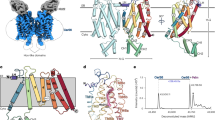
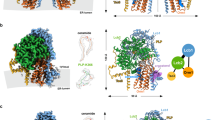
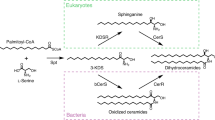
Abstract
Ceramides are essential lipids involved in forming complex sphingolipids and acting as signaling molecules. They result from the N-acylation of a sphingoid base and a CoA-activated fatty acid, a reaction catalyzed by the ceramide synthase (CerS) family of enzymes. Yet, the precise structural details and catalytic mechanisms of CerSs have remained elusive. Here we used cryo-electron microscopy single-particle analysis to unravel the structure of the yeast CerS complex in both an active and a fumonisin B1-inhibited state. Our results reveal the complex’s architecture as a dimer of Lip1 subunits bound to the catalytic subunits Lag1 and Lac1. Each catalytic subunit forms a hydrophobic crevice connecting the cytosolic site with the intermembrane space. The active site, located centrally in the tunnel, was resolved in a substrate preloaded state, representing one intermediate in ceramide synthesis. Our data provide evidence for competitive binding of fumonisin B1 to the acyl-CoA-binding tunnel.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All density maps and models were deposited to the EM Data Bank (https://www.ebi.ac.uk/emdb/) and the PDB (https://www.rcsb.org/) and are publicly available under the following accession numbers: PDB 8QTN and EMD-18652 for ScCerS apo; PDB 8QTR and EMD-18653 for FB1-bound ScCerS. The CerS model is available from ModelArchive at https://www.modelarchive.org/doi/10.5452/ma-i2sme. All structural data were visualized with ChimeraX and protein interactions were analyzed with the help of PDBe PISA73. The 2D ligand–protein interaction diagrams were calculated using LigPlotZ74. The MS proteomics data were deposited to the ProteomeXchange Consortium through the PRIDE partner repository and are publicly available (PXD054198 and PXD054237). Other databases used were UniProt (https://www.uniprot.org/), AlphaFold-Multimer (https://cosmic-cryoem.org/tools/alphafoldmultimer/) and AlphaFold. Source data are provided with this paper.
Code availability
No code was generated.
References
Hannun, Y. A. & Obeid, L. M. Many ceramides. J. Biol. Chem. 286, 27855–27862 (2011).
Dadsena, S. et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 10, 1832 (2019).
Pettus, B. J., Chalfant, C. E. & Hannun, Y. A. Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta 1585, 114–125 (2002).
Hannun, Y. A. & Obeid, L. M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 175–191 (2018).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Morad, S. A. F. & Cabot, M. C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 (2013).
Coant, N., García-Barros, M., Zhang, Q., Obeid, L. M. & Hannun, Y. A. AKT as a key target for growth promoting functions of neutral ceramidase in colon cancer cells. Oncogene 37, 3852–3863 (2018).
Canals, D. et al. Ceramide launches an acute anti-adhesion pro-migration cell signaling program in response to chemotherapy. FASEB J. 34, 7610–7630 (2020).
Jana, A., Hogan, E. L. & Pahan, K. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 278, 5–15 (2009).
Choi, M. J. & Maibach, H. I. Role of ceramides in barrier function of healthy and diseased skin. Am. J. Clin. Dermatol 6, 215–223 (2005).
Sribney, M. Enzymatic synthesis of ceramide. Biochim. Biophys. Acta 125, 542–547 (1966).
Morell, P. & Radin, N. S. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A’s by brain microsomes. J. Biol. Chem. 245, 342–350 (1970).
Mullen, T. D., Hannun, Y. A. & Obeid, L. M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441, 789–802 (2012).
Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 (2003).
Körner, C. & Fröhlich, F. Compartmentation and functions of sphingolipids. Curr. Opin. Cell Biol. 74, 104–111 (2022).
Breslow, D. K. et al. Orm family proteins mediate sphingolipid homeostasis. Nature 463, 1048–1053 (2010).
Schäfer, J.-H. et al. Structure of the ceramide-bound SPOTS complex. Nat. Commun. 14, 6196 (2023).
Megyeri, M. et al. Yeast ceramide synthases, Lag1 and Lac1, have distinct substrate specificity. J. Cell Sci. 132, jcs228411 (2019).
Clausmeyer, L. & Fröhlich, F. Mechanisms of nonvesicular ceramide transport. Contact 6, 25152564231208250 (2023).
Limar, S. et al. Yeast Svf1 binds ceramides and contributes to sphingolipid metabolism at the ER cis-Golgi interface. J. Cell Biol. 222, e202109162 (2023).
Funato, K. & Riezman, H. Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast. J. Cell Biol. 155, 949–960 (2001).
Kumagai, K. et al. CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 280, 6488–6495 (2005).
Winter, E. & Ponting, C. P. TRAM, LAG1 and CLN8: members of a novel family of lipid-sensing domains? Trends Biochem. Sci. 27, 381–383 (2002).
Venkataraman, K. et al. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 277, 35642–35649 (2002).
Laviad, E. L. et al. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 283, 5677–5684 (2008).
Riebeling, C., Allegood, J. C., Wang, E., Merrill, A. H. & Futerman, A. H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 278, 43452–43459 (2003).
Mizutani, Y., Kihara, A. & Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 (2005).
Tidhar, R. et al. Eleven residues determine the acyl chain specificity of ceramide synthases. J. Biol. Chem. 293, 9912–9921 (2018).
Sociale, M. et al. Ceramide synthase Schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep. 22, 967–978 (2018).
Guillas, I. et al. Human homologues of LAG1 reconstitute Acyl-CoA-dependent ceramide synthesis in yeast. J. Biol. Chem. 278, 37083–37091 (2003).
Vallée, B. & Riezman, H. Lip1p: a novel subunit of acyl-CoA ceramide synthase. EMBO J. 24, 730–741 (2005).
Muir, A., Ramachandran, S., Roelants, F. M., Timmons, G. & Thorner, J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. eLife 3, 944–956 (2014).
Fresques, T. et al. Regulation of ceramide synthase by casein kinase 2-dependent phosphorylation in Saccharomyces cerevisiae. J. Biol. Chem. 290, 1395–1403 (2015).
Kim, J. L., Ben-Dor, S., Rosenfeld-Gur, E. & Futerman, A. H. A novel C-terminal DxRSDxE motif in ceramide synthases involved in dimer formation. J. Biol. Chem. 298, 101517 (2022).
Spassieva, S. et al. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J. Biol. Chem. 281, 33931–33938 (2006).
Zelnik, I. D. et al. Computational design and molecular dynamics simulations suggest the mode of substrate binding in ceramide synthases. Nat. Commun. 14, 2330 (2023).
Wang, E., Norred, W. P., Bacon, C. W., Riley, R. T. & Merrill, A. H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 266, 14486–14490 (1991).
Pascoa, T. C. et al. Structural basis of the mechanism and inhibition of a human ceramide synthase. Preprint at bioRxiv https://doi.org/10.1101/2023.12.02.569723 (2023).
Færgeman, N. J. et al. Acyl-CoA-binding protein, Acb1p, is required for normal vacuole function and ceramide synthesis in Saccharomyces cerevisiae. Biochem. J. 380, 907–918 (2004).
Gaigg, B. et al. Depletion of acyl-coenzyme A-binding protein affects sphingolipid synthesis and causes vesicle accumulation and membrane defects in Saccharomyces cerevisiae. Mol. Biol. Cell 12, 1147–1160 (2001).
Merrill, A. H., Sullards, M. C., Wang, E., Voss, K. A. & Riley, R. T. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 109, 283–289 (2001).
Xie, T. et al. Structure and mechanism of a eukaryotic ceramide synthase complex. EMBO J. 42, e114889 (2023).
Sui, X. et al. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature 581, 323–328 (2020).
Wang, L. et al. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature 581, 329–332 (2020).
Kageyama-Yahara, N. & Riezman, H. Transmembrane topology of ceramide synthase in yeast. Biochem. J. 398, 585–593 (2006).
Fröhlich, F., Olson, D. K., Christiano, R., Farese, R. V. & Walther, T. C. Proteomic and phosphoproteomic analyses of yeast reveal the global cellular response to sphingolipid depletion. Proteomics 16, 2759–2763 (2016).
Muir, A., Ramachandran, S., Roelants, F. M., Timmons, G. & Thorner, J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. eLife 3, e03779 (2014).
Tkach, J. M. et al. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol. 14, 966–976 (2012).
Turner, N. et al. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun. 9, 3165 (2018).
Janke, C. et al. A versatile toolbox for PCR‐based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Fröhlich, F., Christiano, R. & Walther, T. C. Native SILAC: metabolic labeling of proteins in prototroph microorganisms based on lysine synthesis regulation. Mol. Cell. Proteom. 12, 1995–2005 (2013).
Olsen, J. et al. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods 4, 709–712 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Tusher, V. G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121 (2001).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Ejsing, C. S. et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl Acad. Sci. USA 106, 2136–2141 (2009).
Esch, B. M. et al. Uptake of exogenous serine is important to maintain sphingolipid homeostasis in Saccharomyces cerevisiae. PLoS Genet. 16, e1008745 (2020).
Goedhart, J. SuperPlotsOfData—a web app for the transparent display and quantitative comparison of continuous data from different conditions. Mol. Biol. Cell 32, 470–474 (2021).
Januliene, D. & Moeller, A.Single-particle cryo-EM of membrane proteins. Methods Mol. Biol. 2302, 153–178 (2021).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Bryant, P., Pozzati, G. & Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 13, 1265 (2022).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low- resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in PHENIX. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Sehnal, D. et al. MOLE 2.0: advanced approach for analysis of biomacromolecular channels. J. Cheminform. 5, 39 (2013).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Acknowledgements
We thank members of the F.F. and A.M. labs for their valuable comments on the manuscript. This work was funded by the German Research Foundation DFG (SFB1557 P6 to F.F., P11 to A.M., FR 3647/2-2, INST 190/201-1 [503478512] and FR 3647/4-1 [491484150] to F.F. and INST.190/196-1 FUGG [455249649], MO2752/3-6 [37802001] and SPRUNG Stay inspired 15-76251-2-04/22 (10743/2022) to A.M.), the BMBF 532 01ED2010 to A.M. and the RISE UP! program of the Boehringer Ingelheim Foundation to F.F. S.V. acknowledges support from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 803952). J.-H.S. is supported by a fellowship from the Friedrich Ebert foundation.
Author information
Authors and Affiliations
Contributions
J.H.S., L.C., C.K., V.N.W., B.M.E., S.W., J.S. and Y.A. carried out all experiments. J.H.S., L.C., C.K., V.N.W., B.M.E. and S.W. performed the data analysis. J.H.S., L.C., C.K., J.S., Y.A. and F.F. performed data visualization. F.F., A.M. and D.J. conceptualized the project. J.H.S., L.C., C.K., F.F. and A.M. wrote the original draft of the manuscript. J.H.S., L.C., C.K., S.V., F.F. and A.M. reviewed and edited the text. F.F., A.M. and S.V. secured funding for this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Michael Airola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro functional characterization of the yeast ceramide synthase complex.
A Pfam-based ___domain annotation of the CerS subunits. B Summary of the mass spectrometric analysis of the sample GST-Acb1 (E). C Summary of the mass spectrometric analysis of the CerS sample (F). D Size-exclusion chromatography profile of GDN-solubilized CerS complex. E Coomassie-blue stained SDS-PAGE gel of purified GST-Acb1. N = 1. F Coomassie-blue stained SDS-PAGE gel of GDN-solubilized CerS complex of indicated fraction from (D). N = 1. G Representative micrograph and 2D class averages from negative-stain TEM. 100 nm scale bar. 36 micrographs were taken and 4170 partikel are used for the 2D classes. H Relative CerS enzyme activity measurement with or without the specific CerS-inhibitor fumonisin B1 (FB1). Control with addition of BSA and the Fatty-acid-Coenzyme A transport protein Acb1. n = 3 technically independent samples for H and data are presented as mean values ± SD. Exact P-values are shown in Supplementary Table 4. I Label free proteomics of yeast cells expressing Lag1-msGFP2 compared to untagged control cells. In the volcano plot, the protein abundance ratios of Lag1-msGFP2 over control cells are plotted against the negative log10 of the P-value of the two-tailed t-test for each protein. J Chemical structures of FB1 (Fumonisin-B1), PHS (phytosphingosine) and C26:0 (hexacosanoic acid).
Extended Data Fig. 2 Cryo-EM analysis and validation of the ceramide synthase apo complex.
A Processing workflow for the CerS in its apo state. All processing steps were performed in cryoSPARC. Representative cryo-EM micrograph and 2D-class averages are given. 50 nm scale bar in micrograph. B FSC-curve with gold-standard criterion at 0.134 and 3.0 Å overall resolution. C Local resolution estimation (threshold 0.16). D Angular particle distribution.
Extended Data Fig. 3 Cryo-EM analysis and validation of the FB1-bound ceramide synthase complex.
A Processing workflow for the FB1-bound CerS complex. All processing steps were performed in cryoSPARC. Representative cryo-EM micrograph and 2D-class averages are given. 50 nm scale bar in micrograph. B FSC-curve with gold-standard criterion at 0.134 and 3.2 Å overall resolution. C Local resolution estimation (threshold 0.16). D Angular particle distribution.
Extended Data Fig. 4 Local cryo-EM density map quality of apo and FB1-bound ceramide synthase.
Side-by-side comparison of selected residues and ligands within all cryo-EM density maps of A Lag1 B FB1 (Fumonisin-B1) C Lac1, D Lip1, E Lip1*, F C26:0-His255 (Lag1/Lac1) G PI16:0-lipid (phosphoinositide) and ammonium ion NH4 + , H PE18:0-lipid (phosphoethanolamine). Contouring levels set to 0.16.
Extended Data Fig. 5 Ligand interaction diagram of ceramide synthase.
2D ligand interaction diagram of: A C26:0 in the apo-state with Lag1, B C26:0 in the apo-state with Lac1, C C26:0 in the FB1-bound state with Lag1, D C26:0 in the FB1-bound state with Lac1, E FB1-binding to Lag1, F FB1-binding to Lac1, G PI16:0 binding to Lac1 in the apo-state and H PI16:0 binding to Lac1 in the FB1-bound state. I PI16:0 binding to Lip1 in FB1-bound state and Lip1* in J. Diagrams were calculated and visualized with LigPlot+1.
Extended Data Fig. 6 Analysis of Lac1 Mutants.
Purification of a Lac1-FLAG-Lip1 homodimer analyzed by SDS-PAGE for A Lac1-WT B Lac1_5F C Lac1_PP D Lac1 K389A R392A. Each purification N = 1. E-H Proteomic analysis of the purified complex. Unique peptides of each protein identified are shown in the tables. I Specific CerS enzyme activity measurement in the presence of the fatty-acid-Coenzyme A transport protein Acb1 for a heterodimeric Lac1-Lip1 complex and the respective Lac1_5F and Lac1_PP mutants. n = 3 technically independent samples for I and data are presented as mean values ± SD. Exact P-values are shown in Supplementary Table 4. J Analysis of the co-purified long chain bases with the Lac1_WT-Lip1 heterodimeric complex or the Lac1_5F-Lip1 heterodimeric complex. n = 4 technically independent samples for J and data are presented as mean values ± SD. Exact P-values are shown in Supplementary Table 5. K Tetrad analysis of lac1Δ (blue) lag1Δ (green) cells, expressing Lac1_5F (upper panel) in dark grey and Lac1_PPWLac1_PP (lower panel) in light grey.
Extended Data Fig. 7 Analysis of Lip1 Mutants.
Purification of the heterodimeric CerS complex analyzed by SDS-PAGE for A Lip1_F40R and B Lip1_W50A_F51A. Each purifications N = 1. C Proteomic analysis of the purified complex with Lip1_F40R and in D with Lip1_W50A_F51A. Unique peptides of each protein identified are shown in the tables. E Relative CerS enzyme activity measurement in the presence of the fatty-acid-Coenzyme A transport protein Acb1 for the CerS with either WT Lip1, Lip1_F40R or Lip1_W50A_F51A. n = 3 technically independent samples for E and data are presented as mean values ± SD. Exact P-values are shown in Supplementary Table 4. F Levels of phytosphingosine (PHS), in WT cells, lip1Δ cells expressing LIP1_F40R or lip1Δ cells. n = 3 technically independent samples for F and data are presented as mean values ± SD. Exact P-values are shown in Supplementary Table 8. G Tetrad analysis of lip1Δ (orange) cells, expressing LIP1_WT (upper panel, brown), or LIP1_F40R (lower panel, sand).
Extended Data Fig. 8 Structural conservation of the yeast ceramide synthase.
A Cartoon representation of Lac1 color-coded by sequence-conservation with data from Supplementary Fig. 10a. B Cartoon representation of Lag1 color-coded by sequence-conservation with data from Supplementary Fig. 11. Yellow areas denote regions of no conservation. The catalytic residues are highlighted as spheres. C Luminal view of the cartoon-representation of superimposed Lag1 (yellow) and Lag1 (grey), color-coded by Cα root-mean-square deviation (r.s.m.d.). Catalytic residues and C26:0 are shown as sticks. D Pair-based sequence alignment of Lac1 Saccharomyces cerevisiae (P28496) and Lag1 Saccharomyces cerevisiae (P38703). Sequences are color coded by conservation with a cut-off at 30 %. Catalytic residues are highlighted in red. Non-resolvable residues from cryo-EM are marked in light gray. Alignment was prepared with Jalview. E Comparison of local densities in Lag1 and Lac1 for density-based subunit differentiation. The corresponding sequences are highlighted in yellow in the alignment (D).
Extended Data Fig. 9 AlphaFold2 multimer prediction of the yeast ceramide synthase.
A AlphaFold2 multimer prediction of the yeast CerS (ScLag1: P38703, ScLac1: P28496, ScLip1: Q03579) shown as cartoon-representation, color-coded by the predicted local distance difference test (pLDDT). The low-confidence termini of each subunit are annotated accordingly. Distance measurement between Cα of Lag1-D390 and Lac1-D394 from their respective proposed dimerization motif (DXRSDXE). B Validation parameters of the prediction, including the pLDDT and the PAE (prediction aligned error matrix). The pLDDT plot is color-coded according to the different subunits. The prediction was run locally using AlphaFold v.2.1.0 (ref. 67) in the multimer-mode (template date set to 2020-05-14).
Extended Data Fig. 10 Sequence conservation of the yeast ceramide synthase subunits Lac1 and Lip1.
A Clustal-based multiple-sequence alignment (MSA) of Lac1 Saccharomyces cerevisiae (P28496), Lag1 Arabidopsis thaliana (Q9LDF2), CS hyl-1 Caenorhabditis elegans (G5ED45), TLC ___domain containing protein Caenorhabditis elegans (X5LPS5), CS Dictyostelium discoideum (Q54S87), CS4 Mus musculus (Q9D6J1), CS2-like Danio rerio (E7F9X7), CS2 Homo sapiens (Q96G23), CS1 Homo sapiens (P27544). Sequences are color coded by conservation with a cut-off at 30 %. Catalytic residues are highlighted in red. Sequences were selected by highest sequence identity hits from protein BLAST of selected model organisms. B Sequence of Saccharomyces cerevisiae Lip1 (Q03579). Non-resolvable residues from cryo-EM are marked in light gray. MSAs were prepared with Jalview3.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Tables 1–9.
Source data
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed plates of tetrad dissections.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed Coomassie gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed Coomassie gels and unprocessed plates of tetrad dissections.
Source Data Extended Data Fig. 7
Unprocessed Coomassie gels and unprocessed plates of tetrad dissections.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schäfer, JH., Clausmeyer, L., Körner, C. et al. Structure of the yeast ceramide synthase. Nat Struct Mol Biol 32, 441–449 (2025). https://doi.org/10.1038/s41594-024-01415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01415-2