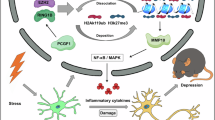
Abstract
Given the unique onset period of adolescent depression, it is crucial to prioritize treatment modalities that are not only effective but also carry low side effects and promote overall health, with exercise emerging as a notable option. High-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) stand out as two prominent forms of exercise, proven to be beneficial in addressing a multitude of disorders. Microglia mediated neuroinflammation is one of the main hypotheses leading to depression. Neuroinflammation leads to abnormal synaptic function, impaired neuroplasticity, and ultimately depressive symptoms. However, the exact mechanisms of exercise in microglia and adolescent depression remain unclear. In this study, we found that exercise can increase the expression of microglial ceramide synthase 1 (CerS1), promote the synthesis of C18 ceramide from sphingosine, and improve depressive-like behaviors in adolescent mice. Overexpression of CerS1 in the CA1 region using microglia specific adeno-associated virus (AAV) inhibits chronic unpredictable mild stress (CUMS) induced neuroinflammation. Meanwhile, overexpression of CerS1 in primary microglia inhibits lipopolysaccharide (LPS) induced neuroinflammation. These results provide new theoretical support for the treatment of adolescent depression with exercise from the perspective of animal models.

Similar content being viewed by others
Introduction
Depression is a globally prevalent mental disorder1, and adolescent depression refers to cases that occur during adolescence. In recent years, the incidence of adolescent depression has been increasing2. Since adolescent depression occurs during a critical stage of physical development and worldview formation, it can have a lifelong impact on affected adolescents3,4. Studies have shown that there are differences in etiology and treatment response between adolescents and adults with depression2,5,6, tricyclic antidepressants and serotonin norepinephrine reuptake inhibitors (SNRI) have imperfect efficacy in the treatment of adolescent depression7,8,9. Hence, it is of great value to discover an effective treatment for adolescent depression. Neuroinflammation gives rise to excitotoxicity and neuronal damage, and is one of the main hypotheses for the occurrence of depression10,11. Microglia are the resident immune cells in the central nervous system and play a dominant role in the neuroinflammatory response12,13. Studies have shown that autopsies of the brains of depressed patients have disclosed microglia activation and neuroinflammation14.
Exercise is a therapeutic approach with low side effects and low cost. Clinical trials have shown that exercise is an effective method for preventing or treating depression15,16,17. Exercise also improves depressive-like behavior in mice18,19,20. Studies have shown that exercise is as effective as psychotherapy and antidepressants21. For non-major depression, the effect of exercise is similar to using antidepressants alone or the combination of the two22. Small trials have shown that exercise is not more effective than psychotherapy or antidepressants23. Therefore, it is recommended that exercise be used in combination with other measures, especially for major depression (MDD)21,24. HIIT and MICT are two of the most popular forms of exercise, demonstrating ameliorative effects on various diseases25,26,27. However, the exact mechanisms of both exercise in microglia and adolescent depression remain unclear.
Sphingolipid metabolism is implicated in the pathophysiology of stress and psychiatric disorders, and ceramide is a core member of sphingolipids28,29. Previous studies revealed that patients with MDD exhibit an increase in ceramide levels in peripheral blood30. Meanwhile, ceramide levels were elevated in the brain and plasma of stress-induced mice31,32. However, stress-induced alterations in microglial ceramide metabolism remain unknown. Ceramides are synthesized from long-chain bases (LCBs), such as sphingosine (SPH), and the enzyme catalyzing this reaction is ceramide synthase (CerS). CerS is mainly located in the endoplasmic reticulum and is highly expressed in the brain, skeletal muscle and internal organs26,33. As an ortholog in mice and humans, CerS shows a strong similarity in sequence and genomic structure, with a highly conserved core catalytic ___domain33,34. CerS includes six members, CerS1 to CerS6, which share LCB substrates and produce ceramides with different chain lengths: CerS2 (C20-C26), CerS3 (C26-C34), CerS4 (C18-C20), CerS5/6 (C14–C16). CerS1 is highly expressed in neurons and synthesizes C18 ceramide33,35. CerS1 inactivation leads to increased LCB and decreased C18 ceramide in the brain, causing neurodegeneration in mice and myoclonic epilepsy with dementia in humans36,37. Overexpression of CerS2 ameliorates this neurodegeneration by decreasing LCB35. This study explores the role and mechanism of microglia in the process of exercise alleviating depressive-like behaviors in adolescent mice, providing new evidence for the coupling of sphingolipid metabolism and neuroinflammation.
Materials and methods
Animals and housing conditions
Male C57BL/6J mice weighing 12–14 g (about 3 weeks) were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). All animal care and experiments were approved by the Shandong University Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The mice were maintained under a 12 h light/dark cycle, with unrestricted access to food and water. The ambient temperature was sustained at 22 ± 2 °C. Every effort was made to minimize the pain and the number of animals utilized in the experiments.
Cell models
In vitro experiments, we used LPS to stimulate primary and BV2 microglia to induce neuroinflammation. Microglia were cultivated in Duchenne’s modified Eagle medium-high glucose medium (#10099141C, Gibco, USA), which contains 10% fetal bovine serum (FBS, #10099141C, Gibco, USA) and penicillin-streptomycin-amphotericin mixture (PSA, #CC033, Macgene, China). Subsequently, the flasks were positioned in an incubator at 37 °C with 5% CO2.
CUMS model
Mice in the CUMS group were housed as per the minor modifications described in the previous literature38. After purchasing, mice were individually housed and acclimatized for one week. Subsequently, the mice were subjected to a daily stress regime for five days per week with a 2-day rest period, lasting for 4 weeks. The mice were maintained under a 12 h light/dark cycle, and the light intensity did not exceed 100 lux. Stresses included (1) 24 h fasting of water and food, (2) standing on an ice-water mixture for 2 min, (3) being subjected to moist sawdust for 24 h, (4) tail pinching for 3 min, (5) cage tilting at 45° for 12 h, (6) restricted activity for 2 h, and (7) horizontal oscillation for 5 min. One stress was randomly applied each day. Meanwhile, control mice were similarly housed individually and ensured to survive under the same conditions as CUMS group.
Treadmill exercise
HIIT and MICT were performed using a treadmill (SA101CM, Jiangsu Saiones, China). Mice were acclimatized to the treadmill from D21 and trained for five days at 5 m/min for 20 min per day. Before the formal training, the maximal velocity (Vmax) was determined based on the incremental exercise test (IET) to calculate the velocities of HIIT and MICT. Mice started running on the treadmill at 8 m/min, increasing the speed by 1 m/min until exhaustion, with the treadmill inclined at 1°. Mice were considered exhausted if they touched the grid five or more times in 1 min. Vmax equaled exhaustion speed. Formal training for MICT and HIIT began at D28 for 4 weeks and ended at D54. Mice were exercised once per day, 5 days per week. MICT training was conducted for 45 min per day, with each session at an exercise rate of 55% Vmax. HIIT training was carried out for 35 min per session (starting with 2 min at an exercise rate of 85% Vmax, followed by 2 min at a recovery rate of 55% Vmax, with an eight times 2–2 min cycle for a total of 32 min, and a 2–1 min cycle of the last 3 min for a total of 35 min). The HIIT and MICT training programs ensured the same running distance. The MICT and HIIT speed calculations and total training protocols are presented in Table 1.
Experimental protocol
First, 3w male C57BL/6J mice were acclimatized for 1 week. As the previously described protocol, CUMS model and treadmill exercise began at D28 for 4 weeks. Exercise after daily stress induction. Behavioral testing began at D56. Mice were sacrificed at D64 for subsequent experiments. For AAV injection, we administered the microglia-specific AAV9-F4/80-CerS1-GFP virus into the CA1 region at D21 and followed the experimental protocol.

Isolation of primary microglia
After isoflurane anesthesia, 3-day-old fetal mice were immersed in alcohol and decapitated. The cerebral cortex was dissected in pre-cooled sterile HBSS, and the meninges and blood vessels were removed. The tissue was cut into 2 mm tissue blocks. Centrifuge at 1000 g for 5 min and remove the supernatant. Add 0.25% trypsin and incubate in a 37 °C incubator for 7–10 min until the tissue becomes flocculent, flipping 2–3 times during the period. Use a pipette tip to blow the flocculent tissue to make the cells into a single-cell suspension, and add complete medium to terminate the digestion. Centrifuge the single-cell suspension and remove the supernatant, then add medium. This is a mixture of microglia and astrocytes. As mentioned previously, these glial cells are placed in a 75 cm2 flask and cultured in glial cell culture medium. Subsequently, the flasks are placed in an incubator at 37 °C with 5% CO2. The medium was refreshed every 3 days. Approximately 11–14 days later, the mixed primary glial cells were shaken at 200 rpm and 37 °C for 4 h to obtain primary microglia.
Flow cytometry sorting of microglia
Mouse brain tissue (n = 3/group) was placed in pre-chilled Hank’s solution. The tissues were cut into ~1 mm fragments, ground to generate a cell suspension, and the cell suspension was further filtered through a 70 μm sieve (431751, Corning, USA) and centrifuged for 10 min at 420 × g. The collected precipitate was resuspended with 4 mL of 30% Percoll solution (GE17-5445-01, Sigma-Aldrich, USA). Subsequently, 4 mL of 70% Percoll solution was gradually added to the bottom of the 30% Percoll mixing solution using a syringe and centrifuged at 500 × g for 20 min (1 portion of 10× HBSS was mixed with nine portions of the Percoll stock solution to prepare an isotonic Percoll suspension, which we designated as 100% Percoll suspension). 100% Percoll was mixed with 1× PBS. Percoll was diluted with 1× PBS to produce Percoll isolates at the density necessary for cell separation. Cells were harvested from the layered interface of different concentrations of Percoll solution and washed once with PBS containing 0.2% BSA. Antibodies for staining were chosen as FITC anti-mouse CD11b (M1/70, Biolegend, USA) or APC anti-mouse CD45 (QA17A26, Biolegend, USA), and CD45-low/CD11b+ microglial cells were sorted using a Moflo Astrios EQ flow cytometer.
Behavioral testing
All behavioral testing was carried out in a separate behavioral testing room, where testers kept the identity of the test group undisclosed. Behavioral testing was performed between 18:00 and 23:00. The interval between tests is 24 h. Behavioral experiments were all recorded, measured, and analyzed by employing a tracking system (TopScan 3.0). Mice were examined in the subsequent behavioral sequence.
Open field test (OFT): after 1 h of laboratory acclimatization, mice were positioned in the center of a 35 cm × 35 cm × 25 cm experimental box (20 × 20). A uniform light (around 100 lux) was used to illuminate the box during the experiment. The mice were permitted to freely explore the field for 10 min to monitor their locomotor activity. The total distance traversed, time expended, and the number of instances the mice entered the central area were documented and analyzed as indicators of locomotor activity and anxiety-like behavior. Between tests, the floor of the vacant field was cleansed with a 75% ethanol solution to hinder the dispersion of olfactory cues.
Sucrose preference test (SPT): for the first 24 h, mice were placed individually in cages containing two bottles of sucrose solution (1%) and then one bottle of sucrose solution was replaced with tap water for the next 24 h. The mice were then deprived of small amounts of food and water for 24 h. Two water bottles, one containing 100 ml of 1% sucrose solution and the other containing 100 ml of tap water, were then placed in the cage and tested after 3 h. Sucrose preference was defined as sucrose consumption/[water consumption + sucrose consumption] × 100%. During the test, the mice were maintained under normal culture conditions, which consisted of normal 12-h light/dark cycle, and the temperature was sustained at 22 °C ± 2 °C.
Elevated positive maze (EPM) test: the EPM apparatus consisted of two open arms, two closed arms, and a central platform. Tested in soft light, the open arms (around 100 lux) were slightly brighter than the closed arms (around 40 lux). The EPM was 50 cm above the floor, and mice were placed through the central area and explored freely in the EPM for 6 min. The time and number of entries of the mice in the open arms were recorded and analyzed using a tracking system as a measure of anxiety-like behavior. Between tests, the EPM was cleaned with a 75% ethanol solution to prevent diffusion of olfactory cues.
Tail suspension test (TST): mice were individually suspended using tape. The tape was positioned 1 cm from the tail tip. Behavioral tests were conducted in uniform light (around 80 lux). Experiments were video-recorded for 6 min and analyzed by a blinded observer. Mice that crawled on their tails were excluded from the experimental analysis. Mobility is defined as the movement of the hind limbs.
Forced swimming test (FST): The experiment was performed in an open glass cylinder (25 cm in height, 18 cm in diameter) filled with water to a depth of 18 cm at a temperature ranging from 22 °C to 25 °C. Behavioral testing were conducted in uniform light (around 80 lux). Each mouse was compelled to swim for 6 min. After each mouse completed swimming, the water in the glass cylinder was replaced to avoid behavioral variations among animals. Mice were regarded as immobile when they ceased swimming and remained static, undertaking only what was requisite to keep their heads above the water surface. The immobility time was documented during the final 5 min of the 6 min training period.
Western blotting
Brain tissue, primary and BV2 microglia were lysed using RIPA buffer with added protease/phosphatase inhibitors. Lysed cells or tissues are centrifuged (20 min, 12,000 rpm, 4 °C) to obtain the lysate. Protein concentration was determined using the Pierce BCA Protein Kit. Protein samples are added to the lanes, separated by electrophoresis on an SDS-PAGE gel, and transferred to a PVDF membrane. The membranes were blocked with TBST buffer containing 5% nonfat milk for 2 h and incubated with primary antibodies overnight at 4 °C. The membranes were subsequently incubated with appropriate HRP-conjugated anti-mouse or anti-rabbit IgG for 1 h at room temperature. Protein bands were ultimately visualized using an enhanced chemiluminescence detection kit (Millipore, USA). Image-J software was employed to carry out pixel quantification of the images. For each sample, intra-run normalization was conducted against the internal actin control. The primary antibodies (diluted in primary antibody diluent) as follows: mouse anti-β-actin (HC201, Transs, 1:5000), mouse anti-CerS1 (DF15450, Affinity Biosciences, 1:500), rabbit anti-p65 (#8242S, CST, 1:1000), rabbit anti-p-p65 (#3033T, CST, 1:1000), rabbit anti-PSD95 (AF5283, Affinity, 1:500), rabbit anti-BDNF (ab108319, Abcam, 1:500), rabbit anti-Synaptophysin (17785-1-AP, Proteintech, 1:500), rabbit anti-p44/42 MAPK(Erk1/2) (#4695, CST, 1:1000), rabbit anti-p-p44/42 MAPK(Erk1/2) (#4370, CST, 1:1000), rabbit anti-JNK (#9252, CST, 1:1000), rabbit anti-p-JNK (#4668, CST, 1:1000), rabbit anti-p38 (#8690, CST, 1:1000), rabbit anti-p-p38 (#4511, CST, 1:1000).
RNA isolation and real-time quantitative PCR
Total RNA from CA1 tissues and primary or BV2 microglia was extracted with TRIZOL reagent (Invitrogen, USA). cDNA was synthesized with the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). Quantitative Real-time PCR was conducted with SYBR Green Realtime PCR Master Mix (TOYOBO CO, Ltd, Japan). The primer sequences employed were presented in Table S1. Each sample was analyzed in duplicate. β-actin expression served as an internal reference for normalization, and the relative quantification of gene expression levels was determined using the 2−△△Ct method.
Immunohistochemistry
Mice were anaesthetized with sodium phenobarbital (50 mg/kg) and transcardially perfused with 50 mL 0.9% NaCl containing heparin sodium salt, followed by perfusion and fixation with 4% paraformaldehyde (PFA). The brains were then removed, post-fixed in 4% PFA overnight at 4 °C, and subjected to graded dehydration. Brain tissue was encased in optimum cutting temperature compound and frozen serial coronal sections were cut. Sections were cut at 15 μm intervals and 4 sections were placed on each adherent slide. For immunofluorescence staining, the sections were blocked with 10% (w/v) donkey serum (#SL050; Solarbio, China) and 0.3% Triton X-100 (#9002-93-1; Solarbio, China) in PBS at 37 °C for 2 h. And then incubated with primary antibodies at 4 °C overnight (Iba1, Ab178680, Abcam, 1:200; MAP2, #4542S, CST, 1:200). On the following day, after washing three times with PBS, sections were incubated with secondary antibodies at room temperature for 1 h (Alexa Fluor 488-conjugated or 594-conjugated antibody, Invitrogen, 1:500). DAPI (C1002, Beyotime, China) was employed for nuclear staining. The sections sealed in antifade mounting medium (P0126, Beyotime, China). Cell slides stained in the same steps. Images were acquired with a confocal microscope (LSM880, Carl Zeiss, Germany) or a fluorescence microscope (Olympus BX51; Olympus, Shinjuku, Japan). The Iba1 fluorescence intensity of six mice from each experimental group and three consecutive brain sections from each mouse were recorded and analyzed using ImageJ (NIH, Bethesda, MD, USA).
RNA sequencing
To perform RNA sequencing (RNA-seq), flow-sorted microglia were harvested and lysed with TRIzol, and total RNA was collected according to the manufacturer’s instructions. RNA-sequencing was performed by the BGI company with the Illumina HiSeq™ 2000 platform (Wuhan, China). The differential expressed genes (DEGs) was confirmed by the bioinformatics service of BGI. Gene Ontology biological process enrichment analysis and the Kyoto Encyclopedia of Genes and Genomes (KEGG) of the DEGs were implemented with the BGI multi-omics system.
Plasmid transfection and ELISA
Plasmid-CerS1 (pCMV-EGFP-Cers1(mouse)-Neo) was acquired from MiaoLingBio, China. We transfected plasmids into microglia using Lipofectamine 2000 reagent (Invitrogen, USA) or jetOPTIMUS reagent (Polyplus, USA) according to the instructions. LPS stimulation was performed after transfection. The concentration of LPS was 500 ng/mL (derived from Escherichia coli O111:B4, L2630, Sigma-Aldrich, USA), and stimulation lasted for 12 h. ELISA kits for IL-1β (MULTI SCIENCES, China) and Tnf-α (MULTI SCIENCES, China) were utilized to quantify the respective cytokines or compounds in cell culture supernatants.
Morphology analysis
Using the Sholl analysis of Fiji-ImageJ to quantify microglial morphology by recording the number of branches. Six mice were recorded per group. Each mouse was analyzed in two consecutive brain slices. Three microglia were randomly selected from each slice and the average number of branches was recorded.
Detection of sphingomyelin and C18 ceramide
Add 10 μL BHT (50 mg/mL) dissolved in methanol, 1 mL chloroform-methanol (1:1) and 0.45 mL water to the primary microglia samples, break it up, vortex mix and centrifuge to collect the lower layer, repeat the extraction of the residue for one time, combine the lower layer of the two collected liquids, evaporate to dryness, add 0.2 mL methanol to re-dissolve it, and pass it through the membrane on the machine. Instrumentation method: (1) Column: Agilent C18 (2.1 mm × 100 mm, 1.8 μm); (2) Column temperature: 35 °C, flow rate: 0.3 mL/min; (3) Acquisition mode: ESI+; (4) Injection volume: 2 μL. The content is calculated according to Eq: W = (C− C0) × V × N/m. W—content of the target substance in the specimen, unit μg/L; C—concentration of the target substance in the specimen assay solution, unit μg/L; C0—concentration of the target in the blank control, unit μg/L; V—volume of fixation, unit mL; N—dilution times; m—sample volume of the specimen, unit mL. In vitro experiments, sphingomyelin and C18 ceramide were added first and then LPS was added after 40 min.
Transmission electron microscopy (TEM)
The ultrastructure of CA1 neurons was observed using transmission electron microscopy (TEM) (Philips Tecnai 20 U-Twin, Holland). Bilateral CA1 tissues were carefully dissected (1 × 1 × 1 mm), fixed with 1% osmium tetroxide for 2 h, and dehydrated with graded ethanol. The tissue was infiltrated overnight with a semi-epoxy-propane mixture and then embedded in resin. Tissues were cut into ultrathin sections (70 nm), stained with 4% uranyl acetate for 20 min and then stained with 0.5% lead citrate on the copper grid.
Statistical analysis
Statistical analysis were carried out using GraphPad Prism version 8 (GraphPad Software, Inc.). The data are presented as the mean ± SD. The normally distributed data were analyzed by unpaired two-tailed Student’s t test for two-group comparisons and one-way ANOVA followed by Tukey post hoc test for more than two groups. The non-normally distributed data were analyzed by Kruskal–Wallis test and Dunn’s multiple comparisons test for more than two groups. A statistically significant difference was determined as P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
HIIT and MICT alleviate depression-like and anxiety-like behaviors in CUMS mice
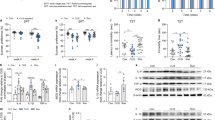
To determine whether HIIT and MICT are effective in improving depressive-like behaviors, we performed behavioral tests on CUMS-treated and treadmill-exercised mice (Fig. 1A). CUMS mice exhibited decreased body weight (Fig. 1B), reduced sucrose consumption in SPT (Fig. 1C), increased immobility time in FST (Fig. 1D) and TST (Fig. 1E), all of which are indicative of depressive-like behaviors. However, both HIIT and MICT were effective in improving these depressive-like behaviors. In the OFT, CUMS did not affect the total distance traveled by the mice (Fig. 1F) but decreased the time and entries of mice exploring the central area (Fig. 1G-I). Furthermore, the results of the EPM test showed that CUMS reduced the time and entries of mice entering the open arm (Fig. 1J-L). Nonetheless, HIIT and MICT reversed the anxiety-like behaviors of CUMS mice in both OFT and EPM test. Taken together, HIIT and MICT ameliorates CUMS-induced depressive and anxiety-like behaviors in mice.
A Experimental protocol. B HIIT and MICT reverses weight loss in CUMS mice (N = 24 per group, one-way ANOVA, D49: F (3, 92) = 29.56; D56: F (3, 92) = 93.42). The red p-value: CUMS/HIIT vs CUMS; the blue p-value: CUMS/MICT vs CUMS. C HIIT and MICT reverses the reduction of sucrose solution consumption in CUMS mice (N = 24 per group, one-way ANOVA, F (3, 92) = 7.137). D, E In CUMS mice, HIIT and MICT reverses the increased immobility time in FST and TST (N = 24 per group, Kruskal-Wallis test). F HIIT and MICT have no significant effect on total distance traveled by the OFT (N = 24 per group, one-way ANOVA, F (3, 92) = 0.1946). In CUMS mice, HIIT and MICT increases the stay time and the number of entries in the central area (N = 24 per group, one-way ANOVA. GF (3, 92) = 6.865; H F (3, 92) = 6.359). I Representative activity tracking in the OFT. In CUMS mice, HIIT and MICT increases the stay time and the number of entries in the open arms during the EPM test (N = 24 per group, one-way ANOVA. J F (3, 92) = 7.260; K F (3, 92) = 7.316). L Representative activity tracking in the EMP test. Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
HIIT and MICT regulates neuroinflammation and neuronal damage in CUMS mice
Next, by immunofluorescence analysis, we observed that exercise reversed the immunoreactivity of Iba1 in the hippocampus and cortex of CUMS mice (Fig. 2A-D). In addition, we observed that HIIT and MICT decreased the mRNA expression of inflammatory factors such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF) (Fig. 2E, F). Meanwhile, exercise inhibited microglial morphological changes in the cornu ammonis 1 (CA1) region of CUMS mice while increasing the number of branches (Fig. S1A, S1B). The TEM analysis revealed significant apoptotic features in the nuclei of neurons (Fig. 2G) and impaired synaptic plasticity (Fig. 2H). However, HIIT and MICT alleviated those abnormalities in CA1 region. HIIT and MICT also reduced neuronal restructuring abnormalities in the CA1 region of CUMS mice, such as reduced density of dendritic spines in Golgi staining (Fig. 2I, J). These findings demonstrate that exercise reduces neuroinflammation and alleviates neuronal damage.
A, B Representative images of Iba1 staining (green) in the hippocampus and cortex. Nuclei (blue), stained with DAPI. The scale bar is 100 μm. Fluorescence intensity analysis of (A) and (B) (N = 6 per group, one-way ANOVA. C F (3, 20) = 28.83; D F (3, 20) = 25.29). QPCR analysis of IL-1β, IL-6 and TNF mRNA levels in the hippocampus and cortex after HIIT and MICT (N = 8 per group, one-way ANOVA. E F (3, 28) = 49.81/43.41/34.28; F F (3, 28) = 36.25/51.51/35.87). G Representative TEM showing the structure of CA1 neuronal nuclei. The scale bar is 2 μm. H Representative TEM of neuronal synapses. The scale bar is 500 nm. I Representative images of dendrites in pyramidal neurons. The scale bar is 5 μm. J Quantification of mushroom spines in neurons (N = 6 per group, one-way ANOVA, F (3, 20) = 13.51). Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
CerS1 is upregulated in microglia of HIIT and MICT mice
In order to accurately investigate the role of microglia in HIIT and MICT mediated antidepressant effect, we sorted out CD11b+CD45low microglia from whole brain of CUMS, HIIT and MICT mice (Fig. 3A) and performed RNA sequencing. As shown in Fig. 3B, C heatmaps were generated showing all differentially expressed genes in HIIT and MICT mice compared to CUMS mice. Next, KEGG analysis observed these differentially expressed genes were enriched in the immune system, nervous system and lipid metabolism (Fig. 3D, E). Gene Ontology analysis revealed that these genes were mainly involved in metabolic process, immune system process and behaviors (Fig. S2A, B). These results suggest that exercise may modulate neuroinflammation and subsequently influence depressive-like behaviors by modulating lipid metabolism in microglia. In the RNA sequencing results, we found that CerS1, an important lipid metabolizing enzyme, was the most significantly upregulated in HIIT mice (log2 (fold change) = 14.00 with a Q-value < 0.05) and MICT mice (log2 (fold change) = 15.25 with a Q-value < 0.05). However, the function of CerS1 in microglia is still unknown. Subsequently, we analyzed the expression of CerS1 in microglia of CUMS, HIIT and MICT mice. As shown, consistent with the sequencing results, CerS1 expression was decreased in CUMS mice, whereas exercise significantly increased CerS1 expression. Notably, HIIT increased CerS1 expression more effectively than MICT (Fig. 3F). Sequencing results suggested that exercise decreased the expression of microglial Cers4 and Cers6 in CUMS mice (Cers4 in MICT/HIIT mice: log2 (fold change) = –1.38/–1.82. Cers6 in MICT/HIIT mice: log2 (fold change) = –2.14/–2.54). This differential expression may be a compensatory effect resulting from the use of the same metabolic substrates by different CerS isoforms. In vitro experiments, we used LPS to stimulate primary microglia mediated inflammation to mimic the neuroinflammation caused by CUMS in vivo. We found that LPS stimulation significantly reduced the protein level of CerS1 (Fig. 3G).
A An experimental protocol for FACS and a gate strategy for CD11b+CD45Low microglia were presented. B, C Heat map of differentially expressed genes in microglia after HIIT and MICT. D, E KEGG analysis of the differentially expressed genes in HIIT and MICT. F CerS1 mRNA levels in microglia by QPCR analysis (N = 3 per group, one-way ANOVA, F (3, 12) = 89.70). G Representative western blot images showing the protein level of CerS1 (N = 3 per group, two-sided Student’s t tests, T (4) = 4.948). The mock treatment was a blank control with PBS added. Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
Microglial CerS1 attenuates LPS-induced inflammation and restores plasticity in a neuron-microglia co-culture
To investigate the mechanism through which CerS1 regulates microglia-mediated neuroinflammation, we employed LPS-activated primary microglia and BV2 microglia to simulate inflammation in the brain. Firstly, the CerS1 expression plasmid was transfected into LPS-induced primary microglia, and the transfection efficiency was assayed (Fig. 5G). It was discovered that the overexpression of CerS1 led to a decrease in the expression of IL-1β, IL-6, and TNF at the mRNA (Fig. 4A) or protein levels (Fig. 4B). Subsequently, CerS1 was overexpressed in BV2 microglia, and the same results as in primary microglia were obtained (Figs. 4C, D and S2A). Simultaneously, the overexpression of CerS1 in primary microglia mitigated inflammation-induced damage to primary neurons during co-culture (Fig. 4F, G). Moreover, it also enhanced the protein levels of brain-derived neurotrophic factor (BDNF), Synaptophysin, and Postsynaptic density protein 95 (PSD95) in primary neurons during co-culture (Fig. 4H, I).
A, B QPCR analysis and ELISA analysis of IL-1β, IL-6 and TNF levels in primary microglia after overexpression of CerS1 (N = 3 per group, one-way ANOVA. A F (2, 6) = 183.4/445.3/78.4; B F (2, 6) = 107.2/350.0). QPCR analysis and ELISA analysis of IL-1β, IL-6 and TNF levels in BV2 microglia after overexpression of CerS1 (N = 3 per group, one-way ANOVA. C F (2, 6) = 135.9/161.9/132.6; D F (2, 6) = 192.9/308.0). E Microglia and neurons co-culture model diagrams. F Representative images of primary neurons morphology. Neurons (green) are stained with MAP2. Nuclei (blue) are stained with DAPI. Scale bar is 20 μm. G Analysis of neurons branches number (N = 24 per group, one-way ANOVA, F (2, 69) = 12.93). H, I Representative western blot images showing the protein levels of BDNF, Synaptophysin and PSD95 (N = 3 per group, one-way ANOVA, F (2, 6) = 11.72/16.37/25.17). Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
CerS1 regulates neuroinflammation possibly via C18 ceramide/sphingosine and the NF-κB/MAPK pathways
SPH and C18 ceramide are the main substrates and products of CerS1-mediated metabolic processes, and their metabolic relationships are key to our exploration of microglial CerS1 function. Therefore, we used High Performance Liquid Chromatography to detect SPH and C18 ceramide in cell lysates of primary microglia. Overexpression of CerS1 in primary microglia increases C18 ceramide while decreasing SPH (Fig. 5A, B). Assays showed that the addition of 10 μM C18 ceramide and SPH to primary microglia for 24 h did not significantly impact cell viability (Fig. S3B, C). Based on these findings, we employed this concentration and added to primary microglia. Analysis reveals that SPH increased LPS-induced inflammation, but C18 ceramide had no significant effect (Fig. 5C-F). Notably, Sphingosine-1-Phosphate, which is produced through the phosphorylation of SPH, also exhibits a pro-inflammatory effect in microglia39,40. Therefore, we speculate that CerS1 to alleviate neuroinflammation by metabolizing the ceramide-shared inflammatory substrate SPH to C18 ceramide. Then, we examined several signaling pathways associated with microglia activation, including NF-κB and MAPK pathways. It was found that overexpression of CerS1 inhibited LPS-induced phosphorylation of p38, JNK, ERK and p65. The above results suggest that microglial CerS1 may be an important target for regulating neuroinflammation.
A Determination of C18 ceramide content by HPLC (N = 3 per group, one-way ANOVA, F (2, 6) = 16.30). B Determination of SPH content by HPLC (N = 3 per group, one-way ANOVA, F (2, 6) = 45.04). C, D QPCR analysis and ELISA analysis of IL-1β, IL-6 and TNF levels in primary microglia after adding C18 ceramide (N = 3 per group, one-way ANOVA. C F (2, 6) = 429.0/355.4/35.39; D F (2, 6) = 197.4/352.1). E, F QPCR analysis and ELISA analysis of IL-1β, IL-6 and TNF levels in primary microglia after adding SPH (N = 3 per group, one-way ANOVA. E F (2, 6) = 505.7/327.4/207.4; F F (2, 6) = 304.4/717.0). G, H Representative western blot images showing the protein levels of CerS1, p-p65, p-ERK, p-p38 and p-JNK (N = 3 per group, one-way ANOVA, F (2, 6) = 15.46/29.08/31.90/29.15/21.16). Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
Microglia CerS1 is a key regulator of depressive-like and anxiety-like behaviors in CUMS mice
The CA1 region is a crucial factor in the neuropathology of depression41,42. To ascertain whether CerS1 is associated with depressive-like behaviors, we specifically induced CerS1 overexpression in microglia by injecting the microglia-specific AAV9-F40/80-CerS1-GFP virus (Fig. 6A). We injected the virus into the hippocampus CA1 region at D21 (Fig. 6B). Five weeks post-injection, a substantial amount of green fluorescence (GFP) was observed, indicating the successful targeting of the virus (Fig. 6C). The virus effectively infects microglia and enhances CerS1 expression in the CA1 region (Fig. 6D-F). Subsequently, we found that CerS1 successfully reduced CUMS-induced neuroinflammation (Fig. 6G). As demonstrated, CerS1 alleviated depressive-like behaviors caused by CUMS. CerS1 increased sucrose consumption (Fig. 6H) and decreased the immobility time in FST (Fig. 6I) and TST (Fig. 6J). Simultaneously, CerS1 increased the time and entries of mice exploring the central area in OFT (Fig. 6K-N). The results of the EPM test also revealed that CerS1 increased the time and entries of mice entering the open arm (Fig. 6O-Q). Collectively, microglial CerS1 mitigates CUMS induced depressive-like behaviors and anxiety-like behaviors in mice, exhibiting therapeutic effects similar to those of HIIT and MICT.
A Schematics of AAV vectors engineered to overexpress CerS1. B An experimental paradigm for behavioral responses in virus-infected mice. C Schematic representation of bilateral CerS1-AAV injection sites in the CA1 region of the hippocampus. Scale bar, 2 mm. GFP, Green fluorescent protein. D Colocalization of GFP (green) and Iba1 (red), scale bar, 20 μm. E Iba1+/GFP+ percentage statistics. F Representative western blot images of CerS1 in the CA1 region (N = 6 per group, two-sided Student’s t tests, T (10) = 5.501). G QPCR analysis of IL-1β, IL-6 and TNF mRNA levels in the CA1 (N = 8 per group, one-way ANOVA, F (3, 28) = 20.17/30.12/27.24). H Microglial CerS1 reverses the reduction of sucrose solution consumption in CUMS mice (N = 12 per group, one-way ANOVA, F (3, 44) = 12.41). In CUMS mice, microglial CerS1 reverses the increased immobility time in FST and TST (N = 12 per group, one-way ANOVA. I F (3, 44) = 15.65; J F (3, 44) = 11.43). K The total distance traveled by OFT was not significantly affected by CerS1 (N = 12 per group, one-way ANOVA, F (3, 44) = 0.1505). In CUMS mice, microglial CerS1 augments the duration of stay and the quantity of entries in the central area during the OFT test (N = 12 per group, one-way ANOVA. LF (3, 44) = 13.85; M F (3, 44) = 10.75). N Represents the tracking of activity in the OFT. In CUMS mice, microglial CerS1 escalates the duration of stay and the number of entries in the open arms during the EPM test (N = 12 per group, one-way ANOVA. O F (3, 44) = 11.58; P F (3, 44) = 13.61). Q Represents the tracking of activity in the EMP test. The open arm is indicated in yellow. Data are shown as the mean ± s.d. P < 0.05 is statistically significant.
Discussion
HIIT and MICT are popular modes of exercise that offer different mechanisms and low side effect options for the treatment of adolescent depression43. Our study demonstrates that exercise serves as an effective adjunctive treatment for adolescent depression, and its metabolic-improving effects may exert a preventive effect on depression. We further reveal that microglial CerS1 may be a potential therapeutic target for adolescent depression. Several studies have shown that HIIT is superior to MICT, especially in metabolic regulation44,45. This is consistent with our finding that microglial CerS1 expression is higher in HIIT than in MICT. HIIT has a higher intensity than MCIT and can produce more intense metabolic stress. These characteristics allow HIIT to reach thresholds that activate certain biological processes and promote metabolic responses or gene expression.
Sphingolipid metabolism has an important role in psychiatric disorders46. The antidepressants amitriptyline and fluoxetine ameliorate depressive-like behavior in mice through two mechanisms: reducing hippocampal ceramide concentration through inhibition of sphingomyelinase (ASM) activity31, which hydrolyzes sphingomyelin to ceramide47; inducing autophagy in hippocampal neurons via endoplasmic reticulum ceramide accumulation48. The classical drug Saikosaponin improves cortical sphingolipid metabolism and neurovascular coupling inhibition via apolipoprotein E in CUMS mice49. Sortilin deletion ameliorates depressive-like behavior in mice by reducing ASM trafficking and ceramide levels50. Microglia constitute only 10% of brain cells, and sphingolipid metabolism also exists in other cell types. Thus, the changes in ceramide levels of brain tissue do not effectively reflect changes in microglia. As immune cells, the sphingolipid metabolism of microglia in inflammatory states may be different from other cell types. Our study uncovers the unique sphingolipid metabolism pattern of microglia in inflammatory conditions.
Ceramides can be generated by reacylation of SPH29. SPH accumulation induces neuronal death and astrocyte apoptosis35,51. Phosphorylation products of SPH also exacerbate inflammation40,52. Studies have shown that C8 ceramide induces microglial BDNF production but does not induce Tnf and IL-1β production53. C2 ceramide has no impact on LPS-induced NO release or iNOS expression in microglia54. Consistently, we found that SPH has a pro-inflammatory effect in LPS-induced microglia, while C18 ceramide has no significant effect. Microglial C18 ceramide content is significantly lower than SPH content (Fig. 5A, B). Theoretically, the even lower extracellular secretion of C18 ceramide by microglia suggests that its direct effects on neurons may be relatively limited. The main mechanism by which microglial CerS1 alleviates neuronal damage is to reduce SPH and inhibit neuroinflammation. CerS isoforms share the same metabolic substrates; there are complex compensatory mechanisms between them, such as the changes of CerS4, CerS6 and CerS1 in the sequencing results. Studies have reported that sphingolipid metabolism has brain region specificity55,56 and our RNA sequencing was performed for whole-brain microglia. This study did not continue to explore the relationship between microglial CerS isoforms and the brain region specificity of sphingolipid metabolism. We will take it as part of the future research plan.
In summary, we found that exercise can increase the expression of microglial CerS1, promote the synthesis of C18 ceramide from SPH, suppress neuroinflammation, and improve depressive-like behaviors in adolescent mice. In conclusion, our study provides a new perspective for the treatment of adolescent depression.
Data and materials availability
Raw sequencing data have been deposited in the National Center for Biotechnology Information database, with project ID PRJNA1196104. Uncropped blots are provided in Supplementary Data 1. Source data are provided in Supplementary Data 2. Any additional data used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Malhi, G. S. & Mann, J. J. Depression. Lancet 392, 2299–2312 (2018).
Paus, T., Keshavan, M. & Giedd, J. N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 9, 947–957 (2008).
Bae, B. I., Jayaraman, D. & Walsh, C. A. Genetic changes shaping the human brain. Dev. Cell 32, 423–434 (2015).
Wu, T., Song, F., Cao, W., Liu, C. & Jia, S. Comparative efficacy of antidepressant medication for adolescent depression: a network meta-analysis and systematic review. BMC Psychiatry 25, 471 (2025).
Miller, L. & Campo, J. V. Depression in adolescents. N. Engl. J. Med. 385, 445–449 (2021).
Spear, L. P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 (2000).
Cipriani, A. et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet 388, 881–890 (2016).
Jane Garland, E., Kutcher, S., Virani, A. & Elbe, D. Update on the use of SSRIs and SNRIs with children and adolescents in clinical practice. J. Can. Acad. Child Adolesc. Psychiatry 25, 4–10 (2016).
Hazell, P., O’Connell, D., Heathcote, D., Robertson, J. & Henry, D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. BMJ 310, 897–901 (1995).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 (2009).
Shelton, R. C. et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 16, 751–762 (2011).
Bie, B. et al. Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat. Neurosci. 17, 223–231 (2014).
Nikolopoulos, D. et al. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann. Rheum. Dis. https://doi.org/10.1136/ard-2022-223506 (2023).
Steiner, J. et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157 (2008).
Dunn, A. L., Trivedi, M. H., Kampert, J. B., Clark, C. G. & Chambliss, H. O. Exercise treatment for depression: efficacy and dose response. Am. J. Prev. Med. 28, 1–8 (2005).
Noetel, M. et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ 384, e075847 (2024).
Rasmussen, O. V., Lopez, J., Udsen, P. & Espersen, O. Doctors involved with torture. Lancet 1, 1112 (1988).
Liu, L. et al. Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol. Psychiatry 29, 2031–2042 (2024).
Liu, R. et al. Effects of aerobic exercise on depression-like behavior and TLR4/NLRP3 pathway in hippocampus CA1 region of CUMS-depressed mice. J. Affect. Disord. 341, 248–255 (2023).
Wu, J. et al. Regular exercise ameliorates high-fat diet-induced depressive-like behaviors by activating hippocampal neuronal autophagy and enhancing synaptic plasticity. Cell Death Dis. 15, 737 (2024).
Kvam, S., Kleppe, C. L., Nordhus, I. H. & Hovland, A. Exercise as a treatment for depression: a meta-analysis. J. Affect Disord. 202, 67–86 (2016).
Recchia, F. et al. Comparative effectiveness of exercise, antidepressants and their combination in treating non-severe depression: a systematic review and network meta-analysis of randomised controlled trials. Br. J. Sports Med. 56, 1375–1380 (2022).
Cooney, G. M. et al. Exercise for depression. Cochrane Database Syst. Rev. 2013, CD004366 (2013).
Mura, G., Moro, M. F., Patten, S. B. & Carta, M. G. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 19, 496–508 (2014).
Houttu, V. et al. Deep phenotyping of patients with MASLD upon high-intensity interval training. JHEP Rep. 7, 101289 (2025).
Sabaghi, A., Ebrahimi, B., Yousofvand, N. & Hoseini, R. Comparative effects of moderate-intensity continuous training and high-intensity interval training on ADHD symptoms and behavioral inhibition in children. Eur. J. Pediatr. 184, 183 (2025).
Yao, Y. et al. High-intensity interval training ameliorates postnatal immune activation-induced mood disorders through KDM6B-regulated glial activation. Brain Behav. Immun. 120, 290–303 (2024).
Werner, F. et al. Psychosomatic-psychotherapeutic treatment of stress-related disorders impacts the sphingolipid metabolism towards increased sphingosine and sphingosine-1-phosphate levels. Eur. Arch. Psychiatry Clin. Neurosci. https://doi.org/10.1007/s00406-025-01985-2 (2025).
Kornhuber, J., Muller, C. P., Becker, K. A., Reichel, M. & Gulbins, E. The ceramide system as a novel antidepressant target. Trends Pharm. Sci. 35, 293–304 (2014).
Kornhuber, J. et al. High activity of acid sphingomyelinase in major depression. J. Neural Transm.112, 1583–1590 (2005).
Gulbins, E. et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat. Med. 19, 934–938 (2013).
Schumacher, F. et al. Stress induces major depressive disorder by a neutral sphingomyelinase 2-mediated accumulation of ceramide-enriched exosomes in the blood plasma. J. Mol. Med. 100, 1493–1508 (2022).
Park, J. W., Park, W. J. & Futerman, A. H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 (2014).
Holmes, R. S., Barron, K. A. & Krupenko, N. I. Ceramide synthase 6: comparative analysis, phylogeny and evolution. Biomolecules 8, https://doi.org/10.3390/biom8040111 (2018).
Spassieva, S. D. et al. Ectopic expression of ceramide synthase 2 in neurons suppresses neurodegeneration induced by ceramide synthase 1 deficiency. Proc. Natl. Acad. Sci. USA 113, 5928–5933 (2016).
Vanni, N. et al. Impairment of ceramide synthesis causes a novel progressive myoclonus epilepsy. Ann. Neurol. 76, 206–212 (2014).
Zhao, L. et al. A deficiency of ceramide biosynthesis causes cerebellar Purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 7, e1002063 (2011).
Antoniuk, S., Bijata, M., Ponimaskin, E. & Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci. Biobehav. Rev. 99, 101–116 (2019).
Aoki, M., Aoki, H., Ramanathan, R., Hait, N. C. & Takabe, K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat. Inflamm. 2016, 8606878 (2016).
Karunakaran, I. et al. Neural sphingosine 1-phosphate accumulation activates microglia and links impaired autophagy and inflammation. Glia 67, 1859–1872 (2019).
Lin, P. Y., Ma, Z. Z., Mahgoub, M., Kavalali, E. T. & Monteggia, L. M. A synaptic locus for TrkB signaling underlying ketamine rapid antidepressant action. Cell Rep. 36, 109513 (2021).
Wu, Y. et al. Subregion-specific, modality-dependent and timescale-sensitive hippocampal connectivity alterations in patients with first-episode, drug-naive major depression disorder. J. Affect. Disord. 305, 159–172 (2022).
Wewege, M., van den Berg, R., Ward, R. E. & Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes. Rev. 18, 635–646 (2017).
Isanejad, A., Nazari, S., Gharib, B. & Motlagh, A. G. Comparison of the effects of high-intensity interval and moderate-intensity continuous training on inflammatory markers, cardiorespiratory fitness, and quality of life in breast cancer patients. J. Sport Health Sci. 12, 674–689 (2023).
Yuan, Z. et al. HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J. Physiol. Biochem. 78, 641–652 (2022).
Kalinichenko, L. S. et al. Neutral sphingomyelinase mediates the co-morbidity trias of alcohol abuse, major depression and bone defects. Mol. Psychiatry 26, 7403–7416 (2021).
Muller, C. P. et al. Paradoxical antidepressant effects of alcohol are related to acid sphingomyelinase and its control of sphingolipid homeostasis. Acta Neuropathol. 133, 463–483 (2017).
Gulbins, A. et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol. Psychiatry 23, 2324–2346 (2018).
Song, Y. et al. Saikosaponin antidepressant mechanism: improving the sphingolipid metabolism in the cortex via Apolipoprotein E and triggering neurovascular coupling. Phytomedicine 132, 155829 (2024).
Chen, S. J. et al. Sortilin deletion in the prefrontal cortex and hippocampus ameliorates depressive-like behaviors in mice via regulating ASM/ceramide signaling. Acta Pharm. Sin. 43, 1940–1954 (2022).
Kanno, T. & Nishizaki, T. Sphingosine induces apoptosis in hippocampal neurons and astrocytes by activating caspase-3/-9 via a mitochondrial pathway linked to SDK/14-3-3 protein/Bax/cytochrome c. J. Cell Physiol. 226, 2329–2337 (2011).
Tanaka, S. et al. Sphingosine 1-phosphate signaling in perivascular cells enhances inflammation and fibrosis in the kidney. Sci. Transl. Med. 14, eabj2681 (2022).
Nakajima, K., Tohyama, Y., Kohsaka, S. & Kurihara, T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J. Neurochem. 80, 697–705 (2002).
Yang, M. S., Jou, I., Inn-Oc, H. & Joe, E. Sphingomyelinase but not ceramide induces nitric oxide synthase expression in rat brain microglia. Neurosci. Lett. 311, 133–136 (2001).
Zoicas, I. et al. Ceramides affect alcohol consumption and depressive-like and anxiety-like behavior in a brain region- and ceramide species-specific way in male mice. Addict. Biol. 25, e12847 (2020).
Zoicas, I. et al. Anxiety and depression are related to higher activity of sphingolipid metabolizing enzymes in the rat brain. Cells 9, https://doi.org/10.3390/cells9051239 (2020).
Acknowledgments
We thank the Translational Medicine Core Facility of Shandong University for instrumental support. This study was supported by funding from the National Natural Science Foundation of China (No. 82071267 and 82371179), the Natural Science Foundation of Shandong Province (No. ZR2024MH131), and Foundation of Jinan (No. 2021GXRC103).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.H., W.Z., N.L. Performed experiments: N.L., Y.Y. Data collection and analysis: N.L., Y.Y., J.D., X.Q., L.W. Investigation: N.L., Y.Y., D.W., F.P., F.M., Y.Z. Funding acquisition: A.H., W.Z., Writing—original draft: N.L. Writing—review & editing: A.H., W.Z., Supervision: A.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Liubov Kalinichenko, Christian Mueller, Karina Luduvico, and the other anonymous reviewer for their contribution to the peer review of this work. Primary Handling Editors: Eliana Scemes and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., Yao, Y., Du, J. et al. Exercise improves depressive-like behavior in adolescent mice by regulating sphingosine and ceramide metabolism through microglial CerS1. Commun Biol 8, 941 (2025). https://doi.org/10.1038/s42003-025-08347-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08347-7