). (c) Analysis of PNMA1, PNMA4, and α-tubulin (loading) protein levels by immunoblot. Shown below is a quantification of the ratio of PNMA1 and PNMA4 in knockouts vs. wild type (set to 1) from n = 3 biological replicates. ** denotes presence of an unfortunate cross-reacting band present in all lanes of the α-PNMA4 immunoblot. Error bars indicate SEM. (d) Male wild type mice (gray), Pnma1−/− (cyan), or Pnma4−/− (purple) were crossed biweekly to CF-1 female fertility tester mice (N = 5 pairs for each genotype-timepoint combination). (e) Female wild type mice (gray), Pnma1−/− (cyan), or Pnma4−/− (purple) were crossed to B6D21/J male fertility tester mice (N = 5 pairs for each genotype-timepoint combination). Pup numbers for each cross were recorded. This is the same experiment used to generate the data in Figs. 2a and 3a, but here average pup count per litter is plotted. Statistical significance was determined by student’s t-test (n.s. p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001). Error bars indicate SEM. Exact p-values available in source data.
Extended Data Fig. 4 Mice lacking Pnma1 or Pnma4 exhibit normal neurobehavioral and muscular traits.
(a-g) Six-month-old male wild type (N = 11), Pnma1−/− (N = 11), Pnma4−/− (N = 8), and Pnma1−/− Pnma4−/− double mutant mice (N = 11) were tested in a panel of behavioral assays. (a) Short-term memory was assessed using the spontaneous alternation percentage in a Y maze. (b) Long-term memory was assessed with the fear conditioning test. (c-e) The open field test was used to assess indicators of anxiety (c, center time) and hyperactivity (d, distance travelled and e, vertical counts). (f) We assessed muscle strength by measuring inverted hang time on a grid and (g) grip strength force. All graphs indicate mean +/− SEM. Statistical significance was assessed by multiple t-test analysis with correction for multiple comparisons or one-way ANOVA with correction for multiple comparisons (n.s. p > 0.05, * p ≤ 0.05, ** p ≤ 0.01).
Extended Data Fig. 5 Defective testicular and ovarian characteristics in Pnma1 and Pnma4 mutants.
(a) Representative PAS-stained testis sections by age. Quantified in Fig. 2. (b) Representative PAS-stained ovary sections by age. Quantified in Fig. 3 and Extended Data Fig. 6. (c, d) Testes were fixed, paraffin embedded, and sectioned. Sections from 12-month samples were analyzed by TUNEL and DAPI staining. (a) Images and (b) quantifications (from n wild type-like tubules) of PLZF-positive cells (red arrows) are shown. Statistical significance was determined by Mann-Whitney test and exact p-values are shown. Error bars indicate SD.
Extended Data Fig. 6 Several ovarian features are not dramatically altered in Pnma1 and Pnma4 mutants.
(a-e) Ovaries from control (pooled wild type and heterozygote, gray), Pnma1−/− (cyan), Pnma4−/− (purple), or Pnma1−/− Pnma4−/− double mutant (red) mice were fixed, embedded, and PAS-stained. The following features were quantified per unit area (mm2) at the indicated times: (a) primary follicles, (b) secondary follicles, (c) corpora lutea, (d) post-ovulation follicles, and (e) atretic follicles. Statistical significance was determined by determined by one-way ANOVA with correction for multiple comparisons. Error bars indicate SEM. (f-i) GV oocytes were collected from ovaries of two-month-old wild-type and Pnma1−/− Pnma4−/− mice. We then injected mRNA encoding mClover-MAP4 (microtubule-binding protein) and H2B-mScarlet (histone) and imaged oocytes live for ~18 hours. (f) Representative images of the first meiotic division from the metaphase I (MI) plate (t = 0 min) to anaphase I (AI, t = 20 min). Misaligned chromosomes were counted at t = 0 min and lagging chromosomes at t = 12 min. (g) Time in hours (hr) between nuclear envelop breakdown to AI onset. (h) AI lagging chromosome quantification. (i) Misaligned chromosomes at on MI plate quantification (J) Misaligned chromosomes at on MII plate quantification (4 hours after meiosis I completion). (k, l) Continued data from Fig. 3. As in f-j, but GV oocytes were collected at 7 months. (k) Misaligned chromosomes at on MI plate quantification. (l) Misaligned chromosomes at on MII plate quantification (4 hours after meiosis I completion). Statistical significance was determined by determined by student’s t-test. Error bars indicate SEM (n.s. p > 0.05, * p ≤ 0.05).
Extended Data Fig. 7 Single-cell analysis of Pnma1-5 expression in aging mouse ovaries.
Ovaries from young (4.5 month, magenta, N = 5), peri-estropause (10.5 month, orange, N = 7), and post-estropause (15.5 month, gray, N = 5) wild type C57BL/6 J mice were analyzed by single-cell RNAseq. Uniquely mapping reads for Pnma1-5 loci were assigned to ovarian tissue types based on clustering analysis. Note that the majority of values = 0 and lie underneath the x axis. Total n of each cell type and percentage of positive cells is indicated above the plots. Statistical significance was determined by Mann-Whitney test (n.s. p > 0.05, exact p-values are provided for significant comparisons up to p < 0.0001). Error bars indicate SEM. Comparisons were not run between conditions with no positive cells.
Extended Data Fig. 8 Pnma1 and Pnma4 are expressed in subpopulations of mouse ovarian cells.
(a, b) Single cell RNA-seq was performed in n = 8 ovaries from adult C57BL/6 J mice. (a) UMAP plot showing distinct ovarian cell populations represented by different colors with Pnma1 and Pnma4-positive cells denoted in black. (b) Average expression of Pnma1 and Pnma4 by sample across different ovarian cell populations. Statistical significance was determined by one-way ANOVA followed by Tukey’s post-hoc test. Significant differences were defined at P < 0.05. (n.s. p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001). Error bars indicate SEM.
Extended Data Fig. 9 PNMA1 and PNMA4 form capsid-sized particles.
(a, b) His-tagged PNMA1 (a) and PNMA4 (b) were expressed in E. coli and affinity purified on nickel resin. Imidazole elutions (15 ml) were concentrated and run over a Superose 6 (HiScale 80 ml) size exclusion column. Shown are UV absorbance traces of the run and Coomassie-stained SDS-PAGE of the fractions. The fractions used for TEM are noted in red. (c) Experimental setup: 3V5-tagged PNMA1, PNMA4, and ARC (control exported VLP) expression plasmids were transfected into HEK-293T cells. mCherry (control non-capsid) was co-expressed from the transfected plasmid. Cells were collected and lysed. Lysates were fractionated on 10–50% sucrose density gradients with continuous monitoring at 260 nm. (d) PNMA1, PNMA4, ARC (anti-V5), and mCherry protein levels in each fraction were analyzed by immunoblot. This experiment was repeated independently 3 times with similar results.
Extended Data Fig. 10 PNMA4 packages its own mRNA in a capsid-like particle.
(a) Lysate was prepared from eight testes (collected at 3 months) from either wild type or control (lacking Pnma4) mice (3 biological replicates each). Lysate was fractionated by velocity (247,000 g, 3 hours) over a double sucrose cushion (25% and 70%). The 70% meniscus was further fractionated by isopycnic centrifugation on an iodixanol step gradient. PNMA4 was IPed from iodixanol fractions 8 and 9, RNA was extracted IP beads, and sequenced. Shown is a volcano plot of log2 fold change plotted against -log10 p-value of RNA-seq reads comparing wild type and controls. Values represent a ratio of enrichment between the wild type and control IPs. The further right the value, the more enriched the mRNA in the wild type IP. Genes with a log2 fold change greater than 1 and an enrichment p-value of less than 10e-4 are colored magenta. (b) Model for PNMA1 and PNMA4 function.
Supplementary information
Supplementary Information
Supplementary Table 1
Supplementary Table 1
Human variation of PNMA1 and PNMA4 is causally associated with reproductive defects. We gathered information on the traits associated with the PNMA1 and PNMA4 genes using the Open Targets Genetics database containing GWAS summary statistics28. This database predicts the causal genes of traits using the V2G and L2G pipelines27. In the database, we queried the PNMA1 and PNMA4 genes to collect the causally associated traits from GWASs. We then filtered traits by P < 5 × 10−8 and merged them into a table (Supplementary Table 1) labeled with the queried genes. Columns indicate gene, study ID, reported trait, (first) author of study, date, study number, index variant ID (chromosome, base, change), index variant RSID, P value, beta (effect size and direction), lower beta confidence limit, upper beta confidence limit and pipeline score.
Source data
Source Data Fig. 1
Source data used for Fig. 1
Source Data Fig. 2
Source data used for Fig. 2
Source Data Fig. 3
Source data used for Fig. 3
Source Data Fig. 4
Source data used for Fig. 4
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 4
Unprocessed western blots
Source Data Extended Data Fig. 1
Source data used for Extended Data Fig. 1
Source Data Extended Data Fig. 2.1
Unprocessed western and northern blots for Extended Data Fig. 2
Source Data Extended Data Fig. 2.2
Unprocessed western and northern blots for Extended Data Fig. 2
Source Data Extended Data Fig. 3
Source data used for Extended Data Fig. 3
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3
Source Data Extended Data Fig. 4
Source data used for Extended Data Fig. 4
Source Data Extended Data Fig. 5
Source data used for Extended Data Fig. 5
Source Data Extended Data Fig. 6
Source data used for Extended Data Fig. 6
Source Data Extended Data Fig. 7
Source data used for Extended Data Fig. 7
Source Data Extended Data Fig. 8
Source data used for Extended Data Fig. 8
Source Data Extended Data Fig. 9
Unprocessed Coomassie gels and western blots for Extended Data Fig. 9
Source Data Extended Data Fig. 10
Source data used for Extended Data Fig. 10
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wood, T.W.P., Henriques, W.S., Cullen, H.B. et al. The retrotransposon-derived capsid genes PNMA1 and PNMA4 maintain reproductive capacity. Nat Aging 5, 765–779 (2025). https://doi.org/10.1038/s43587-025-00852-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-025-00852-y
This article is cited by
-
Ancient viruses sustain mammalian reproduction
Nature Aging (2025)