Abstract
Background: Histone deacetylase 4 (HDAC4) and histone deacetylase 5 (HDAC5) are two isoforms of class IIa HDACs, and LMK235 is an HDAC inhibitor with higher selectivity for HDAC4/5. This study aimed to explore the expression and subcellular localization of HDAC4/5 and determine the mechanisms underlying the impact of LMK235 on ventricular remodelling post-MI. Methods: The MI model was established by left anterior descending branch (LAD) ligation, and LMK235 or vehicle was intraperitoneally injected daily for 21 days. Cardiac function was determined by echocardiography. Inflammation was evaluated by HE staining and measuring inflammatory cytokine expression, and fibrosis was evaluated by Masson staining and measuring fibrotic biomarker expression. Results: We found that LMK235 ameliorated cardiac dysfunction post-MI by suppressing inflammation and fibrosis, and LMK235 inhibited upregulation of lysine-specific demethylase 1 (LSD1) expression post-MI. In macrophages, LMK235 attenuated lipopolysaccharide (LPS) - induced inflammatory cytokine expression and inhibited LSD1 expression, while overexpression of LSD1 abrogated the anti-inflammatory effect of LMK235. In cardiac fibroblasts, LMK235 attenuated transforming growth factor-β1 (TGF-β1) - induced fibrotic biomarker expression and inhibited LSD1 expression, while overexpression of LSD1 abrogated the antifibrotic effect of LMK235. Conclusion: LMK235 attenuates chronic inflammation and fibrosis post-MI, leading to improved cardiac function. The anti-inflammatory effect of LMK235 may result from inhibition of the LSD1-NF-κB pathway in macrophages. The antifibrotic effect of LMK235 may result from inhibition of the LSD1-Smad2/3 pathway in cardiac fibroblasts.
Similar content being viewed by others
Introduction
As myocardial infarction (MI) progresses, chronic inflammation and interstitial fibrosis gradually develop in the border zone, and this process is described as ventricular remodelling1.
HDAC4 and HDAC5 are two isoforms of class IIa HDACs2. In cardiomyocytes, protein kinase D (PKD) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signalling promote phosphorylation-dependent nuclear export of HDAC4/53,4,5, whereas protein kinase A (PKA) signalling promotes nuclear retention of HDAC4/56,7,8, However, the expression and subcellular localization of HDAC4/5 in infarcted heart tissue have not yet been determined.
To date, HDAC inhibitors have been shown to improve pressure overload- or MI-induced ventricular remodelling by regulating cardiomyocyte hypertrophic growth, macrophage polarization and fibroblast-myofibroblast transformation9,10,11,12,13,14. LMK235 is a novel hydroxamate-based HDAC inhibitor with higher selectivity for HDAC4/5 than other HDACs15. Previously, many studies explored the antiproliferative and proapoptotic effects of LMK235 on different cancer cells, which suggested that LMK235 hold promise in cancer therapy16,17,18. Bin Li et al. reported that LMK235 improved intestinal dysfunction by inhibiting the ghrelin/E2F1/NF-κB pathway in an intestinal sepsis model19. Chenxi Zhu et al. reported that LMK235 treatment significantly attenuated transverse aortic constriction (TAC)-induced cardiac hypertrophy and AngII-induced cardiomyocyte hypertrophic growth, indicating its potential use in cardiovascular disease20.
As the first identified histone demethylase, LSD1 have been reported to interact with HDAC5 in many studies. As reported by Chunyu Cao et al., HDAC5 directly interacted with and stabilized the LSD1 protein, and HDAC5 promoted the stability of USP28 thus indirectly preventing the ubiquitination-dependent degradation of LSD1 in breast cancer cell lines21. Sulforaphane, an HDAC inhibitor, facilitated LSD1 ubiquitination and degradation in an HDAC5-dependent manner in breast cancer cells22. Bo Hu et al. suggested that HDAC5 directly mediated LSD1 deacetylation and stabilization in hepatocellular carcinoma cell lines23. LSD1 also play an important role in ventricular remodelling, Jin-Ling Huo et al.. claimed that LSD1 was increased in failing human and mouse hearts, and myofibroblast-specific deletion of LSD1 significantly alleviated cardiac hypertrophy and fibrosis by inhibiting TGF-β1-Smad2/3 signalling in a TAC model24.
Here, we aimed to examine the expression and subcellular localization of HDAC4 and HDAC5 in infarcted heart tissue, and hypothesized that LMK235 ameliorated chronic inflammation and fibrosis post-MI by inhibiting LSD1-related pathway.
Materials and methods
Experimental design
We conducted two-stage experiments in vivo. In the first stage, male SD rats were divided into four groups: sham group, MI-7days group, MI-14days group and MI-21days group (n = 6 for each group). Western blotting and immunofluorescence were performed to determine the expression and subcellular localization of HDAC4/5. In the second stage, 44 male SD rats were divided into four groups: (1) the sham group (n = 8), which received daily intraperitoneal injections of vehicle from the day of the sham operation until 21 days postoperation; (2) the sham + LMK235 group (n = 8), which received daily intraperitoneal injections of LMK235 from the day of the sham operation until 21 days postoperation; (3) the MI group (n = 14), which received daily intraperitoneal injections of vehicle from the day of LAD ligation until 21 days post-MI ; and (4) the MI + LMK235 group (n = 14), which received daily intraperitoneal injections of LMK235 from the day of LAD ligation until 21 days post-MI. Echocardiography, Staining and Western blotting were performed to assess cardiac function, inflammatory repsonse, interstitial fibrosis and LSD1 expression.
For in vitro experiments, primary mouse cardiac fibroblasts (MCFs) were divided into four groups: NC group, TGF-β1 group, TGF-β1 + LMK235 group and TGF-β1 + LMK235 + LSD1-OE group. Western blotting was performed to examine the fibrotic biomarker expression and LSD1-Smad2/3 pathway activation. RAW264.7 murine macrophage cell line were divided into 4 groups: NC group, LPS group, LPS + LMK235 group and LPS + LMK235 + LSD1-OE group. Flow cytometry and Western blotting were performed to examine the macrophage polarization, inflammatory biomarker expression and LSD1-NF-κB pathway activation.
The experimental design of our study is presented in Supplementary Fig. 1.
Chemicals and reagents
LMK235 (CAS Number: 1418033-25-6, purity 99.61%) was purchased from MCE Co. Ltd, dissolved in dimethylsulfoxide (DMSO, Solarbio, China, D8371) and separately stored at -80℃ in advance. The chemical structure of LMK235 is provided in Supplementary Fig. 215. The vehicle contained 50% saline, 40% PEG300 (MCE, China), 5% Tween-80 (MCE, China) and 5% DMSO in accordance with the instructions of MCE Co. Ltd. The lentiviral vectors were designed and purchased from BIOMEDICAL Co. Ltd.
Animal preparation
All animal experiments complied with the criteria approved by the Animal Ethics Committee of Wenzhou Medical University (Number wydw2021-0274, date of ethics approval: 2021.8.5) and conformed to the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health, all methods are reported in accordance with ARRIVE guidelines.
MI model
Male Sprague-Dawley rats (SPF class, 220–250 g, 6–8 weeks old) were used for experiments. After being anaesthetized by continuously inhaling a mixture of 2% isoflurane and oxygen, and mechanically ventilated (Inspira, Harvard Apparatus, Hollisone, MA), the heart was exposed, and the left anterior descending branch (LAD) was ligated. The criterion of a successful MI model were as follows: (1) the left ventricle became pale and significantly hypokinetic, and (2) the ECG displayed an elevated ST segment.
First, we performed a preliminary experiment. Non-infarcted left ventricles and infarcted left ventricles collected at 7, 14 and 21 days post-MI were examined to determine the expression and subcellular localization of HDAC4/5 (n = 6 for each group). 30 male SD rats were prepared for the preliminary experiment, and 6 of them sacrificed during or after operation.
Then, 44 male SD rats were divided into four groups: sham group (n = 8), sham + LMK235 group (n = 8), MI group (n = 14) and MI + LMK235 group (n = 14). The number of dead rats in MI group and MI + LMK235 group were both 4, and the survival rate were both approximately 71%. The interval of the intraperitoneal injections was at least 24 h to avoid seroperitoneum and adhesive ileus. The dose of LMK235 used was 5 mg/kg/day19,20,25,26.
Culture of MCFs and RAW264.7 cells
MCFs were obtained from Procell Life Science & Technology Co. Ltd. and cultured in DMEM (Gibco, Thermo Fisher Scientific) containing 10% (v/v) FBS. To verify the antifibrotic effect of LMK235, three plates of MCFs were treated with 10 ng/ml TGF-β1 for 48 h27, and two plates were pretreated with 1.0 µM LMK235 for 24 h.
The RAW264.7 murine macrophage cell line were obtained from Procell Life Science & Technology Co. Ltd. and maintained in DMEM supplemented with 10% (v/v) FBS. To verify the anti-inflammatory effect of LMK235, three plates of RAW264.7 cells were treated with 100 ng/ml LPS for 48 h28, and two plates were pretreated with 1.0 µM LMK235 for 24 h.
To induce overexpression of LSD1, the last plate of MCFs or RAW264.7 cells were transduced with lentiviral vector carrying LSD1 reporter gene (LV-shLSD1) at a multiplicity of infection (MOI) of 10 prior to TGF-β1 / LPS stimulation and LMK235 treatment29. For comparison, other plates of MCFs or RAW264.7 cells were transduced with original lentiviral vector with empty gene (LV-shNC) according to manufacturer’s protocol.
Both cell lines were incubated in a humidified incubator (37 °C, 5% CO2). RAW264.7 cells were used for experiments between passages 2 and 5, and MCFs were used for experiments within 4 passages.
Echocardiography
Prior to sacrifice, the rats were anaesthetized (urethane, 1.5 g/kg intraperitoneally injected) and subjected to echocardiographic examinations 21 days after ligation. Short-axis views of M-mode echocardiograms were recorded at the papillary muscle level through the anterior and posterior walls to measure the left ventricular end-diastolic dimension (LVEDd) and left ventricular end-systolic dimension (LVESd), and the data were determined by averaging three consecutive cardiac cycles. Left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were obtained according to the Teicholz formula based on the LVEDd and LVESd. Left ventricular ejection fraction (LVEF) = (LVEDV-LVESV)/LVEDV×100%30.
All measurements were performed by experienced technicians who were blinded to the study groups.
Haematoxylin and eosin (HE) staining and Masson’s trichrome staining
The ventricles were quickly excised and washed with PBS. The ventricles were embedded in paraffin and sectioned into 5-µm-thick slices. HE Staining was performed to evaluate the extent of inflammatory infiltration in the border zone, and Masson’s trichrome staining was performed to evaluate the fibrotic area in the border zone. The inflammatory infiltration area and the fibrotic area of each group were calculated using Image J software.
Immunofluorescence analysis
The left ventricles were quickly excised and washed with PBS, dehydrated with 30% sucrose, embedded in OCT compound, and sectioned into 5-µm-thick frozen slices. Heart tissue was incubated with 10% blocking serum and 0.3% Triton X-100 at room temperature for 1 h. Then, the specimens were incubated with primary antibodies against HDAC4 (dilution 1:100; ABclonal, A0179) or HDAC5 (dilution 1:100; ABclonal, A0632). Alexa Fluor 488-conjugated goat anti-rabbit (dilution 1:100; Yeasen) secondary antibodies were added, and the specimens were incubated in the dark at room temperature for 1 h. Finally, the specimens were immersed in anti-fluorescence quenching sealant containing 4,6-diamidino-2-phenylindole (DAPI) and sealed with a coverslip.
Flow cytometry
After the indicated treatments in different groups, RAW264.7 cells were harvested and suspended in PBS. The cells were incubated with FITC-conjugated anti-mouse F4/80 antibodies (dilution 1:200; BioLegend, 105113) and APC-conjugated anti-mouse CD86 antibodies (dilution 1:200; BioLegend, 123107) in the dark at 4 °C for 1 h. After being washed with PBS, the cells were fixed and permeabilized with a BD Cytofix/Cytoperm plus fixation/permeabilization kit (BD Pharmingen, #554715). The cells were analyzed by CytoFLEX (Beckman Counter, Model A00-1-1102). The data were analyzed by FlowJo software. Only F4/80+ RAW264.7 cells were labelled as macrophages, and CD86+F4/80+ RAW264.7 cells were labelled as inflammatory macrophages (M1 phenotype)31,32,33. The proportion of CD86-positive macrophages = CD86+F4/80+ cells%/total F4/80+ cells%×100%.
Western blotting
The border zones of infarcted hearts or cells (RAW264.7 and MCFs) were lysed with RIPA lysis buffer (Solarbio, China) containing PMSF (Solarbio, China) and protease inhibitors (Applygen, China) at a ratio of 100:1:1. A BCA protein assay kit (Beyotime, China) was used to measure the protein concentration, and the proteins were diluted with double-distilled water and 5× loading buffer to balance the concentration of each sample. Each protein sample was separated by SDS–PAGE and transferred to a PVDF membrane, which was blocked in 5% nonfat milk and incubated with primary antibodies at 4 °C overnight and secondary antibodies at room temperature for 1 h. The PVDF membrane was cropped prior to antibody incubation. Antibodies against HDAC5 (A0632), HDAC4 (A0179), phospho-HDAC4-S246 (AP0280), lysine-specific demethylase 1 (LSD1, A8711), TGF-β1 (A2124), α-smooth muscle actin (α-SMA, A7248), Smad2 (A7699D), phospho-Smad2-S465/467 (p-Smad2, AP0925), Smad3 (A19115), phospho-Smad3-S423/425 (p-Smad3, AP0727), NF-κB p65 (p65, A10609), phospho-p65/RelA-S276 (p-p65, AP0123), α-tubulin and GAPDH were purchased from ABclonal. Antibodies against tumour necrosis factor alpha (TNF-α, 17590-1-AP), interleukin-6 (IL-6, 66146-1-Ig), interleukin-1β (IL-1β, 16806-1-AP), collagen I (14695-1-AP) and collagen III (22734-1-AP) were purchased from Proteintech. Phospho-HDAC5-S498 (ab240644) was purchased from Abcam. Cleaved-interleukin-1β (cleaved-IL-1β, 83186) was purchased from CST. The grey level of each band were calculated using Image J software.
Statistical analysis
The data are expressed as the mean ± SD. The outcomes of the MI groups (MI-7days/MI-14days/MI-21days) and the sham group were compared using one-way analysis of variance followed by Dunnett’s test for multiple comparisons between MI groups and sham group. The following outcomes of each group were compared using one-way analysis of variance followed by Tukey’s test for multiple comparisons between each group. Statistical analysis was performed using SPSS 22 software and GraphPad Prism 8 software. Differences were considered statistically significant when P < 0.05, P < 0.01, P < 0.001.
Results
HDAC4 and HDAC5 were gradually upregulated and underwent phosphorylation-dependent nuclear export post-MI
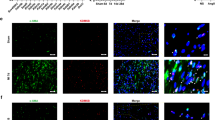
First, we examined the expression and subcellular localization of HDAC4 and HDAC5 in the border zone by western blotting and immunofluorescence analysis. Western blot analysis showed that the expression of HDAC4 and HDAC5 was gradually upregulated in the border zone from 7 days post-MI until 21 days post-MI, while the phosphorylation levels of HDAC4 and HDAC5 were upregulated from 14 days post-MI until 21 days post-MI (P < 0.01, Fig. 1A-D, Supplementary Fig. 3A-D). Immunofluorescence analysis showed that HDAC4 and HDAC5 colocalized with the nucleus in noninfarcted heart tissue, while HDAC4 and HDAC5 did not colocalize with the nucleus in the border zone of infarcted heart tissue (Fig. 1E, Supplementary Fig. 3E).
HDAC5 was gradually upregulated and underwent phosphorylation-dependent nuclear export post-MI. A-D, Western blot results and statistical analysis of LAP-TGF-β1, HDAC5 and p-HDAC5 (S498) in noninfarcted hearts and infarcted left ventricles 7, 14, and 21 days post-MI (n = 6 for each group). The band of LAP-TGF-β1, HDAC5 and GAPDH were from the same gel, and the band of p-HDAC5 (S498) were from the other gel. E, Immunofluorescence analysis of HDAC5 in noninfarcted heart tissue and the border zone of infarcted heart tissue. The data are presented as the mean ± SD, ☨P<0.05 vs. sham group, *P < 0.01 vs. sham group. The samples derive from the same experiment and that blots were processed in parallel. The original blots are presented in Supplementary Figs. 5–9.
LMK235 improved left ventricular dysfunction 21 days after MI
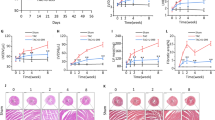
Echocardiography revealed that the LVEDd and LVESd of MI rats were significantly increased, whereas the LVEF of MI rats was significantly decreased compared with that in the sham group (P < 0.001). Three weeks of LMK235 administration slightly reversed the increases in LVEDd and LVESd and improved the decrease in LVEF (P < 0.01), indicating that LMK235 improved cardiac dysfunction post-MI (Fig. 2A-G).
LMK235 improved left ventricular dysfunction 21 days after MI. A-D, Representative images of M-mode echocardiography of rats in each group. E-G, Statistical analysis of LVESd, LVEDd and LVEF (n = 8 for sham group and sham + LMK235 group, while n = 10 for MI group and MI + LMK235 group). The data are presented as the mean ± SD, *P < 0.001 vs. the sham group, #P < 0.01 vs. the MI group.
Comparisons between the sham group and sham + LMK235 group indicated that LMK235 alone exerted little effect on physiological cardiac function (P > 0.05, Fig. 2A-B, E-G).
LMK235 attenuated chronic inflammation in the border zone 21 days after MI
The extent of inflammatory infiltration was quantified by HE staining and measuring the expression of TNF-α, IL-6, cleaved-IL-1β and pro-IL-1β. The extent of cardiac inflammatory infiltration was significantly increased in the MI group compared with the sham group, but was markedly relieved by LMK235 treatment post-MI (P < 0.001, Fig. 3A-B). The expression of inflammatory cytokines was significantly elevated post-MI (P < 0.001), and LMK235 attenuated the increases in the expression of inflammatory cytokines post-MI (P < 0.001, Fig. 3C-G). Thus, LMK235 exerted anti-inflammatory effects in the border zone of the infarcted heart tissue.
LMK235 attenuated chronic inflammation in the border zone 21 days after MI. A-B, Representative images of HE staining in each group at a magnification of 40×, and statistical analysis of inflammatory infiltration area (n = 3 for each group). C-G, Western blot results and statistical analysis of TNF-α, IL-6, cleaved-IL-1β and pro-IL-1β expression (n = 5 for sham group and sham + LMK235 group, while n = 7 for MI group and MI + LMK235 group). The band of TNF-α, cleaved-IL-1β and GAPDH were from the same gel, and the band of IL-6 and pro-IL-1β were from the other two gels. The data are presented as the mean ± SD, ☨P < 0.05 vs. the sham group, *P < 0.001 vs. the sham group, #P < 0.001 vs. the MI group. The samples derive from the same experiment and that blots were processed in parallel. The original blots are presented in Supplementary Figs. 10–16.
Comparisons between the sham group and sham + LMK235 group indicated that LMK235 hardly affected the inflammatory response in non-infarcted heart tissue (P > 0.05, Fig. 3A-G).
LMK235 attenuated interstitial fibrosis in the border zone 21 days after MI
The extent of interstitial fibrosis was quantified by Masson’s trichrome staining and measuring the expression of LAP-TGF-β1, collagen I, and collagen III. The percentage of the fibrotic area was significantly increased in the MI group compared with the sham group (P < 0.001), but this change was markedly reversed by LMK235 treatment post-MI (P < 0.001, Fig. 4A-D, F). Consistently, LMK235 treatment attenuated the increase in the expression of fibrotic biomarkers post-MI (P < 0.001, Fig. 4E, G-I). Thus, LMK235 exerted antifibrotic effects in the border zone of the infarcted heart tissue.
LMK235 attenuated interstitial fibrosis in the border zone 21 days after MI, and LMK235 inhibited upregulation of LSD1 in the border zone 21 days after MI. A-D & F, Representative images of Masson’s trichrome staining in each group at magnifications of 40× and 100× and statistical analysis of the fibrotic area (n = 3 for each group). E & G-K, Western blot results and statistical analysis of collagen I, collagen III, LAP-TGF-β1 and LSD1 expression (n = 5 for sham group and sham + LMK235 group, while n = 7 for MI group and MI + LMK235 group). The band of collagen I, LAP-TGF-β1 and GAPDH (Fig. 4E) were from the same gel, the band of LSD1 and GAPDH (Fig. 4J) were from the same gel, and the band of collagen III were from the other gel. The data are presented as the mean ± SD, *P < 0.001 vs. the sham group, #P < 0.001 vs. the MI group. The samples derive from the same experiment and that blots were processed in parallel. The original blots are presented in Supplementary Figs. 17–23.
Comparisons between the sham group and sham + LMK235 group indicated that LMK235 hardly affected interstitial fibrosis in non-infarcted heart tissue (P > 0.05, Fig. 4A-I).
LMK235 inhibited the upregulation of LSD1 expression in the border zone 21 days after MI
Furthermore, we found that LSD1 was upregulated in the border zone post-MI (P < 0.001), and LMK235 inhibited LSD1 expression in both normal and infarcted hearts (P < 0.001, Fig. 4J-K).
LMK235 suppressed LPS-induced inflammatory macrophage polarization in RAW264.7 cells, while overexpression of LSD1 abrogated the anti-inflammatory effect of LMK235
Mouse monocytes/macrophages are identified primarily by the F4/80 antigen, and CD86 is a sensitive and specific marker of inflammatory macrophage polarization (M1 phenotype)31,32,33. By performing flow cytometry and Western blotting, we confirmed that LMK235 suppressed LPS-induced inflammatory macrophage polarization and inflammatory cytokine expression in RAW264.7 cells (P < 0.01, Fig. 5A-E). We further confirmed that LMK235 suppressed the expression of LSD1, the experssion and phosphorylation of p65, thus inhibiting NF-κB target gene expression (P < 0.01, Fig. 5F-J), and lentiviral vector mediated overexpression of LSD1 abrogated the inhibitory effect of LMK235 on NF-κB pathway activation, inflammatory cytokine upregulation and inflammatory macrophage polarization (P < 0.01, Fig. 5A-J).
LMK235 suppressed LPS-induced inflammatory macrophage polarization and inflammatory cytokine expression by inhibiting NF-κB pathway in RAW264.7 cells, while overexpression of LSD1 abrogated the anti-inflammatory effect of LMK235. A-B, Flow cytometry results and statistical analysis of the percentage of CD86+ macrophages (n = 4 for each group). C-J, Western blot results and statistical analysis of IL-6, TNF-α, LSD1, p-p65 and p65 expression in RAW264.7 cells in each group. n = 4 for each group. The band of IL-6 and GAPDH (Fig. 5C) were from the same gel, the band of TNF-α were from the other gel. The band of LSD1, t-p65 and GAPDH (Fig. 5F) were from the same gel, and the band of p-p65 were from the other gel. The data are presented as the mean ± SD, *P < 0.001 vs. the NC group, #P < 0.01 vs. the LPS group, ☨P < 0.01 vs. the LPS + LMK235 group. The samples derive from the same experiment and that blots were processed in parallel. The original blots are presented in Supplementary Figs. 24–32.
These results suggested that the anti-inflammatory effect of LMK235 was mediated by the inhibition of LSD1-NF-κB pathway in macrophages.
LMK235 suppressed TGF-β1-induced fibroblast-myofibroblast transformation in MCFs, while overexpression of LSD1 abrogated the antifibrotic effect of LMK235
We confirmed that LMK235 suppressed TGF-β1-induced fibrotic biomarker expression in MCFs, and LMK235 suppressed the TGF-β1-induced upregulation of LSD1 and phosphorylation of Smad2/3 (P < 0.01, Fig. 6A-H). While lentiviral vector mediated overexpression of LSD1 abrogated the inhibitory effect of LMK235 on Smad2/3 pathway activation and fibrotic biomarker upregulation (P < 0.01, Fig. 6A-H).
LMK235 suppressed TGF-β1-induced fibroblast-myofibroblast transformation by inhibiting Smad2/3 pathway in MCFs, while overexpression of LSD1 abrogated the antifibrotic effect of LMK235. A‒H, Western blot results and statistical analysis of collagen I, collagen III, α-SMA, LSD1, p-Smad2, Smad2, p-Smad3 and Smad3 expression in MCFs in each group. n = 4 for each group. The band of collagen I, α-SMA and α-tublin (Fig. 6A) were from the same gel, the band of collagen III were from the other gel. The band of LSD1, t-Smad2 and α-tublin (Fig. 4J) were from the same gel, and the band of p-Smad2, p-Smad3 and t-Smad3 were from the other three gels. The data are presented as the mean ± SD, *P < 0.001 vs. the NC group, #P < 0.01 vs. the TGF-β1 group, ☨P < 0.01 vs. the TGF-β1 + LMK235 group. The samples derive from the same experiment and that blots were processed in parallel. The original blots are presented in Supplementary Figs. 33–46.
These results suggested that the antifibrotic effect of LMK235 was mediated by the inhibition of LSD1-Smad2/3 pathway in cardiac fibroblasts.
Discussion
The major findings of the present study were as follows: (1) HDAC4/5 expression was gradually upregulated, and HDAC4/5 underwent phosphorylation-dependent nuclear export in the border zone post-MI. (2) LMK235 ameliorated left ventricular dysfunction post-MI by restraining chronic inflammation and interstitial fibrosis in the border zone. (3) In RAW264.7 cells, LMK235 might suppress LPS-induced inflammatory macrophage polarization by inhibiting LSD1-NF-κB pathway activation. (4) In primary MCFs, LMK235 might suppress TGF-β1-induced fibroblast-myofibroblast transformation by inhibiting LSD1-Smad2/3 pathway activation.
The expression of HDAC4 and HDAC5 was gradually upregulated post-MI in a time-dependent manner. This finding is consistent with the variations of HDAC1 and HDAC2 expression in the chronic MI model13,14. In addition, both HDAC4 and HDAC5 were phosphorylated and exported from the nucleus to the cytoplasm post-MI, which is consistent with previous investigations of HDAC4/5 in cardiomyocytes3,4,5.
LMK235 is a novel hydroxamate-based HDAC inhibitor with nanomolar inhibition of HDAC4 and HDAC515. In the present study, LMK235 attenuated chronic inflammation and interstitial fibrosis in the border zone 21 days after MI, which contributed to the preservation of cardiac function. To date, a variety of HDAC inhibitors have been reported to improve cardiac function and block ventricular remodelling under conditions of pressure overload or MI34. Classic HDAC inhibitors, including TSA and VPA, can ameliorate ventricular remodelling by regulating cardiomyocyte hypertrophic growth10,12. In addition, TSA and SAHA preserve cardiac function post-MI by promoting the polarization and recruitment of reparative (M2) macrophages11. Furthermore, some novel HDAC inhibitors, including mocetinostat and givinostat, have been shown to reverse ventricular remodelling by suppressing fibroblast-myofibroblast transformation13,14,35.
Several articles have reported the interaction between HDAC4/5 and LSD1. Chunyu Cao et al. reported that HDAC5 directly interacted with and stabilized the LSD1 protein, and HDAC5 promoted the stability of USP28 thus indirectly preventing the ubiquitination-dependent degradation of LSD1 in breast cancer cell lines21. Sulforaphane, an HDAC inhibitor, facilitated LSD1 ubiquitination and degradation in an HDAC5-dependent manner in breast cancer cells22. Bo Hu et al. suggested that HDAC5 directly mediated LSD1 deacetylation and stabilization in hepatocellular carcinoma cell lines23. So we hypothesized that the anti-inflammatory effect and antifibrotic effect of LMK235 might be mediated by the inhibition of LSD1-related pathway in macrophages and cardiac fibroblasts.
Macrophages are critical players in the immune system initiating appropriate immune responses in MI and can exhibit two major phenotypes: inflammatory phenotype (M1) and reparative phenotype (M2)31,32,33. Reports have suggested that HDAC5 can positively regulate the NF-κB pathway in both macrophages and inflammatory mouse models. Recently, Bin Li et al. reported that LMK235 improved intestinal dysfunction by inhibiting the ghrelin/E2F1/NF-κB pathway in an intestinal sepsis model19. Chonghui Xu et al. revealed that the presence of HDAC5 contributed to persistent NF-κB pathway activation by disrupting PP2A activity36. Notably, Bo Hu et al. suggested that in hepatocellular carcinoma cell lines, HDAC5 directly mediated LSD1 deacetylation and stabilization, and LSD1 mediated p65 demethylation and stabilization to maintain NF-κB activation23, indicating that LMK235 might suppress NF-κB pathway activation by inhibiting LSD1 expression in macrophages. Here, we confirmed that LMK235 might attenuate LPS-induced inflammatory macrophage polarization and inflammatory cytokine expression by suppressing LSD1-NF-κB pathway activation.
Cardiac fibroblasts are the most abundant interstitial cells in the myocardium. Under conditions of MI stress, the proliferation, migration and myofibroblast transformation of cardiac fibroblasts are the major forces promoting scar formation in the infarcted area, as well as interstitial fibrosis in the border zone37. Ample evidence has shown that HDAC inhibitors can inhibit cardiac fibroblast activation and collagen deposition in response to pressure overload or MI38. In a uninephrectomy plus deoxycorticosterone acetate–salt (UNX/DOCA)-induced diastolic dysfunction model, givinostat blocked cardiac fibroblast-myofibroblast differentiation by inhibiting the recruitment of bromodomain-containing protein 4 (BRD4) to the regulatory elements of profibrotic genes35. In a chronic MI model, mocetinostat suppressed cardiac fibroblast-myofibroblast transformation by inhibiting IL-6/Stat3 signalling13,14. Moreover, in vitro studies of other types of fibroblasts revealed that the inhibitory effect of TSA on myofibroblast transformation was largely dependent on HDAC439,40. Recently, Ya Gao et al. reported that LMK235 significantly alleviated hypertrophic scar formation in vivo, and HDAC5 knockdown inhibited TGF-β1-induced Smad2/3 phosphorylation and fibroblast activation in vitro41. Jin-Ling Huo et al.. claimed that in addition to HDAC inhibitors, myofibroblast-specific deletion of LSD1 significantly alleviated cardiac hypertrophy and fibrosis by inhibiting TGF-β1-Smad2/3 signalling in a TAC model24, indicating a positive relationship between LSD1 and the TGF-β1-Smad2/3 pathway. In our study, we confirmed that LMK235 might attenuate TGF-β1-induced fibroblast-myofibroblast transformation by inhibiting LSD1-Smad2/3 pathway activation.
Several defects and limitations should be mentioned for this study. First, although we confirmed the anti-inflammatory and antifibrotic effect of LMK235 in vivo, and confirmed the anti-inflammatory and antifibrotic effect of LMK235 were mediated by LSD1-related pathway in vitro, we had to admit that in vivo experiments were relatively insufficient to illustrate the validation of in vitro conclusions for myocardial infarction model, so we will perform immunofluorescence in vivo or perform flow cytometry using primary macrophages or fibroblasts to strengthen our conclusion in the future. Second, it would be better if HDAC4 or HDAC5 was specifically knocked out in vivo or in vitro to further confirm that the cardioprotective effect of LMK235 was primarily mediated by catalytic inhibition of HDAC4 and HDAC5. Third, the process of ventricular remodelling is mediated by dynamic crosstalk between macrophages and cardiac fibroblasts, as well as other cells, so the impact of LMK235 on the interaction between different cells and intracellular signalling requires further investigation. Finally, given that the clinical application of other HDAC inhibitors has not been as successful as expected, pharmacological data, such as pharmacokinetics and side effects, need to be identified and documented.
Conclusion
This study demonstrated that LMK235 could ameliorate cardiac dysfunction post-MI by suppressing inflammatory macrophage polarization and fibroblast-myofibroblast differentiation. The anti-inflammatory effect of LMK235 may result from the inhibition of the LSD1-NF-κB pathway in macrophages, and the antifibrotic effect of LMK235 may result from the inhibition of the LSD1-Smad2/3 pathway in cardiac fibroblasts. The Graphical Abstract is presented in Supplementary Fig. 4.
Data availability
The data and supportive information is available within the article and supplementary material.
References
Suthahar, N., Meijers, W. C., Sillje, H. H. W. & de Boer, R. A. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr. Heart Fail. Rep. 14 (4), 235–250 (2017).
Weeks, K. L. & Avkiran, M. Roles and post-translational regulation of cardiac class IIa histone deacetylase isoforms. J. Physiol. 593 (8), 1785–1797 (2015).
Bossuyt, J. et al. Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ. Res. 102 (6), 695–702 (2008).
Davis, F. J., Gupta, M., Camoretti-Mercado, B., Schwartz, R. J. & Gupta, M. P. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J. Biol. Chem. 278 (22), 20047–20058 (2003).
Sin, Y. Y. & Baillie, G. S. Protein kinase D in the hypertrophy pathway. Biochem. Soc. Trans. 40 (1), 287–289 (2012).
Ha, C. H. et al. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U S A. 107 (35), 15467–15472 (2010).
He, T. et al. Cyclic AMP represses pathological MEF2 activation by myocyte-specific hypo-phosphorylation of HDAC5. J. Mol. Cell. Cardiol. 145, 88–98 (2020).
Sucharov, C. C., Dockstader, K., Nunley, K., McKinsey, T. A. & Bristow, M. beta-adrenergic receptor stimulation and activation of protein kinase A protect against alpha1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J. Card Fail. 17 (7), 592–600 (2011).
Kong, Y. et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 113 (22), 2579–2588 (2006).
Kee, H. J. et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation. 113 (1), 51–59 (2006).
Kimbrough, D. et al. HDAC inhibition helps post-MI healing by modulating macrophage polarization. J. Mol. Cell. Cardiol. 119, 51–63 (2018).
Lee, T. M., Lin, M. S. & Chang, N. C. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am. J. Physiol. Heart Circ. Physiol. 293 (2), H968–H977 (2007).
Nural-Guvener, H., Zakharova, L., Feehery, L., Sljukic, S. & Gaballa, M. Anti-fibrotic effects of class I HDAC inhibitor, mocetinostat is associated with IL-6/Stat3 signaling in ischemic heart failure. Int. J. Mol. Sci. 16 (5), 11482–11499 (2015).
Nural-Guvener, H. F. et al. HDAC class I inhibitor, mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90 + cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 7, 10 (2014).
Marek, L. et al. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J. Med. Chem. 56 (2), 427–436 (2013).
Li, X. et al. Histone deacetylase inhibitor LMK-235-mediated HO-1 expression induces apoptosis in multiple myeloma cells via the JNK/AP-1 signaling pathway. Life Sci. 223, 146–157 (2019).
Oltra, S. S. et al. HDAC5 inhibitors as a potential treatment in breast Cancer affecting very young women. Cancers (Basel) ;12(2):412. (2020).
Wanek, J. et al. Pharmacological inhibition of Class IIA HDACs by LMK-235 in pancreatic neuroendocrine tumor cells. Int. J. Mol. Sci. ;19(10):3128. (2018).
Li, B. et al. HDAC5 promotes intestinal sepsis via the Ghrelin/E2F1/NF-kappaB axis. FASEB J. 35 (7), e21368 (2021).
Zhu, C., Piao, Z. & Jin, L. HDAC5 inhibition attenuates ventricular remodeling and cardiac dysfunction. Orphanet J. Rare Dis. 18 (1), 266 (2023).
Cao, C. et al. Functional interaction of histone deacetylase 5 (HDAC5) and lysine-specific demethylase 1 (LSD1) promotes breast cancer progression. Oncogene. 36 (1), 133–145 (2017).
Cao, C. et al. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int. J. Cancer. 143 (6), 1388–1401 (2018).
Hu, B. et al. CD13 promotes hepatocellular carcinogenesis and sorafenib resistance by activating HDAC5-LSD1-NF-kappaB oncogenic signaling. Clin. Transl Med. 10 (8), e233 (2020).
Huo, J. L. et al. Myofibroblast deficiency of LSD1 alleviates TAC-induced heart failure. Circ. Res. 129 (3), 400–413 (2021).
Pozhidayeva, D. Y. et al. Chronic chemogenetic stimulation of the nucleus accumbens produces lasting reductions in binge drinking and ameliorates alcohol-related morphological and transcriptional changes. Brain Sci. ;10(2). (2020).
Trazzi, S. et al. HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Hum. Mol. Genet. 25 (18), 3887–3907 (2016).
Morine, K. J. et al. Bone morphogenetic protein 9 reduces cardiac fibrosis and improves cardiac function in Heart failure. Circulation. 138 (5), 513–526 (2018).
Wu, S. J. et al. Alteration of cholinergic anti-inflammatory pathway in rat with ischemic cardiomyopathy-modified electrophysiological function of heart. J. Am. Heart Assoc. 6(9):e006510. (2017).
Cao, F. et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 113 (7), 1005–1014 (2006).
Wang, W. E. et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation. 136 (9), 834–848 (2017).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 121 (22), 2437–2445 (2010).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204 (12), 3037–3047 (2007).
Strauss-Ayali, D., Conrad, S. M. & Mosser, D. M. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 82 (2), 244–252 (2007).
Xie, M. & Hill, J. A. HDAC-dependent ventricular remodeling. Trends Cardiovasc. Med. 23 (6), 229–235 (2013).
Travers, J. G. et al. HDAC inhibition reverses preexisting diastolic dysfunction and blocks covert extracellular matrix remodeling. Circulation. 143 (19), 1874–1890 (2021).
Xu, C. et al. Histone deacetylase 5 deacetylates the phosphatase PP2A for positively regulating NF-kappaB signaling. J. Biol. Chem. 297 (6), 101380 (2021).
Chen, W. & Frangogiannis, N. G. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim. Biophys. Acta. 1833 (4), 945–953 (2013).
Schuetze, K. B., McKinsey, T. A. & Long, C. S. Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs. J. Mol. Cell. Cardiol. 70, 100–107 (2014).
Glenisson, W., Castronovo, V. & Waltregny, D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim. Biophys. Acta. 1773 (10), 1572–1582 (2007).
Guo, W., Shan, B., Klingsberg, R. C., Qin, X. & Lasky, J. A. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 297 (5), L864–L870 (2009).
Gao, Y. et al. HDAC5-mediated Smad7 silencing through MEF2A is critical for fibroblast activation and hypertrophic scar formation. Int. J. Biol. Sci. 18 (15), 5724–5739 (2022).
Acknowledgements
Not applicable.
Funding
This work was supported by The Natural Science Foundation of Zhejiang Province (grant number LY19H020006) .
Author information
Authors and Affiliations
Contributions
Fangzhou Lv performed the experiments, analyzed the data and wrote the original manuscript, Laidi Xie assisted in performing the experiments, Lei Li provided the research idea and designed the experiments, Jiafeng Lin revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The present study was approved by the the Animal Ethics Committee of Wenzhou Medical University (Number wydw2021-0274).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, F., Xie, L., Li, L. et al. LMK235 ameliorates inflammation and fibrosis after myocardial infarction by inhibiting LSD1-related pathway. Sci Rep 14, 23450 (2024). https://doi.org/10.1038/s41598-024-74887-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74887-3